1. Introduction
Decreased feed intake and growth performance are common in weaning piglets, and may be attributed to numerous stresses including mixing and moving, dam separation, and changes in diet and feeding systems. Thus, new nutritional strategies aimed at stimulating feed intake and improving digestive functions during this period are required in swine production [
1]. Rice is one of the most abundant cereals, however, as it is a staple food for humans, its use as an animal feed ingredient has been restricted [
2]. Broken rice, a by-product of rice processing, has received considerable attention since its inclusion in piglet diets improved growth performance [
3,
4,
5] and reduced the mortality of nursery piglets [
6]. Broken rice exhibits greater nutrient digestibility compared with corn [
7], thus, it has a higher postprandial glycemic curve [
8]. High glycemic index (GI) carbohydrates promote higher glucose and insulin secretion peaks when compared with low GI carbohydrates, which ultimately results in differences in feed intake [
9].
Extruded cereal-based products, such as extruded corn, broken rice, and wheat, are widely used in swine production, as they improve nutrient digestibility and feed intake following weaning [
6,
10]. In addition, the extrusion process induces several chemical and structural changes in cereal nutrients (e.g., starch), leading to changes in digesta retention times in the intestine [
11]. Extruded cereal grains may also contain resistant starch that is not easily digested or absorbed in the small intestine, but rather passes onto the large intestine. Thus, cereal grain extrusion diets enhance microbiota access to undigested carbohydrates and microbial fermentation in the hindgut, which putatively affects feed intake via specific appetite regulatory factors, such as short-chain fatty acids (SCFAs), glucagon-like peptide-1 (GLP-1), and peptide YY (PYY) [
12]. However, it is unclear how cereal extrusion diets interact with the gut microbiota to influence host feeding physiology.
Therefore, this study was conducted to investigate the effects of different grains (corn or broken rice) and the extrusion processing method (extruded corn or extruded broken rice) on feed intake, nutrient digestibility, and the microbiota in weaned piglets.
2. Materials and Methods
2.1. Ethical Considerations
This study was performed at Sichuan Agricultural University. The experimental protocol was in accordance with the animal care and use committee guidelines of our University (Ethics Approval Code: 20184006), and followed national laws and National Research Council (NRC) guidelines for the care and use of laboratory animals.
2.2. Preparation of Extruded Cereals
Cereal extrusions were prepared as previously described [
10]. Briefly, broken early Indica rice from the Chongqing province (China) and corn from Northeast China were ground using a hammer mill and a 2.5 mm screen, and extruded using a single-screw extruder (model TPH200C, Jiangsu Muyang Group, Yangzhou, China). Samples were steam-cooked at 80 °C for 20 s, then extruded at 125 °C and 100 °C for 5 s each. After this, corn and broken rice were cooled using a counter-flow cooling procedure and ground through a 2.5 mm screen to generate mashed cereal. Intact corn and broken rice were used as controls.
2.3. Animals, Diets, and Management
Sixty-four crossed Duroc × (Landrace × Yorkshire) piglets, weaned at day 28 (±1 day) and having a body weight (BW) of 7.11 kg (±0.16 kg) were used in a 28-day feeding study. Piglets were randomly allocated to one of four dietary treatments, with eight replicates (pens) per treatment group. There were two piglets in each pen (one barrow and one gilt). The four study diets included one control diet containing 62.17% corn (CORN), and three diets with half the corn replaced by: extruded corn (ECORN); broken rice (RICE); and extruded broken rice (ERICE) (
Table 1). Piglets were adapted to a common prestarter diet for 3 days. Each pen was equipped with one self-feeder and two nipples supplying water. Piglets were fed and watered ad libitum and provided with feed four times per day (8:00, 12:00, 16:00, and 20:00). Feeding conditions were observed for an average of 2 h to ensure surplus feed in feeding troughs was not less than 25% of the tank volume. The environmental temperature was maintained at 24–26 °C. Diets were supplied in mashed form and formulated to contain equal quantities of digestible energy, crude protein, and lysine. Diets were formulated to meet or exceed the nutrient requirements as recommended by the NRC [
13].
2.4. Sample Collection
Before study commencement, diets were sampled and stored at −20 °C for chemical analysis. Piglets were weighed on days 1, 14, and 28. Feed consumption was recorded daily on a pen basis to calculate the average daily feed intake (ADFI), the average daily growth (ADG), and the feed conversion ratio (F:G).
In the evening of days 13 and 27, the remaining feed in troughs was weighed and piglets fasted overnight. At 9:00 a.m. on days 14 and 28 of experiment, one piglet (barrow) in each pen was selected for blood collection (while in the fasting state) and marked on the back to denote selection. Blood samples from the jugular vein were collected into heparinized vacuum tubes. Piglets were provided with feed after blood collection, and additional blood samples were collected from marked piglets at 1 h, 2 h, and 4 h post-feeding. Blood samples were centrifuged at 3000× g for 15 min at 4 °C, with plasma stored at −20 °C. The plasma samples collected before feeding and 1 h and 2 h after feeding on day 28 were determined for concentrations of GLP-1 and PYY. The plasma samples collected before feeding and 4 h after feeding were determined for the level of acetate, propionate, and butyrate.
On days 13 and 27, one piglet per pen was selected to provide fecal samples. Feces were collected into sterile tubes, immediately frozen in liquid nitrogen, and stored at −80 °C for SCFA and bacterial analysis. Feces were collected under sterile conditions during sampling.
From day 21, the indigestible marker, chromium (III) oxide (Cr2O3), was added to feed at 0.3% to measure apparent total tract digestibility (ATTD). After a 3-day adaption, freshly voided fecal samples from each pen were collected by hand into plastic bags on days 25–28, and 10% hydrochloric acid was added (10 mL/100 g feces) to fix excreta nitrogen. Samples were maintained on ice and stored at −20 °C. Then, samples were dried in a forced-air oven at 60 °C for 5 days. Dried samples were pooled for each pen and ground using a heavy-duty blender for dry matter (DM), crude protein (CP), ether extract (EE), gross energy (GE), crude ash (ASH), and crude fiber (CF) analysis.
