Immunoexpression of Relaxin and Its Receptors in Stifle Joints of Dogs with Cranial Cruciate Ligament Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. Double Immunofluorescence
2.3. Western Blot
2.4. Histologic Scoring of Immunoreactivity
2.5. Statistical Analysis
3. Results
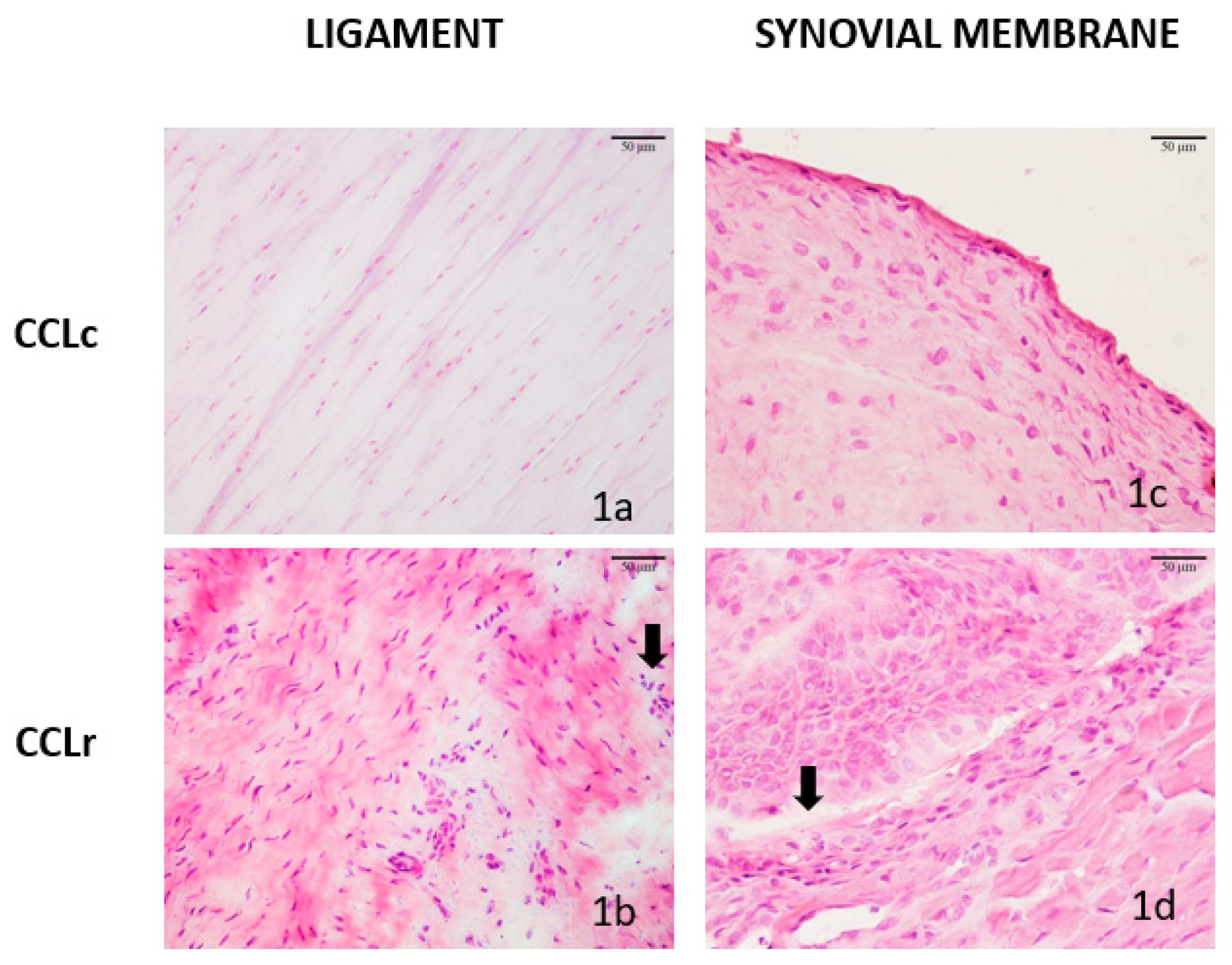
3.1. Histologic Features of CCL and SM Samples
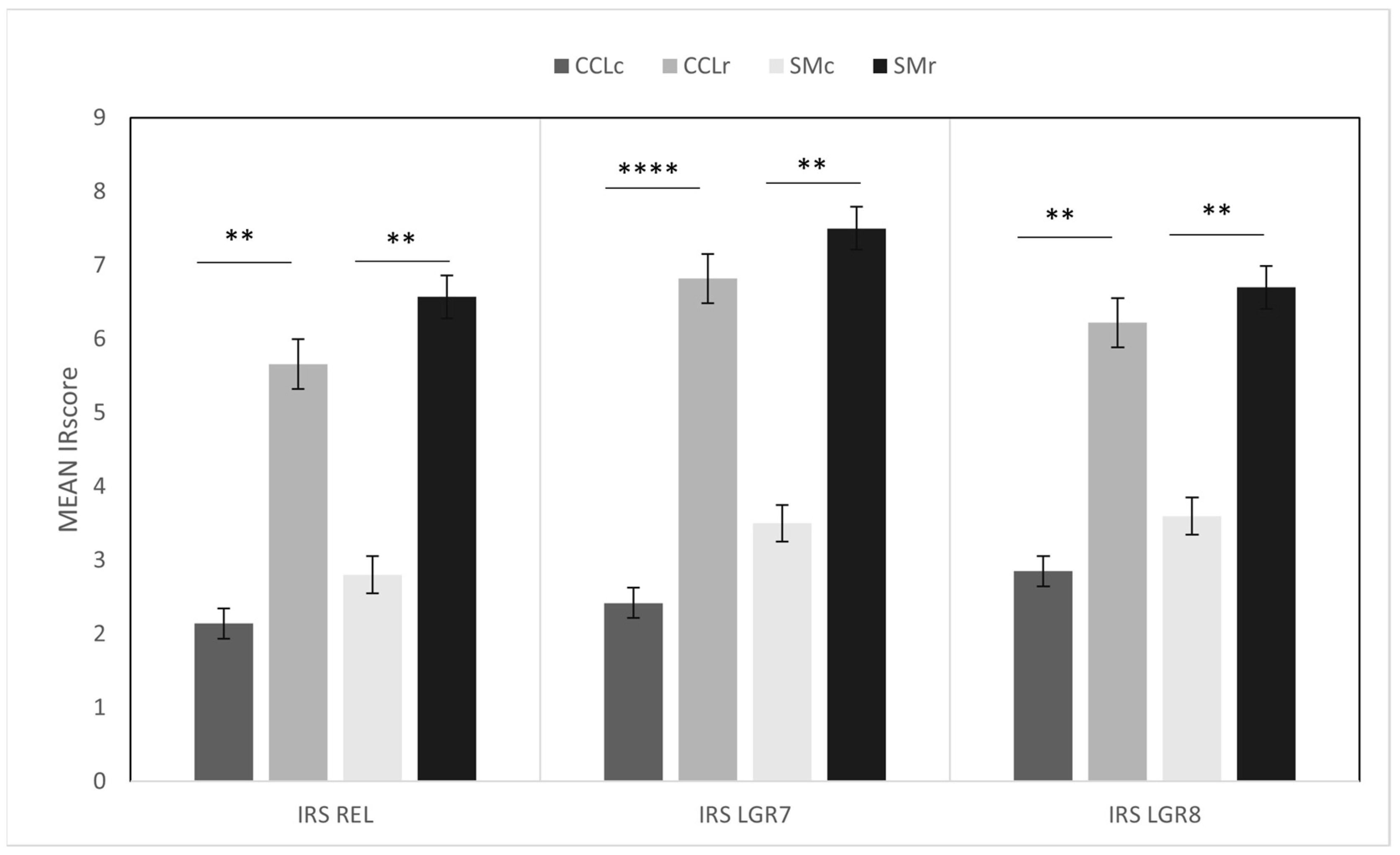
3.2. Immunohistochemistry
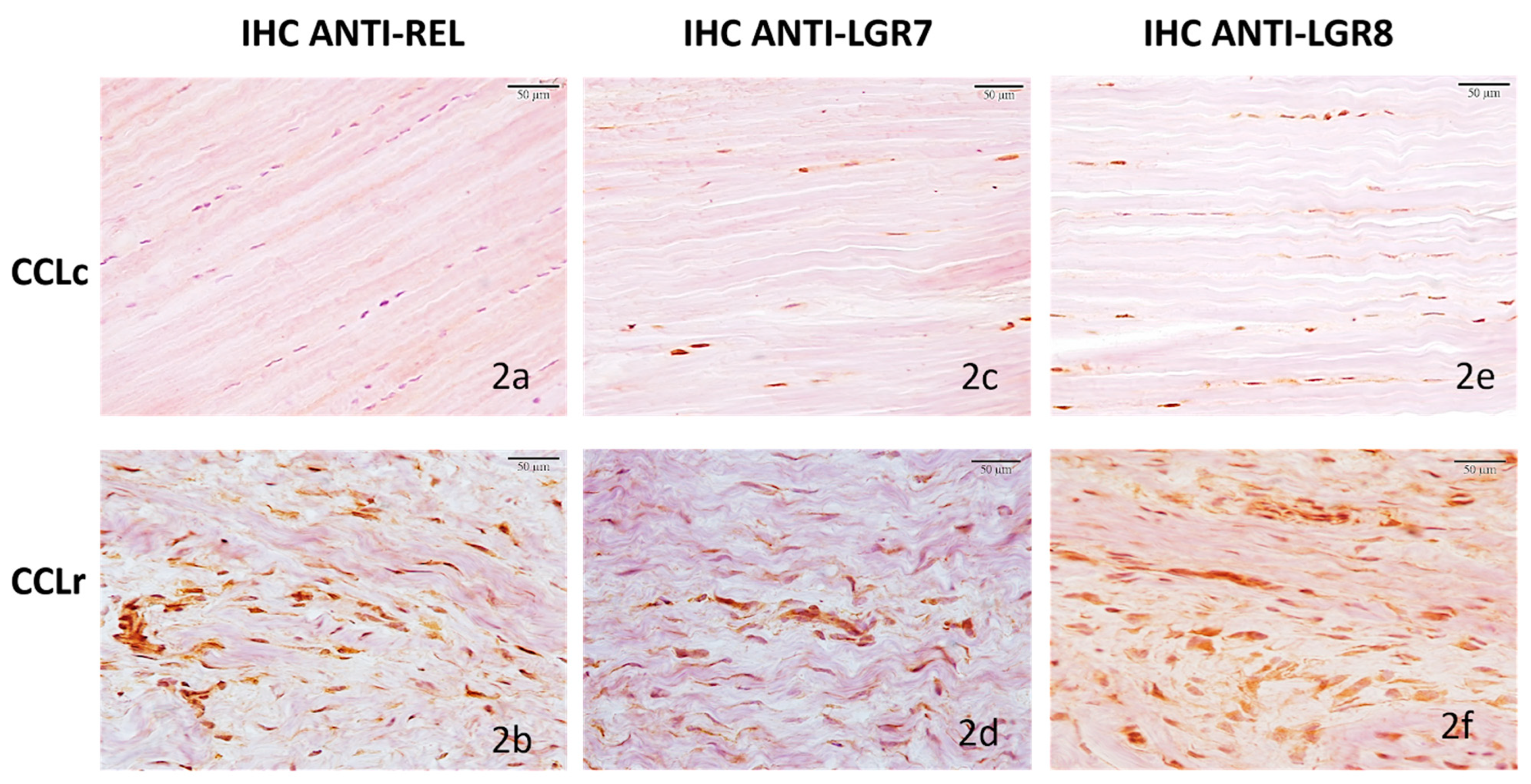
3.2.1. Cranial Cruciate Ligaments (CCL)
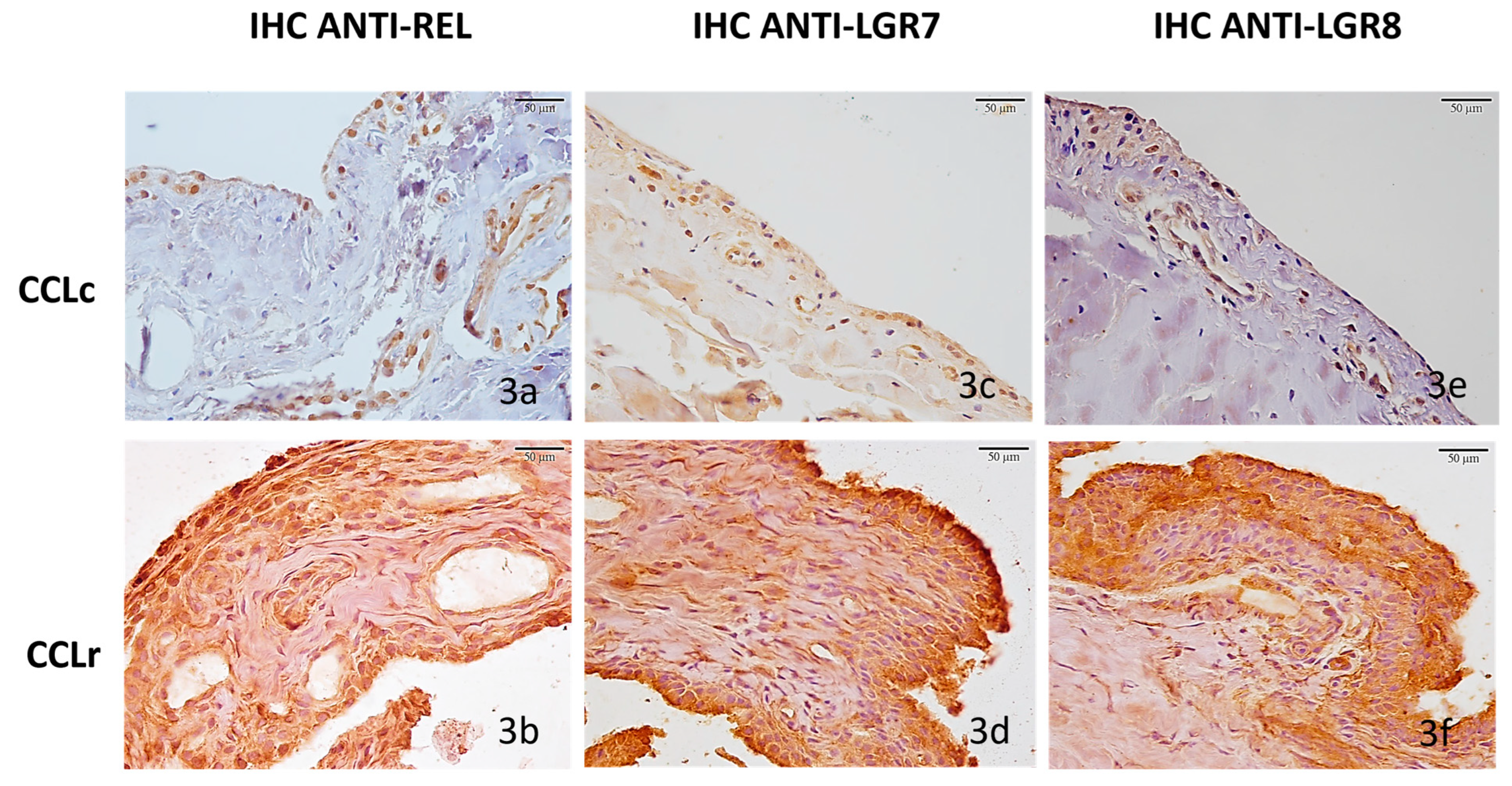
3.2.2. Synovial Membranes (SM)
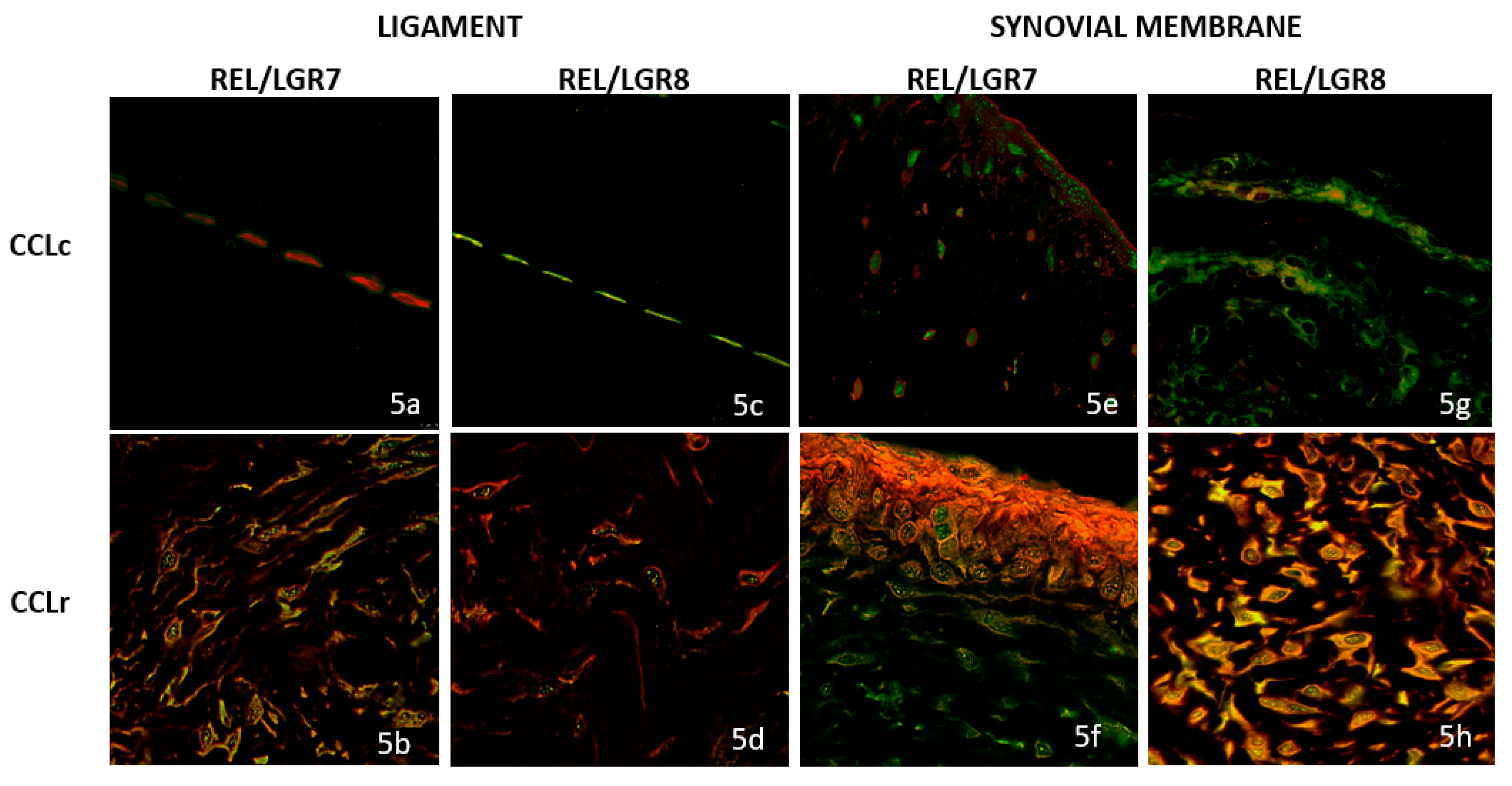
3.3. Double Immunofluorescence (IF) Staining
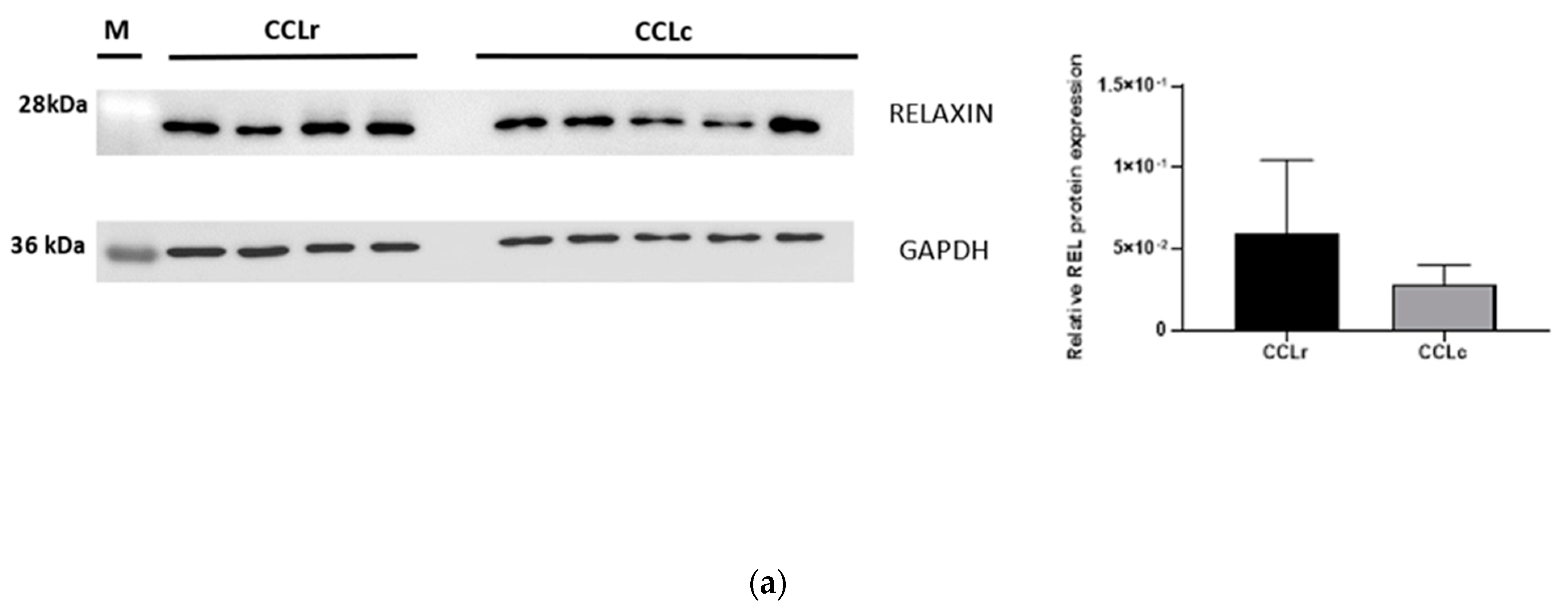
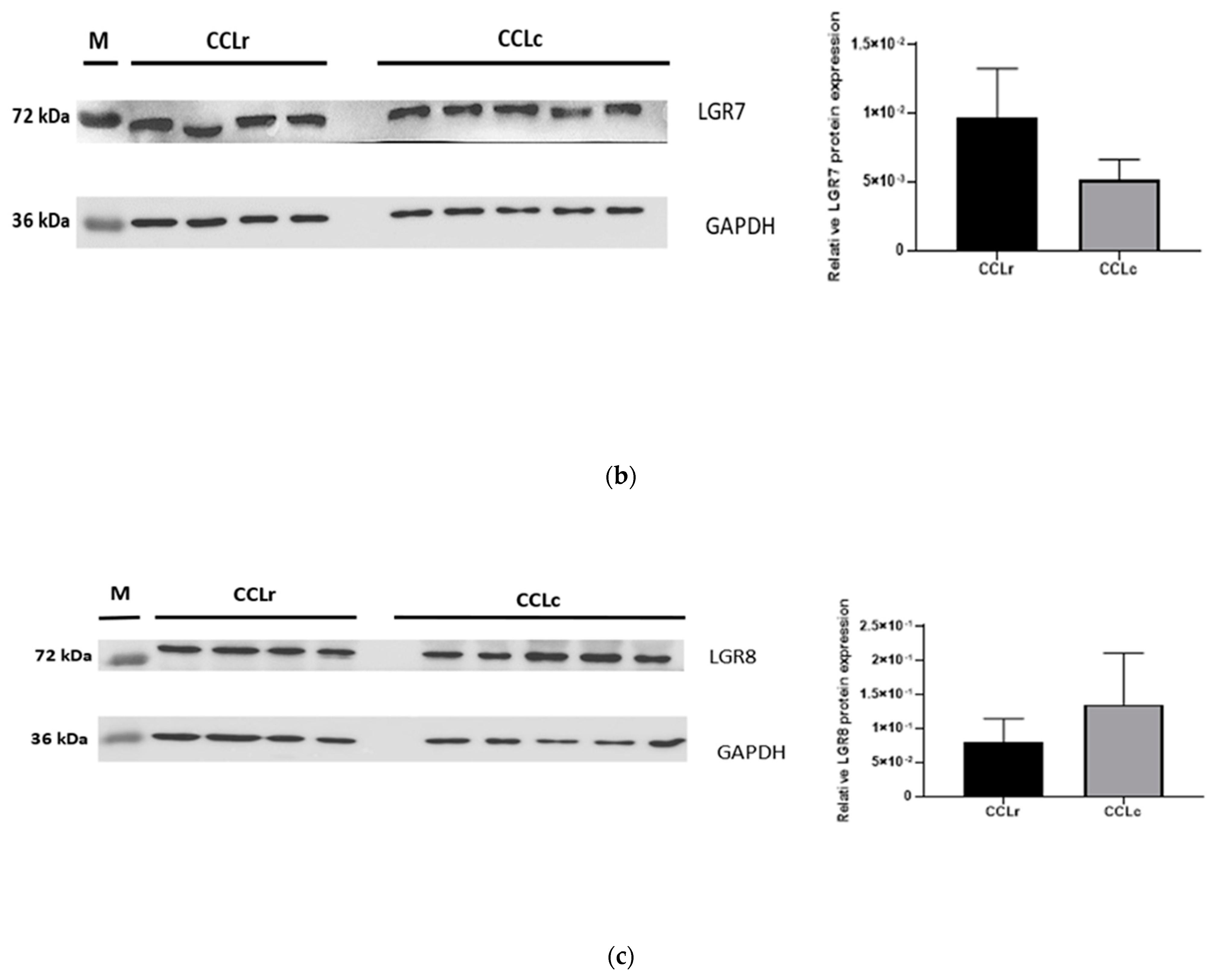
3.4. Western Blot Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayashi, K.; Manley, P.A.; Muir, P. Cranial Cruciate Ligament Pathophysiology in Dogs with Cruciate Disease: A Review. J. Am. Anim. Hosp. Assoc. 2004, 40, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Comerford, E.J.; Smith, K.; Hayashi, K. Update on the Aetiopathogenesis of Canine Cranial Cruciate Ligament Disease. Vet. Comp. Orthop. Traumatol. 2011, 24, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Frank, J.D.; Dubinsky, C.; Hao, Z.; Markel, M.D.; Manley, P.A.; Muir, P. Histologic Changes in Ruptured Canine Cranial Cruciate Ligament. Vet. Surg. 2003, 32, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, K.; Williams, N.; Cook, J.L. Histologic Evidence for a Humoral Immune Response in Synovitis Associated with Cranial Cruciate Ligament Disease in Dogs. Vet. Surg. 2021, 50, 1032–1041. [Google Scholar] [CrossRef]
- Spreng, D.; Sigrist, N.; Schweighauser, A.; Busato, A.; Schawalder, P. Endogenous Nitric Oxide Production in Canine Osteoarthritis: Detection in Urine, Serum, and Synovial Fluid Specimens. Vet. Surg. 2001, 30, 191–199. [Google Scholar] [CrossRef]
- Muir, P.; Manley, P.A.; Hao, Z. Collagen Fragmentation in Ruptured Canine Cranial Cruciate Ligament Explants. Vet. J. 2006, 172, 121–128. [Google Scholar] [CrossRef]
- Niebauer, G.W.; Niedermüller, H.; Skalicky, M. Collagen cross-links in the cruciate ligament of dogs and their relation to pathological cruciate ligament rupture. Zbl. Vet. Med. A 1983, 30, 688–693. [Google Scholar] [CrossRef]
- Neumann, S.; Lauenstein-Bosse, S. Evaluation of Transforming Growth Factor Beta 1 in Dogs with Osteoarthritis. Open Vet. J. 2018, 8, 386–392. [Google Scholar] [CrossRef] [Green Version]
