Antigestagens Mediate the Expression of Decidualization Markers, Extracellular Matrix Factors and Connexin 43 in Decidualized Dog Uterine Stromal (DUS) Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and In Vitro Experiments
2.2. RNA Isolation, Reverse Transcription (RT) and Semi-Quantitative (TaqMan) PCR
2.3. Immunofluorescence (IF) Staining, and Evaluation of Data Using CellProfiler
2.4. Protein Preparation and Western Blot Analysis
2.5. Statistical Analysis
3. Results
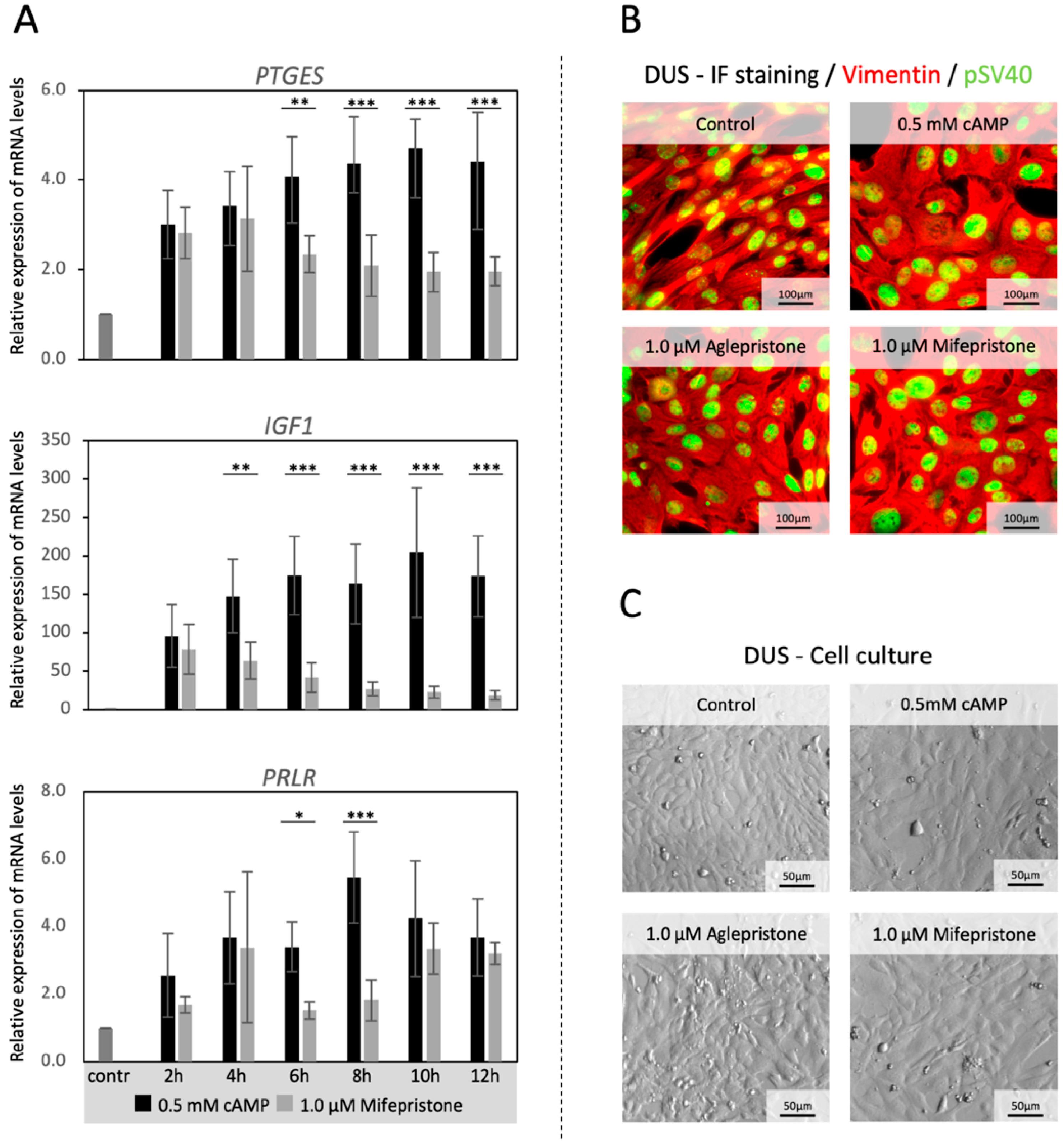
3.1. Time-Dependent Effects of Antigestagen Treatment on the In Vitro Expression of Decidualization Markers
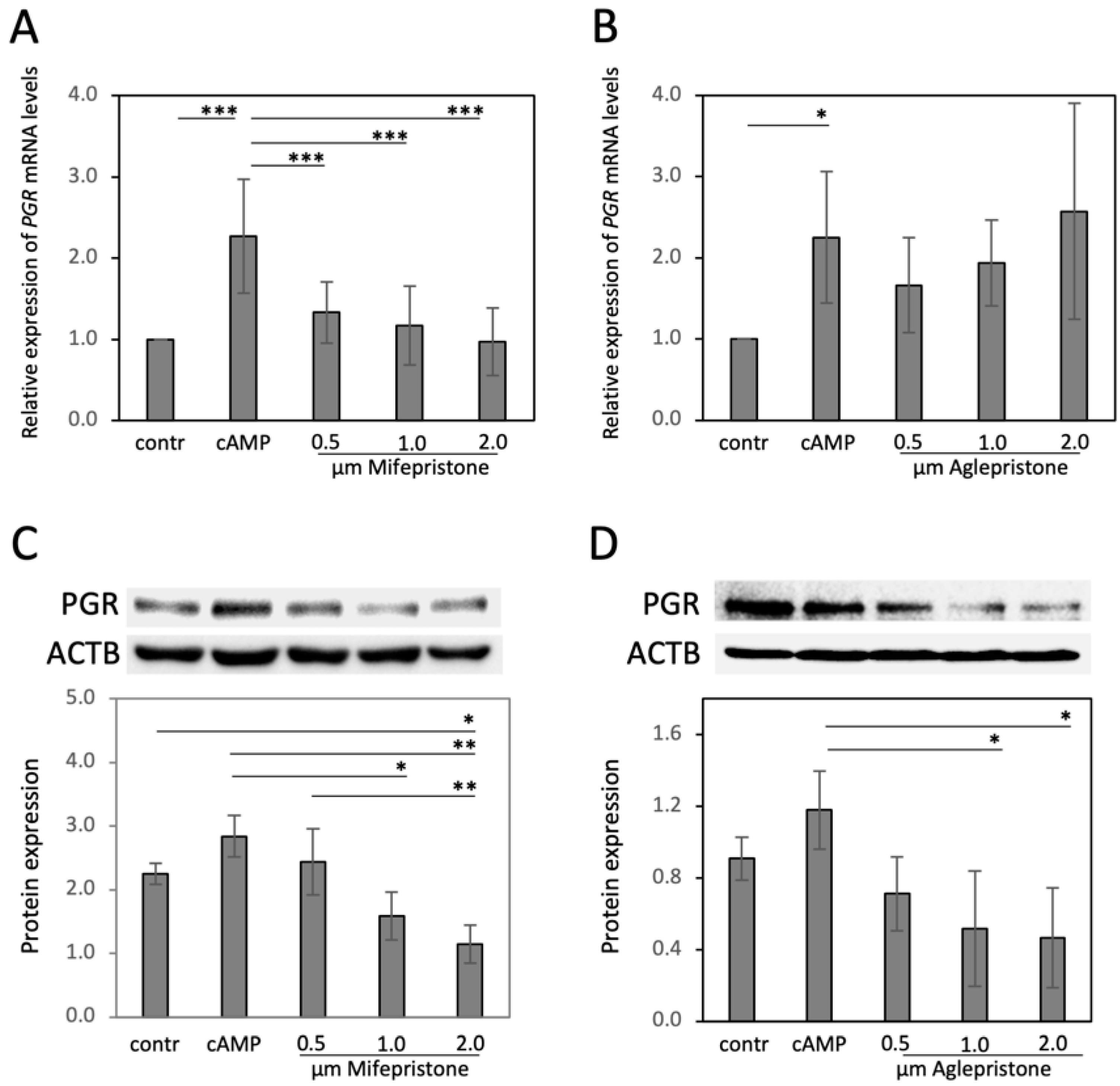
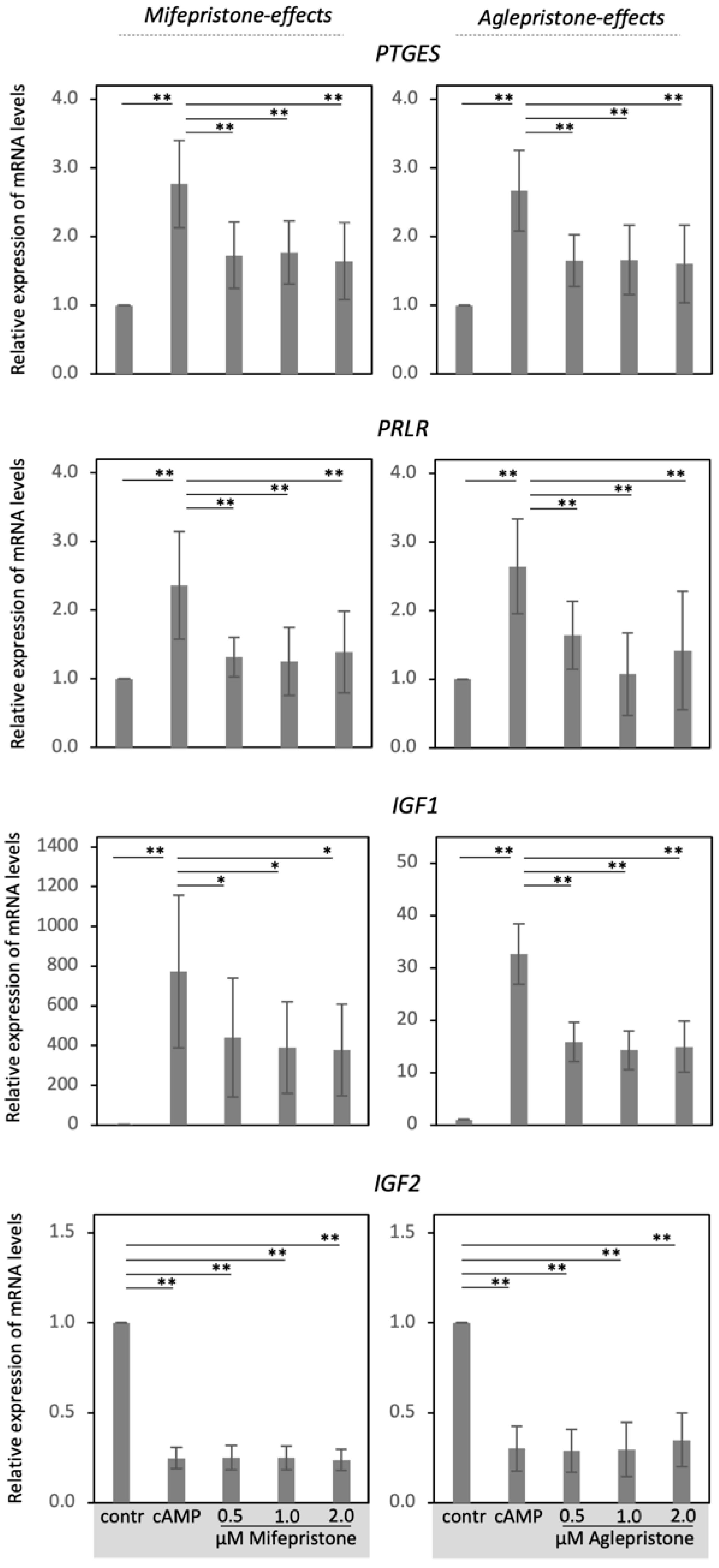
3.2. Antigestagens Modulate the Expression of Decidualization Markers in DUS Cells
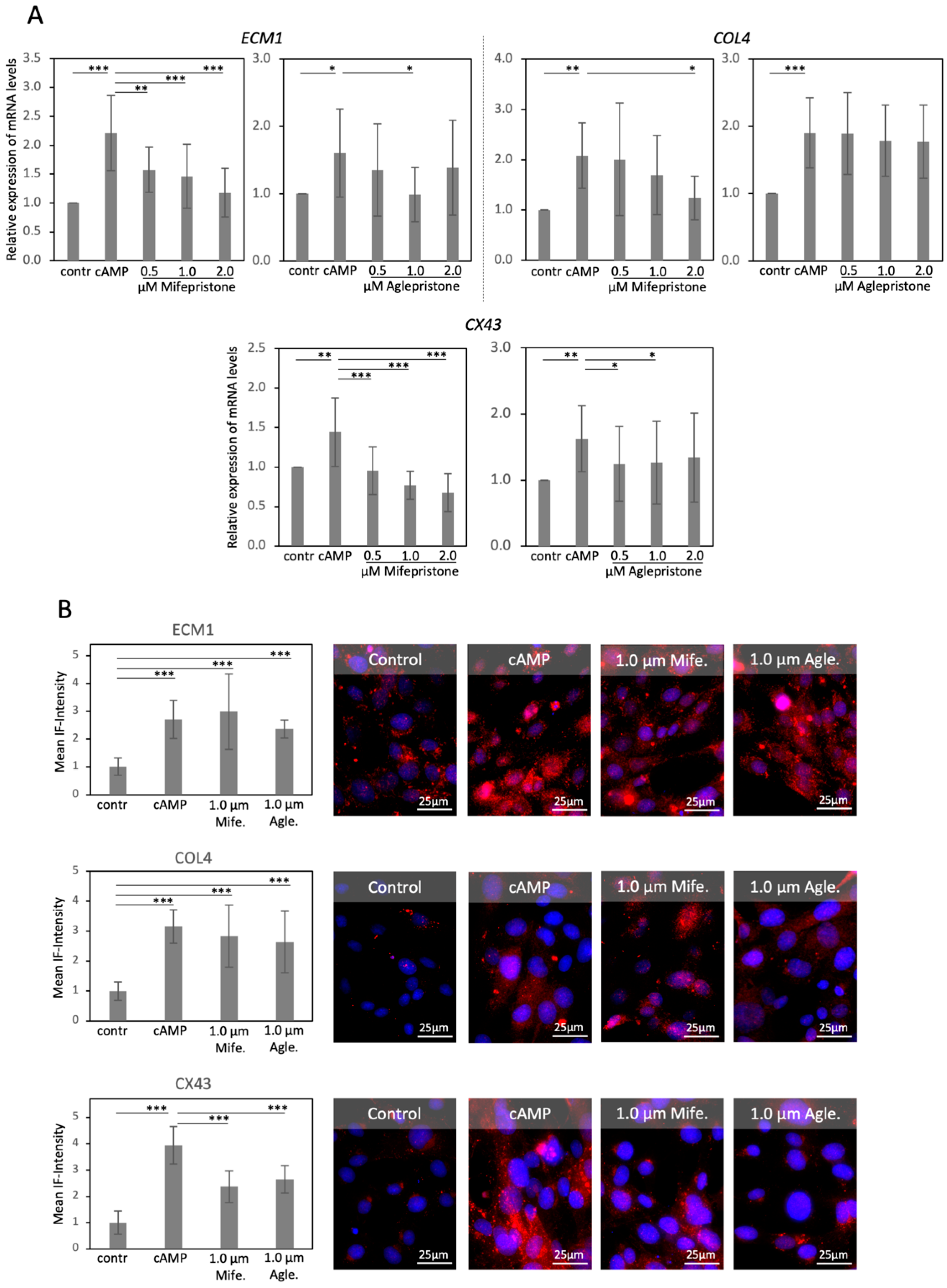
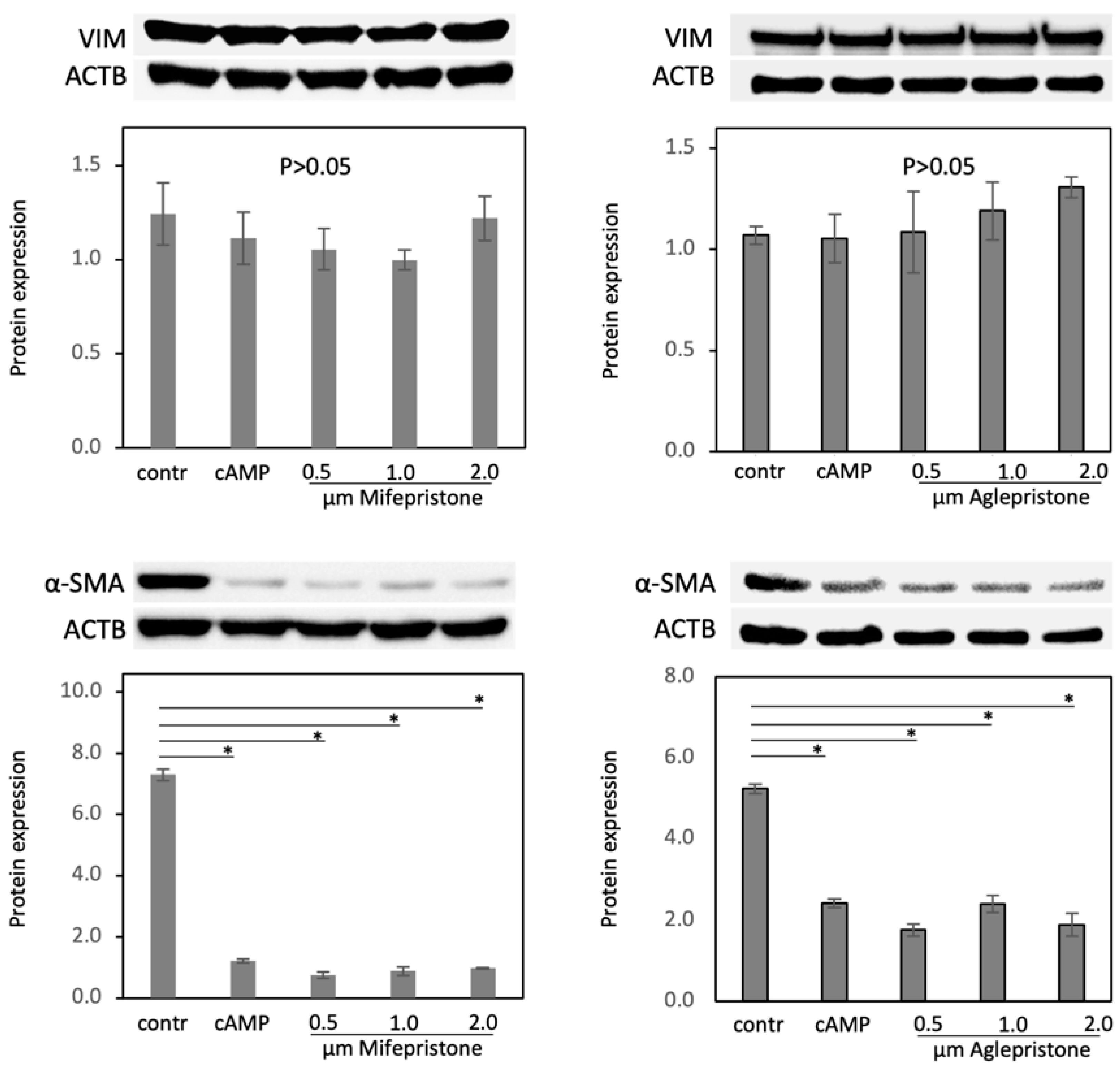
3.3. Antigestagens Modulate the Expression of ECM Factors and CX43, but Not of Mesenchymal Markers
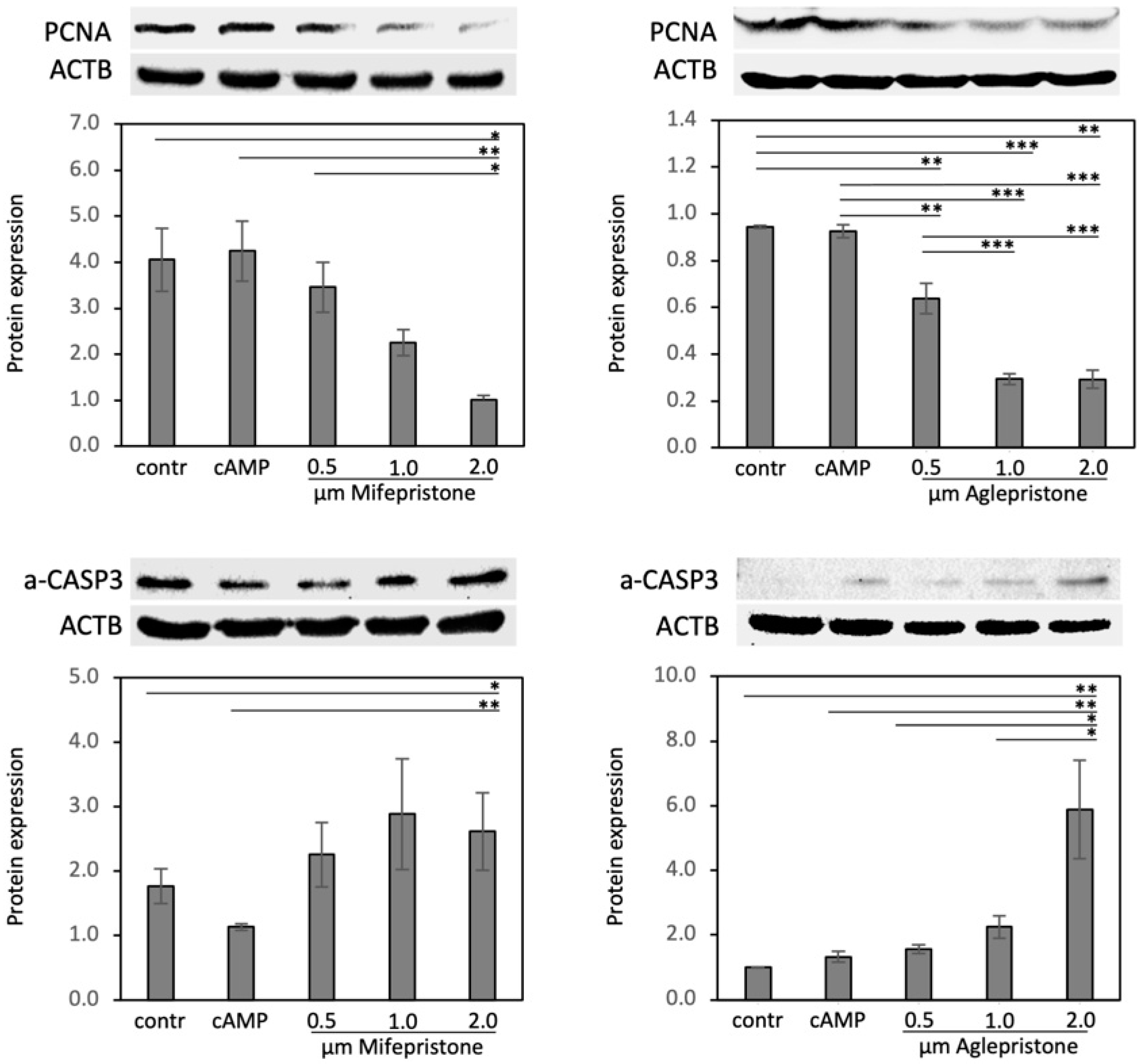
3.4. Antigestagens Modulate the Expression of PCNA and a-CASP3 in Decidualized DUS Cells
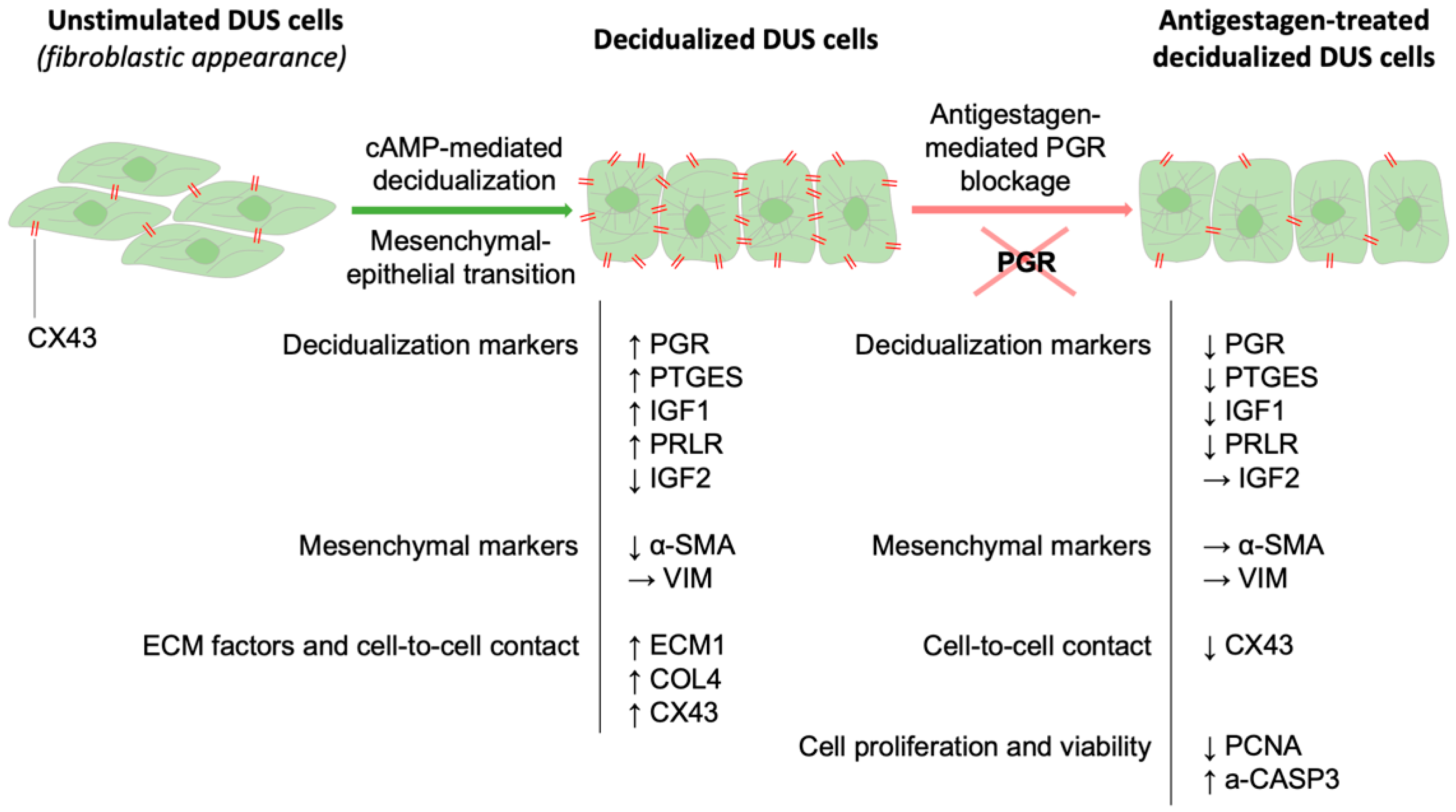
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kautz, E.; Gram, A.; Aslan, S.; Ay, S.S.; Selcuk, M.; Kanca, H.; Koldas, E.; Akal, E.; Karakas, K.; Findik, M.; et al. Expression of genes involved in the embryo-maternal interaction in the early-pregnant canine uterus. Reproduction 2014, 147, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.P.; Gram, A.; Kautz, E.; Graubner, F.R. The Dog: Nonconformist, Not Only in Maternal Recognition Signaling. Adv. Anat. Embryol. Cell Biol. 2015, 216, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Graubner, F.R.; Reichler, I.M.; Rahman, N.A.; Payan-Carreira, R.; Boos, A.; Kowalewski, M.P. Decidualization of the canine uterus: From early until late gestational in vivo morphological observations, and functional characterization of immortalized canine uterine stromal cell lines. Reprod. Domest. Anim. 2017, 52 (Suppl. S2), 137–147. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bany, B.M.; Cross, J.C. Post-implantation mouse conceptuses produce paracrine signals that regulate the uterine endometrium undergoing decidualization. Dev. Biol. 2006, 294, 445–456. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef]
- Graubner, F.R.; Gram, A.; Kautz, E.; Bauersachs, S.; Aslan, S.; Agaoglu, A.R.; Boos, A.; Kowalewski, M.P. Uterine responses to early pre-attachment embryos in the domestic dog and comparisons with other domestic animal species. Biol. Reprod. 2017, 97, 197–216. [Google Scholar] [CrossRef]
- Herington, J.L.; Bany, B.M. Do molecular signals from the conceptus influence endometrium decidualization in rodents? J. Exp. Zool. B Mol. Dev. Evol. 2009, 312, 797–816. [Google Scholar] [CrossRef]
- Kautz, E.; de Carvalho Papa, P.; Reichler, I.M.; Gram, A.; Boos, A.; Kowalewski, M.P. In vitro decidualisation of canine uterine stromal cells. Reprod. Biol.Endocrinol. 2015, 13, 85. [Google Scholar] [CrossRef]