The diarrhea index was measured by the appearance of feces monitored at 9:00, 14:00, and 20:00 daily. The fecal score was defined as 0 for forming or granular feces, 1 for soft but forming feces, 2 for thick, unseparated, unformed liquid feces, and 3 for unshaped, separated liquid feces.
2.5. In Vitro Microbial Fermentation
Fecal microbiota were collected from each pen at day 28 and used in in vitro fermentation trials, conducted as previously described, but with minor modifications [
14]. Briefly, feces were rectally collected, placed in plastic bags, anaerobically preserved in a foam box filled with ice, and transferred to the laboratory within 2 h. Feces (~70 g) were weighed and mixed with 0.9% sterile saline at 1:5 (g/mL). Samples were homogenized for 30 s using a hand mixer and filtered through four sterile cheesecloth layers to collect filtrates, which were transferred to air-tight plastic syringes and incubated in a water bath at 39 °C. The fermentation medium contained the following (per liter): peptone, 0.2 g; NH
4HCO
3, 0.4 g; NaHCO
3, 35 g; Na
2HPO
412H
2O, 9.45 g; K
2HPO
4, 6.2 g; MgSO
47H
2O, 0.6 g; CaCl
22H
2O, 13.2 mg; MnCl
24H
2O, 10 mg; CoCl
26H
2O, 1 mg; FeCl
36H
2O, 8 mg; L-Cysteine HCL, 1 g; and pectin, 120 mg. Syringes were sealed with glycerinum. Fermentation gases were recorded at 0, 3, 6, 9, 12, 18, 21, 24, 36, 48, 60, and 72 h using a glass syringe. Blanks containing inocula without medium were used to calculate background levels. In vitro fermentation characteristics, including the final asymptotic gas volume in feces (
VF), initial fractional rate of degradation at t-value = 0 (
FRD0), fractional rate of gas production at a particular time (
K), and half-life to asymptote (
T1/2), were determined as previously described [
15]. The in vitro production of the SCFAs, acetate, propionate, butyrate, isobutyrate, valerate, and isovalerate were also determined.
2.6. Chemical Analysis and Intakes of Digestible Nutrients
The degree of starch gelatinization (%) as a proportion of total starch for the ingredients corn, extruded corn, broken rice, and extruded broken rice was determined by enzymatic hydrolysis as described previously [
16]. This method involved the incubation of amyloglucosidase (A7095; Sigma-Aldrich, St Louis, MO, USA) with 0.2 g sample followed by photometrical product measurement of the reaction between glucose and the copper reagent, ferricyanide. The reaction followed the principle that gelatinized starch was digested more easily by amyloglucosidase to form glucose. Apparent total tract digestibility (ATTD) was determined using Cr
2O
3 as an indigestible marker. ATTD was evaluated in each cage individually. All diet and feces samples were analyzed in duplicate for Cr (method 990.08), DM (method 930.15), ASH (method 942.05), EE (method 945.16), CP (method 990.03), and CF (method 920.98) according to AOAC procedures (1995). The GE of feed and fecal samples was determined using an adiabatic oxygen bomb calorimetry apparatus (Parr Instrument Co., Moline, IL, USA). ATTD was calculated using the following formula: ATTD (%) = (100 − A1/A2 × F2/F1 × 100), where A1 = Cr content in feed; A2 = Cr content in feces; F1 = nutrient content in feed; and F2 = nutrient content in feces. The intakes of ATTD nutrients were calculated by the feed intake during days 22–28 of the experiment, and the ATTD of nutrients was determined during days 25–28 of the experiment.
2.7. Determining Appetite Hormone Plasma Levels
GLP-1 and PYY plasma levels from marked piglets recorded before feeding and 1 h and 2 h after feeding on day 28 were assessed using commercial kits (BIM Inc., San Francisco, CA, USA) according to the manufacturer’s instructions. Ghrelin and insulin plasma levels recorded before feeding and 4 h after feeding on day 28 were assessed using commercial kits (Jiangsu Meimian Industry Co., Ltd., Nanjing, China) according to the manufacturer’s instructions. Glucose levels before feeding and 1 h, 2 h, and 4 h post-feeding were assessed using commercial kits (Sichuan Maker Biotechnology Inc., Chengdu, China) on an automatic biochemical analyzer (Hitachi 7020, Hitachi High-Technologies Corporation, Tokyo, Japan). For glucose, the minimal detection limit was 0.02 mmol/L.
2.8. Determining SCFA Levels
The SCFA pretreatment of plasma, fecal samples, and fermentation media was conducted according to a previous method [
17]. SCFA levels of acetate, propionate, and butyrate in plasma and feces were determined by gas chromatography (CP-3800GC, Varian, Inc., Walnut Creek, CA, USA), following instructions from a previous method [
18].