- De Bruin, T.; de Rooster, H.; van Bree, H.; Cox, E. Interleukin-8 MRNA Expression in Synovial Fluid of Canine Stifle Joints with Osteoarthritis. Vet. Immunol. Immunopathol. 2005, 108, 387–397. [Google Scholar] [CrossRef]
- Barrett, J.G.; Hao, Z.; Graf, B.K.; Kaplan, L.D.; Heiner, J.P.; Muir, P. Inflammatory Changes in Ruptured Canine Cranial and Human Anterior Cruciate Ligaments. Am. J. Vet. Res. 2005, 66, 2073–2080. [Google Scholar] [CrossRef]
- Malek, S.; Weng, H.Y.; Martinson, S.A.; Rochat, M.C.; Béraud, R.; Riley, C.B. Evaluation of Serum MMP-2 and MMP-3, Synovial Fluid IL-8, MCP-1, and KC Concentrations as Biomarkers of Stifle Osteoarthritis Associated with Naturally Occurring Cranial Cruciate Ligament Rupture in Dogs. PLoS ONE 2020, 15, e0242614. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Maeda, S.; Yonezawa, T.; Matsuki, N. Synovial Fluid Matrix Metalloproteinase-2 and -9 Activities in Dogs Suffering from Joint Disorders. J. Vet. Med. Sci. 2016, 78, 1051–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niebauer, G.W.; Lubec, G. Collagenase activity in the ruptured cruciate ligament in dogs and its inhibition in vitro. Zbl. Vet. Med. A 1980, 27, 628–634. [Google Scholar] [CrossRef]
- Boland, L.; Danger, R.; Cabon, Q.; Rabillard, M.; Brouard, S.; Bouvy, B.; Gauthier, O. MMP-2 as an Early Synovial Biomarker for Cranial Cruciate Ligament Disease in Dogs. Vet. Comp. Orthop. Traumatol. 2014, 27, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Doom, M.; de Bruin, T.; de Rooster, H.; van Bree, H.; Cox, E. Immunopathological Mechanisms in Dogs with Rupture of the Cranial Cruciate Ligament. Vet. Immunol. Immunopathol. 2008, 125, 143–161. [Google Scholar] [CrossRef] [Green Version]
- Carter, S.D.; Bell, S.C.; Bari, A.S.; Bennett, D. Immune Complexes and Rheumatoid Factors in Canine Arthritides. Ann. Rheum. Dis. 1989, 48, 986–991. [Google Scholar] [CrossRef]
- Bari, A.S.; Carter, S.D.; Bell, S.C.; Morgan, K.; Bennett, D. Anti-Type II Collagen Antibody in Naturally Occurring Canine Joint Diseases. Br. J. Rheumatol. 1989, 28, 480–486. [Google Scholar] [CrossRef]
- Menzel, E.J.; Niebauer, G.; Smolen, J.S. Demonstration of C 1 Q-Binding Immune Complexes in Dogs with Arthritis of the Femoro-Tibial Joints Accompanied by Rupture of the Anterior Cruciate Ligaments. Zbl. Vet. Med. B 1980, 27, 658–667. [Google Scholar] [CrossRef]
- Niebauer, G.W.; Menzel, E.J. Immunological Changes in Canine Cruciate Ligament Rupture. Res. Vet. Sci. 1982, 32, 235–241. [Google Scholar] [CrossRef]