- Gram, A.; Boos, A.; Kowalewski, M.P. Uterine and Placental Expression of Canine Oxytocin Receptor During Pregnancy and Normal and Induced Parturition. Reprod. Domest. Anim. 2014, 49, 41–49. [Google Scholar] [CrossRef]
- Vermeirsch, H.; Simoens, P.; Hellemans, A.; Coryn, M.; Lauwers, H. Immunohistochemical detection of progesterone receptors in the canine uterus and their relation to sex steroid hormone levels. Theriogenology 2000, 53, 773–788. [Google Scholar] [CrossRef]
- Vermeirsch, H.; Simoens, P.; Lauwers, H. Immunohistochemical detection of the estrogen receptor-alpha and progesterone receptor in the canine pregnant uterus and placental labyrinth. Anat. Record. 2000, 260, 42–50. [Google Scholar] [CrossRef]
- Kowalewski, M.P.; Beceriklisoy, H.B.; Pfarrer, C.; Aslan, S.; Kindahl, H.; Kucukaslan, I.; Hoffmann, B. Canine placenta: A source of prepartal prostaglandins during normal and antiprogestin-induced parturition. Reproduction 2010, 139, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Gram, A.; Fox, B.; Buchler, U.; Boos, A.; Hoffmann, B.; Kowalewski, M.P. Canine placental prostaglandin E2 synthase: Expression, localization, and biological functions in providing substrates for prepartum PGF2alpha synthesis. Biol. Reprod. 2014, 91, 154. [Google Scholar] [CrossRef] [PubMed]