2.9. Fecal Microbiota Analysis
Fecal samples collected on day 28 were analyzed for microbial diversity by extracting microbial DNA using QIAamp Fast DNA Stool mini kits (Qiagen, Hilden, Germany) according to the manufacturer’s instructions [
19]. DNA concentrations and integrity were assessed on a Nanodrop-1000 and 0.8% agarose gel electrophoresis, respectively. The fusion primers, 338F 5′-ACTCCTACGGGAGGCAGCA-3′ and 806R 5′-GGACTACHVGGGTWTCTAAT-3′ with dual index, were used to amplify the V3–V4 region of bacterial 16S rRNA over 27 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. Amplicon sequencing was performed on an Illumina MiSeq platform (Illumina, San Diego, CA, USA). Paired-end reads from clean data sets were assembled into tags using FLASH (v.1.2.11) [
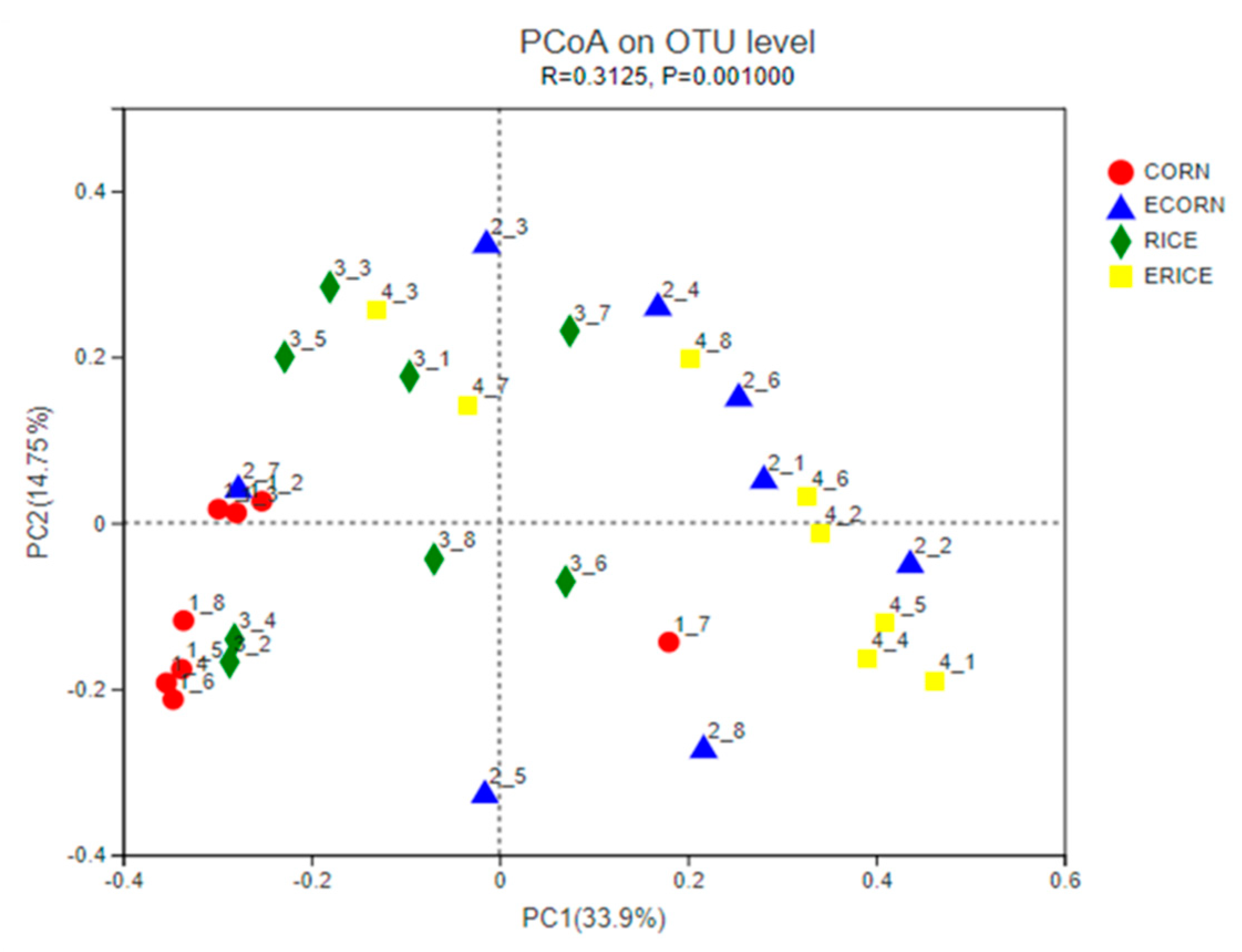
20]. Microbial diversity was assessed using QIIME software and operational taxonomic units (OTUs) were defined at ≥97% sequence homology. Taxonomic composition was generated using QIIME-UCLUST based on the Ribosomal Database Project. Principal coordinate analysis plots (PCoA) were generated at the genus level and were based on the Bray–Curtis dissimilarity. Microbial 16S rRNA sequencing analysis was conducted as previously described [
21].
2.10. Statistical Analysis
Data were analyzed using a completely randomized study design with a 2 × 2 factorial treatment arrangement. Data normality and variance homogeneity were evaluated by Shapiro–Wilk and Levene tests, respectively. Data were analyzed by a mixed-procedure approach using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The fixed effects were cereal type, the extrusion process, and interactions between cereal sources and the extrusion process. The plasma concentrations of glucose, ghrelin, acetate, propionate, butyrate, total SCFAs, GLP-1, and PYY before feeding and at different intervals after feeding were analyzed using the MIXED procedure in SAS 9.4:
where
Yijkl is the response variable,
µ is the overall mean; and
αi,
βj, and
γk are fixed effects of cereal type (corn or broken rice), extrusion process, and time of sample collection, respectively. (
αβγ)
ijk is the interaction among fixed effects,
tƖ is the random effect of the gilt to account for repeated measurements within a piglet and
εijkl is the residual error. Results were expressed as the mean ± standard error. Differences were considered significant at
p < 0.05, whereas 0.05 ≤
p < 0.10 values indicated a tendency.
4. Discussion
In this study, our primary aim was to determine the influence of different grains (corn and broken rice) and an extruded processing diet (extruded corn/extruded rice) on feed intake, growth performance, and associated microbiota changes in weaned piglets. Broken rice is a favorable dietary ingredient due to its high starch and low fiber levels. It was observed that the ATTD levels of DM and GE in RICE and ERICE groups were greater than those in CORN and ECORN groups, respectively, in agreement with a previous study [
10]. Increased ATTD values in the broken rice group may have been due to the lower size of rice starch relative to corn starch [
22]. Rice starch has a smaller starch granule, lower non-starch polysaccharide levels, and lower amylose-to-amylopectin ratios than corn starch, thereby contributing to increased starch digestibility [
23]. Since piglets in the RICE and ERICE groups had greater ATTD levels of nutrients as well as greater ADFI, their intakes of digestible nutrients were also greater, which might be the reason why they had greater performance that CORN and ECORN piglets.