- Niebauer, G.W.; Wolf, B.; Bashey, R.I.; Newton, C.D. Antibodies to Canine Collagen Types I and II in Dogs with Spontaneous Cruciate Ligament Rupture and Osteoarthritis. Arthritis Rheum. 1987, 30, 319–327. [Google Scholar] [CrossRef]
- Kuroki, K.; Williams, N.; Ikeda, H.; Bozynski, C.C.; Leary, E.; Cook, J.L. Histologic Assessment of Ligament Vascularity and Synovitis in Dogs with Cranial Cruciate Ligament Disease. Am. J. Vet. Res. 2019, 80, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P.; Rubin, R.M.; Marshall, J.L. Microvasculature of the Cruciate Ligaments and Its Response to Injury. An Experimental Study in Dogs. J. Bone Jt. Surg. Am. 1979, 61, 1221–1229. [Google Scholar] [CrossRef]
- Warzee, C.C.; Dejardin, L.M.; Arnoczky, S.P.; Perry, R.L. Effect of Tibial Plateau Leveling on Cranial and Caudal Tibial Thrusts in Canine Cranial Cruciate-Deficient Stifles: An in Vitro Experimental Study. Vet. Surg. 2001, 30, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.B.; Pool, R.R.; Arnoczky, S.P.; Lau, R.E. Correlative Biomechanical and Histologic Study of the Cranial Cruciate Ligament in Dogs. Am. J. Vet. Res. 1985, 46, 1842–1854. [Google Scholar]
- Baker, S.J.; Baker, G.J. Surgical versus Nonsurgical Management for Overweight Dogs with Cranial Cruciate Ligament Rupture. J. Am. Vet. Med. Assoc. 2013, 243, 479. [Google Scholar]
- Slocum, B.; Devine, T. Cranial Tibial Thrust: A Primary Force in the Canine Stifle. J. Am. Vet. Med. Assoc. 1983, 183, 456–459. [Google Scholar]
- Wilke, V.L.; Conzemius, M.C.; Rothschild, M.F. SNP Detection and Association Analyses of Candidate Genes for Rupture of the Cranial Cruciate Ligament in the Dog. Anim. Genet. 2005, 36, 519–521. [Google Scholar] [CrossRef]
- Clements, D.N.; Short, A.D.; Barnes, A.; Kennedy, L.J.; Ferguson, J.F.; Butterworth, S.J.; Fitzpatrick, N.; Pead, M.; Bennett, D.; Innes, J.F.; et al. A Candidate Gene Study of Canine Joint Diseases. J. Hered. 2010, 101, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Ayers, D.; Clements, D.N.; Salway, F.; Day, P.J.R. Expression Stability of Commonly Used Reference Genes in Canine Articular Connective Tissues. BMC Vet. Res. 2007, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Hara, Y.; Nezu, Y.; Schulz, K.S.; Tagawa, M. Proinflammatory Cytokine Activities, Matrix Metalloproteinase-3 Activity, and Sulfated Glycosaminoglycan Content in Synovial Fluid of Dogs with Naturally Acquired Cranial Cruciate Ligament Rupture. Vet. Surg. 2006, 35, 369–376. [Google Scholar] [CrossRef]