- Gram, A.; Buchler, U.; Boos, A.; Hoffmann, B.; Kowalewski, M.P. Biosynthesis and degradation of canine placental prostaglandins: Prepartum changes in expression and function of prostaglandin F2alpha-synthase (PGFS, AKR1C3) and 15-hydroxyprostaglandin dehydrogenase (HPGD). Biol. Reprod. 2013, 89, 2. [Google Scholar] [CrossRef] [PubMed]
- Gram, A.; Hoffmann, B.; Boos, A.; Kowalewski, M.P. Expression and localization of vascular endothelial growth factor A (VEGFA) and its two receptors (VEGFR1/FLT1 and VEGFR2/FLK1/KDR) in the canine corpus luteum and utero-placental compartments during pregnancy and at normal and induced parturition. Gen. Comp. Endocr. 2015, 223, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.A.; Edwards, D.P. Mechanism of action of progesterone antagonists. Exp. Biol. Med. 2002, 227, 969–980. [Google Scholar] [CrossRef]
- Beck, C.A.; Zhang, Y.; Weigel, N.L.; Edwards, D.P. Two types of anti-progestins have distinct effects on site-specific phosphorylation of human progesterone receptor. J. Biol. Chem. 1996, 271, 1209–1217. [Google Scholar] [CrossRef]
- Edwards, D.P.; Altmann, M.; DeMarzo, A.; Zhang, Y.; Weigel, N.L.; Beck, C.A. Progesterone receptor and the mechanism of action of progesterone antagonists. J. Steroid Biochem. Mol. Biol. 1995, 53, 449–458. [Google Scholar] [CrossRef]
- Klein-Hitpass, L.; Cato, A.C.; Henderson, D.; Ryffel, G.U. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 1991, 19, 1227–1234. [Google Scholar] [CrossRef]