Previous studies reported that corn-to-rice substitution in piglet diets improved feed intake, ADG, and nutrient digestibility [
2,
24]. In the current study, the RICE group displayed the best growth performance, but no significant differences were observed when compared with the CORN group. This may have been due to differences in feed textures. In previous studies, experimental reagents included rice or brown rice, which contained rice bran, and were classified as “functional foods”. A previous study reported that nursery diet supplementation with 10% rice bran increased growth performance and feed efficiency, similar to conventional diets containing growth-promoting antibiotics [
25].
A previous study also demonstrated that gelatinized starch exhibited greater digestibility than raw starch [
26]. However, in the present study, corn and broken rice extrusion diets did not increase the growth performance of piglets. This may have been due to gelatinization and supplementation levels. A brown rice extrusion diet did not affect piglet ADG; in fact, it decreased the G:F ratio, which was possibly due to reductions in essential amino acid levels [
27]. As previously reported, rice starch had the highest expansion ratio when compared with bean starch and corn starch [
28]. Similarly, a previous study showed that cereal heat-processing exerted no beneficial effects on broiler performance [
29]. The effect of supplementation of extruded material might be dependent on the feeding duration. For example, the feed conversion ratio (FCR) on days 8 to 14 tended to be improved in groups supplemented with non-extruded cereals; this observation was supported by the fact that pigs fed a corn- and rice-rich diet displayed catch-up growth in the second week following weaning [
10]. Moreover, the benefits of extruded materials might be dependent on the types of cereal grains. When the feed ingredients contain high levels of antinutritional factors such as soybean, an extrusion treatment would exert a greater benefit. Therefore, a better use of extruded materials for weaned piglet feed should be identified.
The decreased growth performance of piglets fed extruded ingredients could be attributed to lower feed intake, as FCRs were not influenced by dietary treatments. To gain insights on why the feed intake of piglets in ECORN and ERICE groups were decreased, appetite-related metabolites and hormones were investigated. Blood glucose is a primary signal influencing appetite [
30], and was unaffected by dietary treatments. Insulin is an orexigenic hormone that increases hunger sensations and heightens sucrose palatability, regardless of glucose plasma levels [
31]. Large insulin responses after a high-GI meal cause postprandial glucose depletion, which in turn increases hunger symptoms and promotes body fat accumulation and BW gain. Corn, raw rice grains, and rice could be considered as high-GI ingredients for pigs [
32]. In this study, insulin plasma levels were decreased by broken rice inclusion at 4 h after feeding. However, feed intake was unaffected by broken rice inclusion; thus, alterations in feed intake appeared to be unaffected by insulin in the present study.
The gastric hormone ghrelin produces hunger and crave pangs [
33,
34]. In pigs, serum ghrelin levels can indicate chronic changes in energy status. In this study, ghrelin plasma levels were similar before feeding. At 4 h after feeding, levels were higher in diets supplemented with broken rice (or extruded broken rice). This was possibly due to lower SCFA levels in piglets fed broken rice, as ghrelin plasma levels are negatively regulated by plasma SCFAs [
35,
36]. Moreover, it was previously shown that additional protein supplementation decreased ghrelin plasma levels, suggesting levels may be related to protein digestibility [
37].
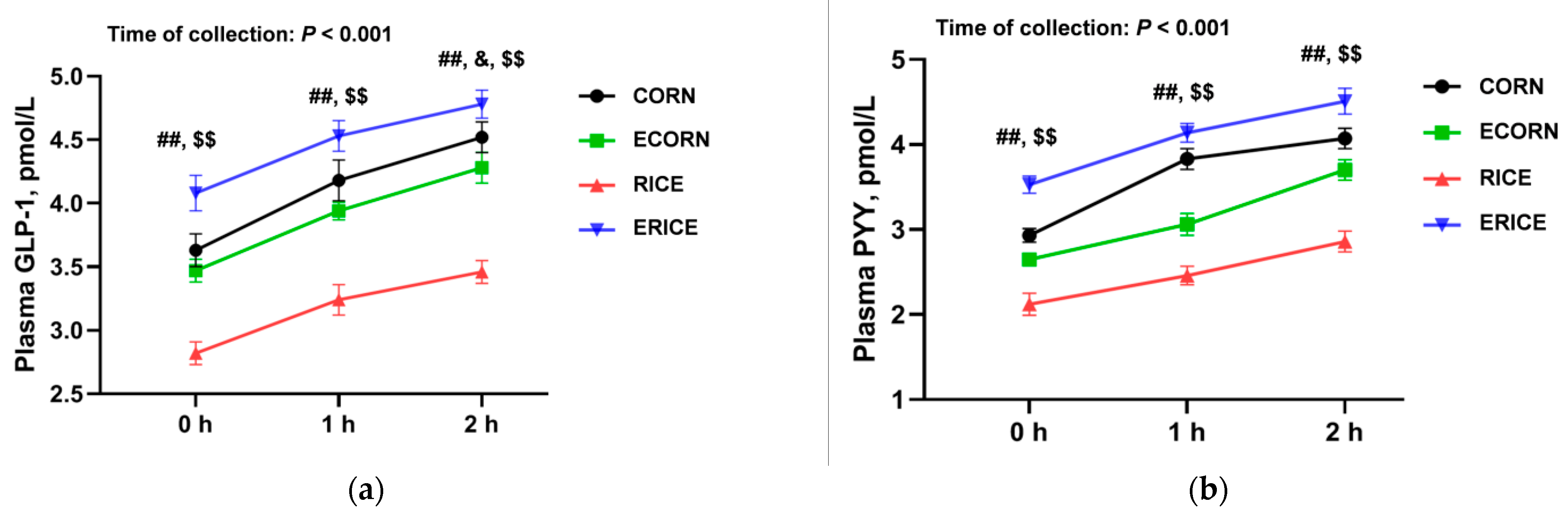
GLP-1 and PYY are the main gut-derived anorexigenic hormones that regulate feeding behavior. GLP-1 and PYY plasma levels were significantly elevated by extruded ingredients, and potentially explained decreased feed intake and growth performance. Interestingly, GLP-1 and PYY levels were mainly affected by gut functions; SCFA-mediated glucose homeostasis and energy metabolism may be stimulated by enteroendocrine L-cells, which produce GLP-1 and PYY. Thus, increased microbiota-derived SCFA levels could in turn stimulate enteroendocrine cells to release GLP-1 [
37] and PYY [
38], while decreasing ghrelin secretion [
39]. In the present study, the nutrient ATTD and feed intake were combined (
Table 4), and we found that extrusion also results in greater nutrient intake such as crude protein; thus, the dietary-extrusion-induced changes of appetite-related hormones could be attributed to the alteration of nutrient intake.