- Hofer, D.; Forterre, S.; Schweighauser, A.; Krayer, M.; Doherr, M.; Schawalder, P.; Zurbriggen, A.; Spreng, D. Selective INOS-Inhibition Does Not Influence Apoptosis in Ruptured Canine Cranial Cruciate Ligaments. Vet. Comp. Orthop. Traumatol. 2009, 22, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Wang, W.; Nair, R.; Kapila, S. Relaxin Induces Matrix-Metalloproteinases-9 and -13 via RXFP1: Induction of MMP-9 Involves the PI3K, ERK, Akt and PKC-ζ Pathways. Mol. Cell. Endocrinol. 2012, 363, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Baccari, M.C.; Calamai, F. Relaxin: New Functions for an Old Peptide. Curr. Protein Pept. Sci. 2004, 5, 9–18. [Google Scholar] [CrossRef]
- Naqvi, T.; Duong, T.T.; Hashem, G.; Shiga, M.; Zhang, Q.; Kapila, S. Relaxin’s Induction of Metalloproteinases Is Associated with the Loss of Collagen and Glycosaminoglycans in Synovial Joint Fibrocartilaginous Explants. Arthritis Res. Ther. 2005, 7, R1–R11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiqvist, I.; Norström, A.; O’Byrne, E.; Wiqvist, N. Regulatory Influence of Relaxin on Human Cervical and Uterine Connective Tissue. Acta Endocrinol. 1984, 106, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.J.; Sherwood, O.D. The Physiological Role of Relaxin in the Pregnant Rat. IV. The Influence of Relaxin on Cervical Collagen and Glycosaminoglycans. Endocrinology 1986, 118, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, J.; Ivance, D.; Konieczko, E.; Cooney, T. Immunohistochemical Detection of Relaxin Binding to the Volar Oblique Ligament. J. Hand Surg. 2006, 31, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Clifton, K.B.; Rodner, C.; Wolf, J.M. Detection of Relaxin Receptor in the Dorsoradial Ligament, Synovium, and Articular Cartilage of the Trapeziometacarpal Joint. J. Orthop. Res. 2014, 32, 1061–1067. [Google Scholar] [CrossRef]
- Wolf, J.M.; Scher, D.L.; Etchill, E.W.; Scott, F.; Williams, A.E.; Delaronde, S.; King, K.B. Relationship of Relaxin Hormone and Thumb Carpometacarpal Joint Arthritis. Clin. Orthop. Relat. Res. 2014, 472, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Galey, S.; Konieczko, E.M.; Arnold, C.A.; Cooney, T.E. Immunohistological Detection of Relaxin Binding to Anterior Cruciate Ligaments. Orthopedics 2003, 26, 1201–1204. [Google Scholar] [CrossRef]