- Kowalewski, M.P.; Pereira, M.T.; Papa, P.; Gram, A. Progesterone receptor blockers: Historical perspective, mode of function and insights into clinical and scientific applications. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2020, 48, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Baan, M.; Taverne, M.A.; Kooistra, H.S.; de Gier, J.; Dieleman, S.J.; Okkens, A.C. Induction of parturition in the bitch with the progesterone-receptor blocker aglepristone. Theriogenology 2005, 63, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Gogny, A.; Fieni, F. Aglepristone: A review on its clinical use in animals. Theriogenology 2016, 85, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Rehrauer, H.; Ay, S.S.; Findik, M.; Boos, A.; Kautz, E.; Kowalewski, M.P. Gene expression profiling of the canine placenta during normal and antigestagen-induced luteolysis. Gen. Comp. Endocrinol. 2019, 282, 113194. [Google Scholar] [CrossRef] [PubMed]
- Graubner, F.R.; Pereira, M.T.; Boos, A.; Kowalewski, M.P. Canine decidualization in vitro: Extracellular matrix modification, progesterone mediated effects and selective blocking of prostaglandin E2 receptors. J. Reprod. Dev. 2020, 66, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Liang, X.; Liang, X.H.; Wang, T.S.; Qi, Q.R.; Deng, W.B.; Sha, A.G.; Yang, Z.M. The mesenchymal-epithelial transition during in vitro decidualization. Reprod. Sci. 2013, 20, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Teo, A.K.K. Replicates in stem cell models-How complicated! Stem Cells 2020, 38, 1055–1059. [Google Scholar] [CrossRef]