Lowered SCFA plasma levels in rice diets may have arisen due to lower indigestible carbohydrate levels, such as fiber and non-starch polysaccharides in corn, which may have escaped digestion in the small intestine and reached the colon to undergo microbial fermentation. Indigestible carbohydrates modulate gut microbiota, as evidenced by higher
Lactobacillus abundance, which is one of the most common lactic acid-producing bacteria in humans and animals [
40].
Corn or rice extrusion diets decrease SCFA plasma levels, possibly due to chemical and structural changes in extruded cereals [
41]. Extrusion of diet elevates carbohydrate and other nutrient digestibility in the small intestine and reduces the supply of fermentable substrates to the large intestine, causing lower SCFA levels [
42]. The SCFA was mainly produced by the gut microbiota, and the differences of serum levels of SCFA could be attributed to alternations of microbiota [
43]. In the present study, compared with the piglets fed with extruded cereal, the piglets in unextruded diet groups showed a higher abundance of
Actinobacteria, a predominant commensal bacterium in the gut [
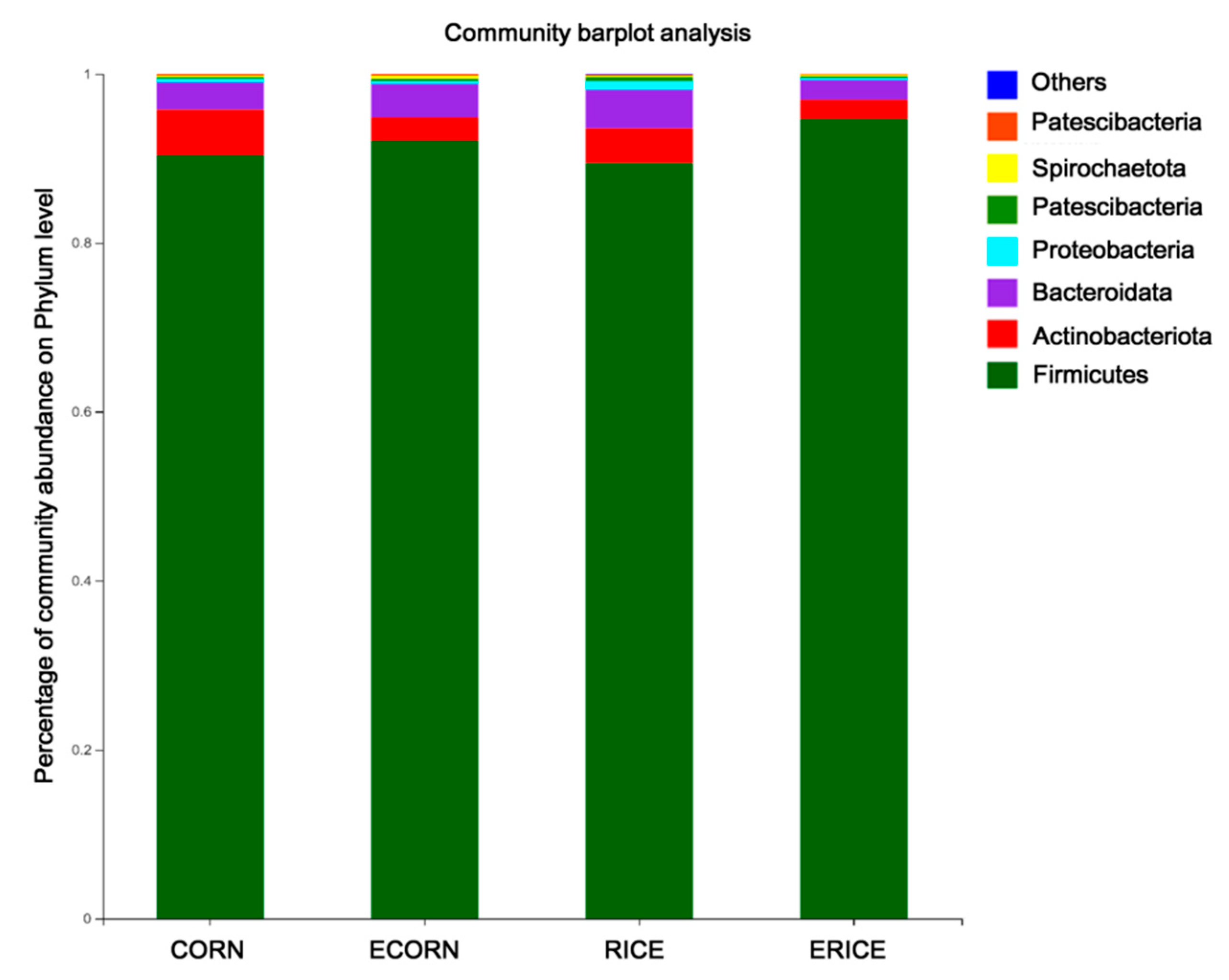
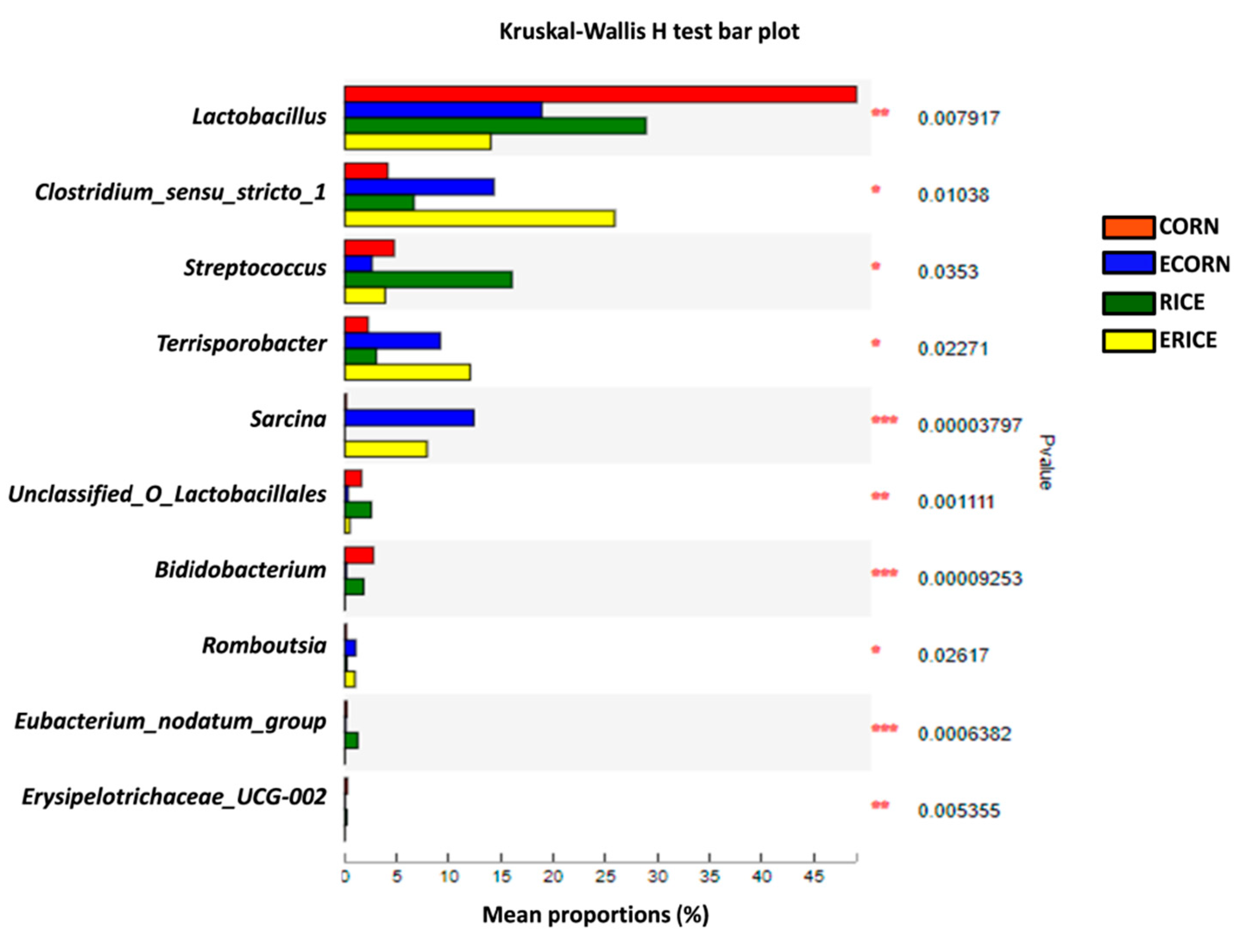
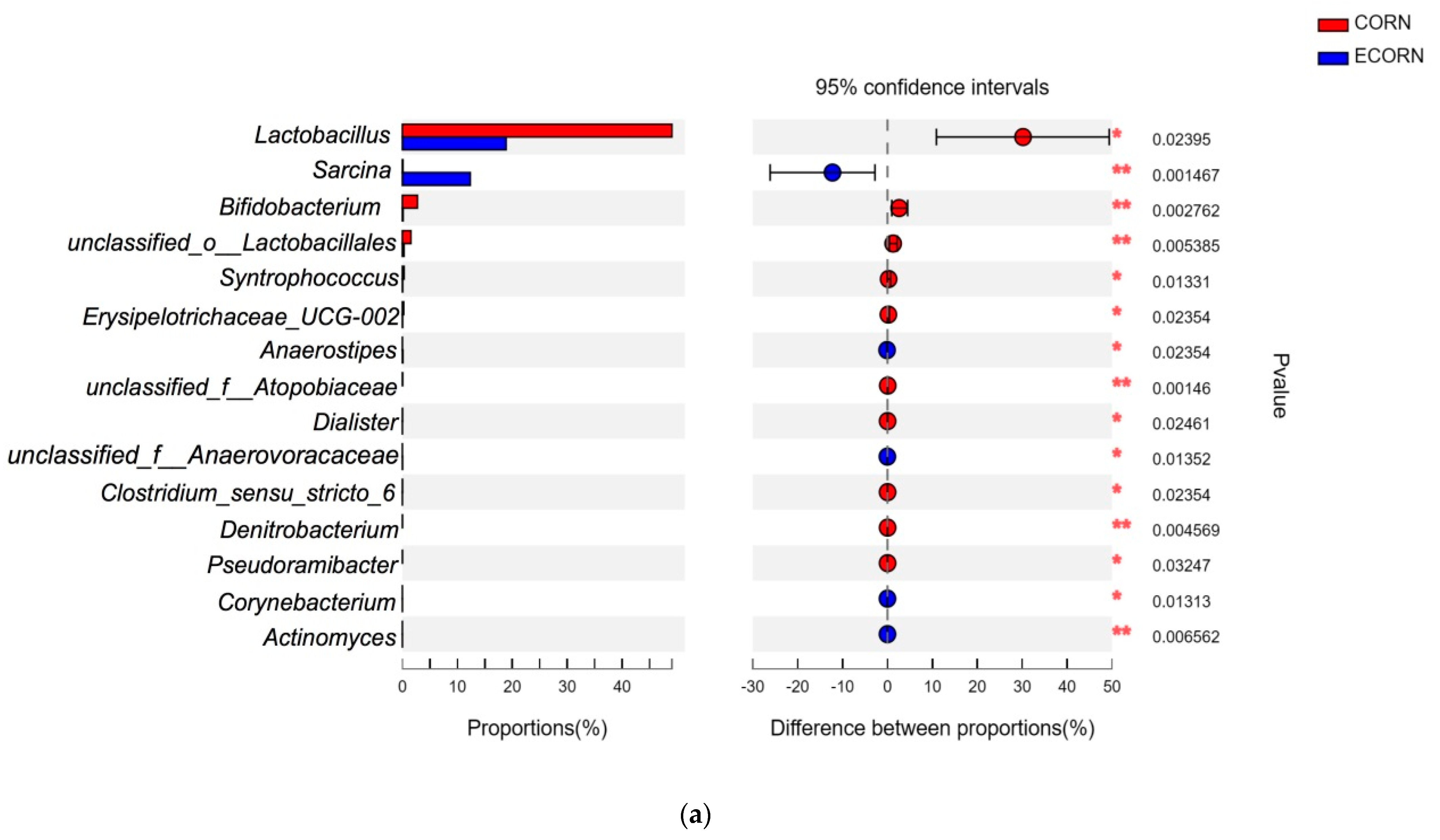
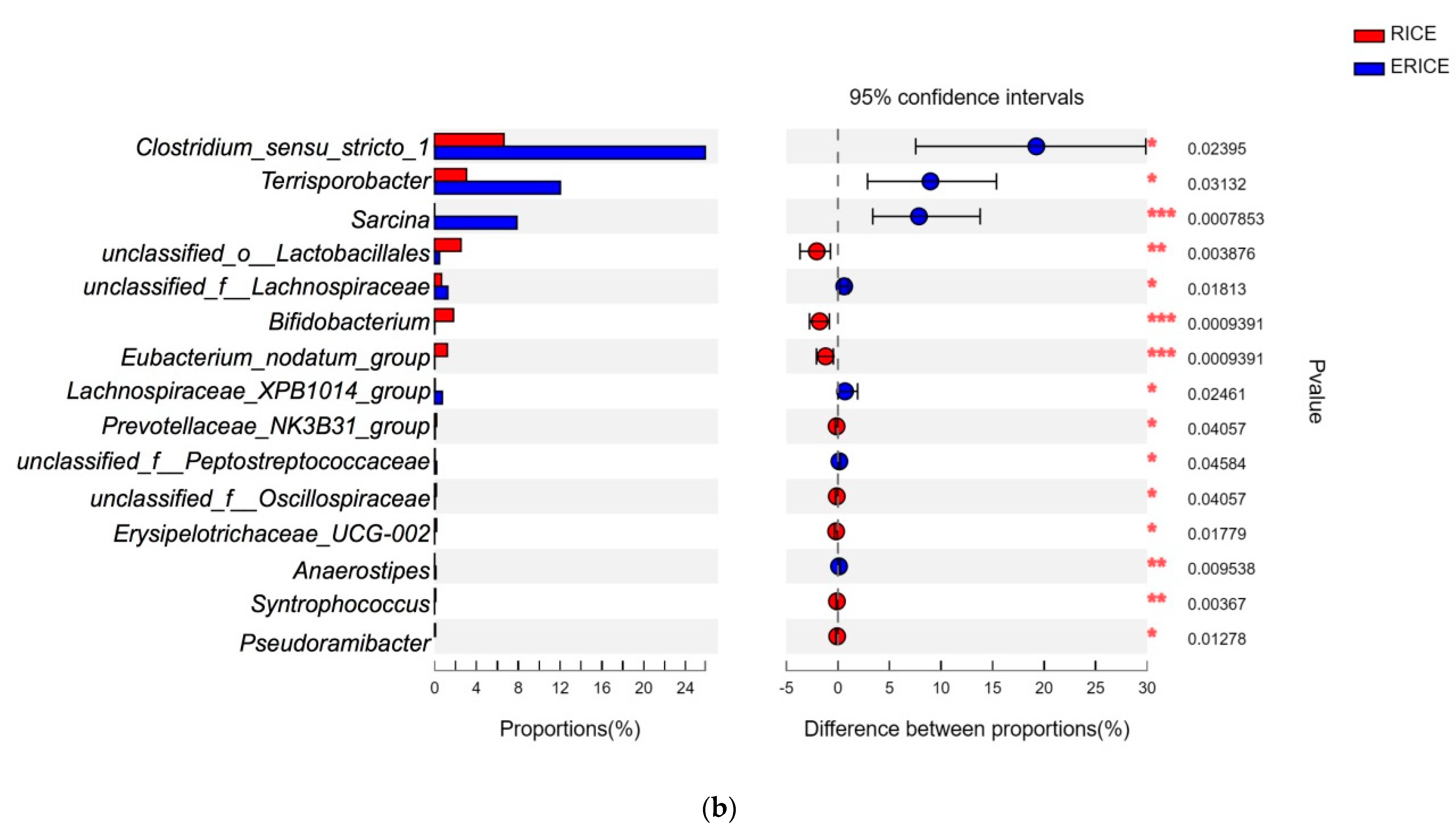
44]. In the present study, we further compared the microbial abundance at the family level between CORN and ECORN, as well as those between RICE and ERICE. One of the most important findings was that more probiotics such as
Lactobacillus and
Bifidobacterium had colonized the gut of piglets fed non-extruded diets compared with piglets fed the extruded diets.