- Parker, E.A.; Meyer, A.M.; Goetz, J.E.; Willey, M.C.; Westermann, R.W. Do Relaxin Levels Impact Hip Injury Incidence in Women? A Scoping Review. Front. Endocrinol. 2022, 13, 827512. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Padrez, K.; Workman, R.; Lindsey, D.P. The Effect of Relaxin on the Female Anterior Cruciate Ligament: Analysis of Mechanical Properties in an Animal Model. Knee 2009, 16, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Lee, R.S.; Benhaim, P.; Finerman, G.A.M.; Hame, S.L. Relaxin Receptors in the Human Female Anterior Cruciate Ligament. Am. J. Sports Med. 2003, 31, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.M. The Influence of Ligamentous Laxity and Gender: Implications for Hand Surgeons. J. Hand Surg. Am. 2009, 34, 161–163. [Google Scholar] [CrossRef]
- Weiss, G. Relaxin in the Male1. Biol. Reprod. 1989, 40, 197–200. [Google Scholar] [CrossRef]
- Feugang, J.M.; Greene, J.M.; Sanchez-Rodríguez, H.L.; Stokes, J.V.; Crenshaw, M.A.; Willard, S.T.; Ryan, P.L. Profiling of Relaxin and Its Receptor Proteins in Boar Reproductive Tissues and Spermatozoa. Reprod. Biol. Endocrinol. 2015, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Agoulnik, A.I. Relaxin and Related Peptides in Male Reproduction. In Relaxin and Related Peptides; Advances in Experimental Medicine and Biology, Book Series; Springer: New York, NY, USA, 2007; Volume 612, pp. 49–64. [Google Scholar]
- Niebauer, G.W.; Shibly, S.; Seltenhammer, M.; Pirker, A.; Brandt, S. Relaxin of Prostatic Origin Might Be Linked to Perineal Hernia Formation in Dogs. Ann. N. Y. Acad. Sci. 2005, 1041, 415–422. [Google Scholar] [CrossRef]
- Merchav, R.; Feuermann, Y.; Shamay, A.; Ranen, E.; Stein, U.; Johnston, D.E.; Shahar, R. Expression of Relaxin Receptor LRG7, Canine Relaxin, and Relaxin-Like Factor in the Pelvic Diaphragm Musculature of Dogs with and Without Perineal Hernia. Vet. Surg. 2005, 34, 476–481. [Google Scholar] [CrossRef]
- Shahar, R.; Shamir, M.H.; Niebauer, G.W.; Johnston, D.E. A Possible Association between Acquired Nontraumatic Inguinal and Perineal Hernia in Adult Male Dogs. Can. Vet. J. 1996, 37, 614–616. [Google Scholar]
- Innes, J. Do Hormones Play a Role in Canine Cruciate Disease? J. Small Anim. Pract. 2003, 44, 520. [Google Scholar]
- Della Valle, G.; Caterino, C.; Aragosa, F.; Micieli, F.; Costanza, D.; di Palma, C.; Piscitelli, A.; Fatone, G. Outcome after Modified Maquet Procedure in Dogs with Unilateral Cranial Cruciate Ligament Rupture: Evaluation of Recovery Limb Function by Use of Force Plate Gait Analysis. PLoS ONE 2021, 16, e0256011. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different Approaches for Interpretation and Reporting of Immunohistochemistry Analysis Results in the Bone Tissue—A Review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinetz, B.G.; Williams, A.J.; Lust, G.; Schwabe, C.; Büllesbach, E.E.; Goldsmith, L.T. Transmission of Relaxin and Estrogens to Suckling Canine Pups via Milk and Possible Association with Hip Joint Laxity. Am. J. Vet. Res. 2008, 69, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; van Bell, C.; Rogers, V.; Cooney, T. The Relationship between Serum Relaxin and Knee Joint Laxity in Female Athletes. Orthopedics 2002, 25, 669–673. [Google Scholar] [CrossRef]
- Adams, P.; Bolus, R.; Middleton, S.; Moores, A.P.; Grierson, J. Influence of Signalment on Developing Cranial Cruciate Rupture in Dogs in the UK. J. Small Anim. Pract. 2011, 52, 347–352. [Google Scholar] [CrossRef]
- Summers, R.J. Recent progress in the understanding of relaxin family peptides and their receptors. Br. J. Pharmacol. 2017, 174, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, S.K.; Lee, S.K.; Kim, J.H.; Fredericson, M. Relaxin Receptor RXFP1 and RXFP2 Expression in Ligament, Tendon, and Shoulder Joint Capsule of Rats. J. Korean Med. Sci. 2016, 31, 983. [Google Scholar] [CrossRef]
- Kapila, S.; Wang, W.; Uston, K. Matrix Metalloproteinase Induction by Relaxin Causes Cartilage Matrix Degradation in Target Synovial Joints. Ann. N. Y. Acad. Sci. 2009, 1160, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Samuel, C.S.; Royce, S.G.; Hewitson, T.D.; Denton, K.M.; Cooney, T.E.; Bennett, R.G. Anti-fibrotic Actions of Relaxin. Br. J. Pharmacol. 2017, 174, 962–976. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Cheon, H.; Cho, H.; Kim, J.; Kang, J.H.; Yang, M.P.; Lee, Y.; Lee, H.; Chang, D. Evaluation of Partial Cranial Cruciate Ligament Rupture with Positive Contrast Computed Tomographic Arthrography in Dogs. J. Vet. Sci. 2008, 9, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Dehghan, F.; Muniandy, S.; Yusof, A.; Salleh, N. Sex-Steroid Regulation of Relaxin Receptor Isoforms (RXFP1 & RXFP2) Expression in the Patella Tendon and Lateral Collateral Ligament of Female WKY Rats. Int. J. Med. Sci. 2014, 11, 180–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, J.P.; Bleedorn, J.A.; Sutherland, B.J.; Sullivan, R.; Kalscheur, V.L.; Ramaker, M.A.; Schaefer, S.L.; Hao, Z.; Muir, P. Arthroscopic Assessment of Stifle Synovitis in Dogs with Cranial Cruciate Ligament Rupture. PLoS ONE 2014, 9, e97329. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P. Blood Supply to the Anterior Cruciate Ligament and Supporting Structures. Orthop. Clin. N. Am. 1985, 16, 15–28. [Google Scholar] [CrossRef]