- Kowalewski, M.P.; Schuler, G.; Taubert, A.; Engel, E.; Hoffmann, B. Expression of cyclooxygenase 1 and 2 in the canine corpus luteum during diestrus. Theriogenology 2006, 66, 1423–1430. [Google Scholar] [CrossRef]
- Kowalewski, M.P.; Michel, E.; Gram, A.; Boos, A.; Guscetti, F.; Hoffmann, B.; Aslan, S.; Reichler, I. Luteal and placental function in the bitch: Spatio-temporal changes in prolactin receptor (PRLr) expression at dioestrus, pregnancy and normal and induced parturition. Reprod. Biol. Endocrinol. 2011, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.P.; Meyer, A.; Hoffmann, B.; Aslan, S.; Boos, A. Expression and functional implications of peroxisome proliferator-activated receptor gamma (PPARgamma) in canine reproductive tissues during normal pregnancy and parturition and at antiprogestin induced abortion. Theriogenology 2011, 75, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Aslan, S.; Kowalewski, M.P. Determination of novel reference genes for improving gene expression data normalization in selected canine reproductive tissues—A multistudy analysis. BMC Vet. Res. 2020, 16, 440. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.P.; Fox, B.; Gram, A.; Boos, A.; Reichler, I. Prostaglandin E2 functions as a luteotrophic factor in the dog. Reproduction 2013, 145, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.P.; Tavares Pereira, M.; Kazemian, A. Canine conceptus-maternal communication during maintenance