Bifidobacteria use complex carbohydrates, which are difficult to digest by the host [
45].
Lactobacillus inhibits the growth of some intestinal pathogens such as
Escherichia coli [
46].
Unclassified_o_lactobacillales is also an SCFA producer during polysaccharide fermentation [
47], and was elevated in non-extruded dietary groups when compared with extruded groups. This explained elevated plasma butyrate levels in the former group. Their proliferation might exert beneficial effects on the health of piglets, as SCFA could act as an energy source of intestinal epithelial cells. On the other hand, extruded ingredients stimulated the relative abundance of
Sarcina,
Clostridium_sensu_strictio_1, and
Terrisporobacter, which exhibit pathogenic potential.
Sarcina causes fatal bloat in animals [
48], and is associated with acute abomasal bloating in young lambs and calves [
49]. Therefore, dietary extrusion may stimulate the colonization of pathogenic microbes as well as decrease the beneficial commensal microbes, and this might be the reason why dietary extrusion causes negative effects on feed intake for piglets.
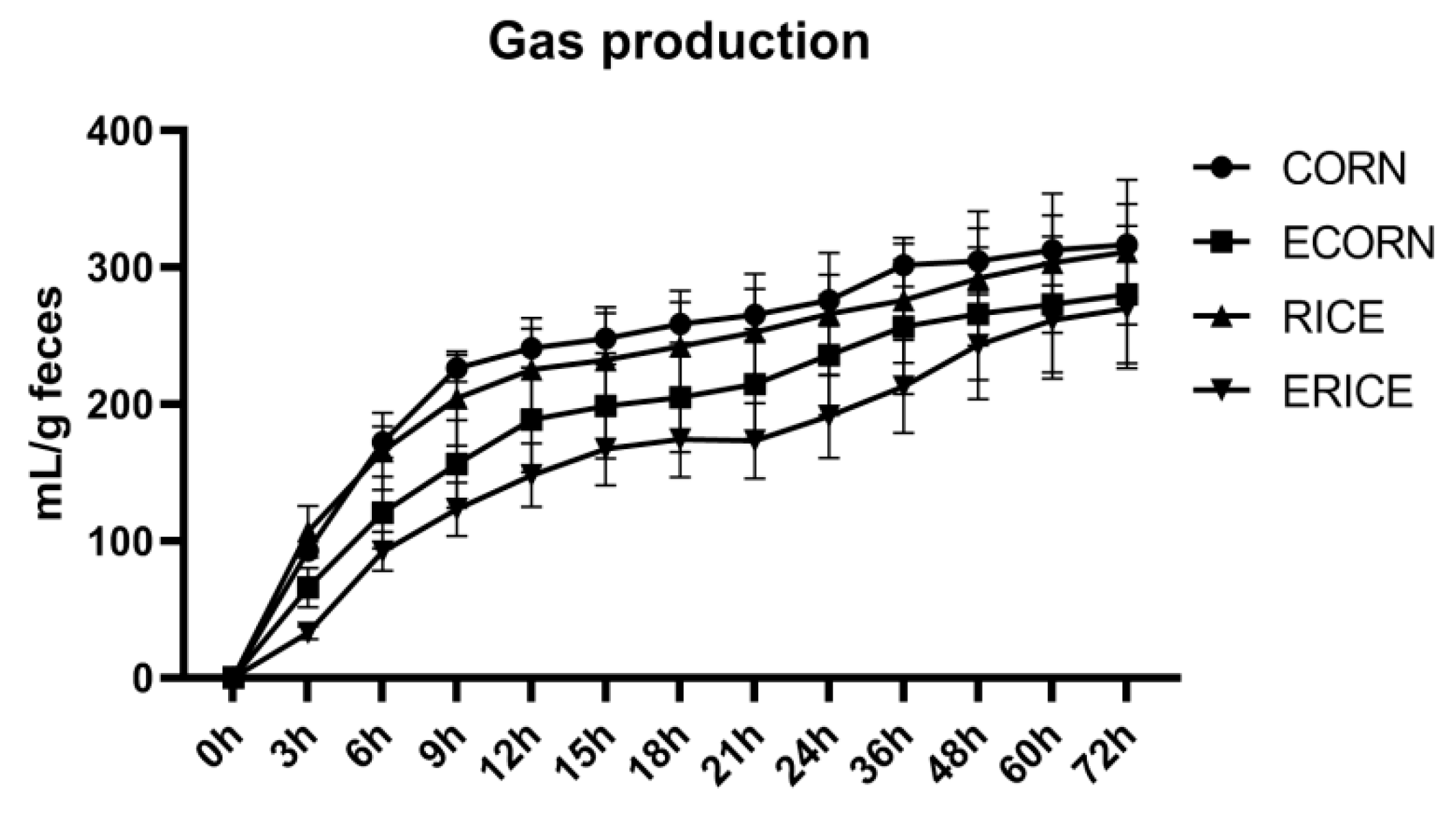
Given the importance of SCFA in mediating the microbial effects on the host physiology, and the fact that determined fecal SCFA concentrations represented less than 3.2% of the predicted hindgut SCFA production [
50], the levels of SCFA in either serum or fecal samples were not able to reflect their SCFA-producing capacity and metabolic activity. An in vitro fermentation trial was performed to further test whether changes in fecal microbiota caused differences in SCFA-producing capacity and other metabolic activities. Results showed that gut microbiota in extruded dietary groups had different fermentation characteristics, e.g., lower SCFA levels, which agreed well with the lower abundance of commensal bacteria producing SCFA. This suggested the gut microbiota had important roles in regulating piglet feed intake when on different extrusion diets. However, why extruded ingredients induced such negative microbiota effects requires further investigation.