- Hayashi, K.; Bhandal, J.; Rodriguez, C.O.J.; Kim, S.Y.; Entwistle, R.; Naydan, D.; Kapatkin, A.; Stover, S.M. Vascular Distribution in Ruptured Canine Cranial Cruciate Ligament. Vet. Surg. 2011, 40, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Jeyabalan, A.; Shroff, S.G.; Novak, J.; Conrad, K.P. The Vascular Actions of Relaxin. In Relaxin and Related Peptides; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 612, pp. 65–87. [Google Scholar] [CrossRef]
- Leo, C.H.; Jelinic, M.; Ng, H.H.; Marshall, S.A.; Novak, J.; Tare, M.; Conrad, K.P.; Parry, L.J. Vascular Actions of Relaxin: Nitric Oxide and Beyond. Br. J. Pharmacol. 2017, 174, 1002–1014. [Google Scholar] [CrossRef] [Green Version]
- Fernihough, J.K.; Innes, J.F.; Billingham, M.E.J.; Holly, J.M.P. Changes in the Local Regulation of Insulin-Like Growth Factors I and II and Insulin-Like Growth Factor-Binding Proteins in Osteoarthritis of the Canine Stifle Joint Secondary to Cruciate Ligament Rupture. Vet. Surg. 2003, 32, 313–323. [Google Scholar] [CrossRef]
- Steinetz, B.G.; Manning, J.P. Influence of Growth Hormone, Steroids and Relaxin on Acid Phosphatase Activity of Connective Tissue. Proc. Soc. Exp. Biol. Med. 1967, 124, 180–184. [Google Scholar] [CrossRef]
| (a) | ||||||||
| No. Case Control Group CCLc | Age (Years) | Breed | Body Weight (kg) | Sex | IRS IHC Ligaments REL; LGR7; LGR8 n = 7 | IRS IHC Synovial Membranes, REL n = 5; LGR7 n = 4; LGR8 n = 5 | ||
| 1 | 5 | MIXED BREED | 23 | FS | 1; 1; 1 | NA | ||
| 2 | 10 | MIXED BREED | 7 | M | 2; 2; 2 | 3; 4; 6 | ||
| 3 | 8 | ROTTWEILER | 36 | M | 2; 1; 4 | 1; 2; 2 | ||
| 4 | 9 | MIXED BREED | 31 | FS | 4; 4; 4 | 4; NA; 2 | ||
| 5 | 6 | MIXED BREED | 8 | FS | 1; 1; 2 | 4; 4; 4 | ||
| 6 | 10 | MIXED BREED | 11 | FS | 2; 4; 2 | NA | ||
| 7 | 4 | ENGLISH BOULEDOGUE | 20 | M | 3; 4; 3 | 2; 4; 4 | ||
| Median 8 Range 4–10 | Median 20 Range 7–36 | 2.62 SE 0.59 2.42 SE 0.57 2.57 SE 0.42 | 2.8 SE 0.58 3.5 SE 0.5 3.6 SE 0.74 | |||||
| (b) | ||||||||
| No. Case Sample Group CCLr | Age (Years) | Breed | Body Weight (kg) | Sex | Surgical Procedure | Time Since Injury (Days) | IRS IHC Ligaments REL; LGR7; LGR8 n = 18 | IRS IHC Synovial Membranes, REL; LGR7;LGR8 n = 14 |
| 1 | 8 | MIXED BREED | 25 | FS | TPLO | UNKNOWN | 4; 4; 4 | 6; 9; 6 |
| 2 | 4 | LABRADOR RETRIVER | 34 | FS | MP TTA + partial meniscectomy | 14 | 9; 9; 6 | 9; 9; 6 |
| 3 | 7 | JACK RUSSEL TERRIER | 6 | M | DE ANGELIS | 70 | 6; 8; 6 | 9; 4; 4 |