and termination of pregnancy, including the role of species-specific decidualization. Theriogenology 2020, 150, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Helmestam, M.; Lindgren, K.E.; Stavreus-Evers, A.; Olovsson, M. Mifepristone-exposured human endometrial endothelial cells in vitro. Reprod. Sci. 2014, 21, 408–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goyeneche, A.A.; Caron, R.W.; Telleria, C.M. Mifepristone inhibits ovarian cancer cell growth in vitro and in vivo. Clin. Cancer Res. 2007, 13, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, D.J.; Wagner, G.P. Single-cell analysis of prostaglandin E2-induced human decidual cell in vitro differentiation: A minimal ancestral deciduogenic signal. Biol. Reprod. 2021, 106, 155–172. [Google Scholar] [CrossRef]
- Heikinheimo, O.; Kontula, K.; Croxatto, H.; Spitz, I.; Luukkainen, T.; Lahteenmaki, P. Plasma concentrations and receptor binding of RU 486 and its metabolites in humans. J. Steroid Biochem. 1987, 26, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Gagne, D.; Pons, M.; Philibert, D. RU 38486: A potent antiglucocorticoid in vitro and in vivo. J. Steroid Biochem. 1985, 23, 247–251. [Google Scholar] [CrossRef]
- Hoffmann, B.; Schuler, G. Receptor blockers—General aspects with respect to their use in domestic animal reproduction. Anim. Reprod. Sci. 2000, 60–61, 295–312. [Google Scholar] [CrossRef]