| 4 | 5 | MIXED BREED | 15 | F | DE ANGELIS | 21 | 9; 9; 9 | 9; 9; 9 |
| 5 | 5 | CAIRN TERRIER | 11 | FS | DE ANGELIS | 60 | 4; 6; 4 | NA |
| 6 | 11 | YORKSHIRE | 5 | M | DE ANGELIS | 60 | 4; 4; 6 | NA |
| 7 | 7 | MIXED BREED | 9 | M | DE ANGELIS | 30 | 4; 4; 4 | 4; 4; 6 |
| 8 | 5 | MIXED BREED | 18 | FS | MP TTA+ partial meniscectomy | 60 | 6; 9; 9 | 2; 6; 6 |
| 9 | 2 | CORSO | 44 | F | MP TTA | 30 | 4; 9; 9 | 4; 9; 9 |
| 10 | 15 | JACK RUSSEL | 6 | FS | De ANGELIS | UNKNOWN | 6; 6; 6 | NA |
| 11 | 7 | BOXER | 33 | FS | DE ANGELIS | 30 | 9; 9; 9 | 6; 9; 9 |
| 12 | 8 | MIXED BREED | 27 | F | DE ANGELIS | 15 | 4; 4; 4 | 6; 6; 6 |
| 13 | 8 | MIXED BREED | 41 | F | MP TTA | 30 | 4; 4; 4 | 9; 9; 9 |
| 14 | 7 | BARBONE MEDIO | 14 | M | DE ANGELIS | 10 | 4; 6; 6 | 9; 9; 6 |
| 15 | 2 | CORSO | 42 | F | MP TTA | 60 | 4; 9; 4 | NA |
| 16 | 5 | AMERICAN STAFFORDSHIRE TERRIER | 17 | FS | MP TTA | 30 | 6; 9; 6 | 6; 4; 6 |
| 17 | 5 | AMERICAN STAFFORDSHIRE TERRIER | 16 | FS | MP TTA | 30 | 9; 6; 9 | 4; 9; 6 |
| 18 | 5 | AMERICAN STAFFORDSHIRE TERRIER | 18 | M | MP TTA | 21 | 6; 9; 9 | 9; 9; 6 |
| Median 6 Range 2–15 | Median 17.5 Range 5–44 | Median 30 Range 10–70 | 5.55 SE 0.48 6.88 SE 0.51 6.33 SE 0.49 | 6.57 SE 0.65 7.5 SE 0.58 6.7 SE 0.42 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restucci, B.; Sgadari, M.; Fatone, G.; Valle, G.D.; Aragosa, F.; Caterino, C.; Ferrara, G.; Niebauer, G.W. Immunoexpression of Relaxin and Its Receptors in Stifle Joints of Dogs with Cranial Cruciate Ligament Disease. Animals 2022, 12, 819. https://doi.org/10.3390/ani12070819
Restucci B, Sgadari M, Fatone G, Valle GD, Aragosa F, Caterino C, Ferrara G, Niebauer GW. Immunoexpression of Relaxin and Its Receptors in Stifle Joints of Dogs with Cranial Cruciate Ligament Disease. Animals. 2022; 12(7):819. https://doi.org/10.3390/ani12070819
Chicago/Turabian StyleRestucci, Brunella, Mariafrancesca Sgadari, Gerardo Fatone, Giovanni Della Valle, Federica Aragosa, Chiara Caterino, Gianmarco Ferrara, and Gert W. Niebauer. 2022. "Immunoexpression of Relaxin and Its Receptors in Stifle Joints of Dogs with Cranial Cruciate Ligament Disease" Animals 12, no. 7: 819. https://doi.org/10.3390/ani12070819
APA StyleRestucci, B., Sgadari, M., Fatone, G., Valle, G. D., Aragosa, F., Caterino, C., Ferrara, G., & Niebauer, G. W. (2022). Immunoexpression of Relaxin and Its Receptors in Stifle Joints of Dogs with Cranial Cruciate Ligament Disease. Animals, 12(7), 819. https://doi.org/10.3390/ani12070819