- Gram, A.; Trachsel, A.; Boos, A.; Kowalewski, M.P. Elevated utero/placental GR/NR3C1 is not required for the induction of parturition in the dog. Reproduction 2016, 152, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Z.; Zhu, C. Effect of mifepristone on the expression of chorionic gonadotropin beta subunit and collagen type IV in female rhesus monkey decidua and villus at early gestation. Zhonghua Nan Ke Xue 2004, 10, 358–361. [Google Scholar] [PubMed]
- Laws, M.J.; Taylor, R.N.; Sidell, N.; DeMayo, F.J.; Lydon, J.P.; Gutstein, D.E.; Bagchi, M.K.; Bagchi, I.C. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development 2008, 135, 2659–2668. [Google Scholar] [CrossRef]
- Orlando-Mathur, C.E.; Bechberger, J.F.; Goldberg, G.S.; Naus, C.C.; Kidder, G.M.; Kennedy, T.G. Rat endometrial stromal cells express the gap junction genes connexins 26 and 43 and form functional gap junctions during in vitro decidualization. Biol. Reprod. 1996, 54, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, J.; Bagchi, I.C.; Bagchi, M.K.; Sidell, N.; Taylor, R.N. Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol. Cell Endocrinol. 2011, 344, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Berga, S.L.; Zou, W.; Sun, H.Y.; Johnston-MacAnanny, E.; Yalcinkaya, T.; Sidell, N.; Bagchi, I.C.; Bagchi, M.K.; Taylor, R.N. Gap junction blockade induces apoptosis in human endometrial stromal cells. Mol. Reprod. Dev. 2014, 81, 666–675. [Google Scholar] [CrossRef]
- Ogle, T.F.; George, P.; Dai, D. Progesterone and estrogen regulation of rat decidual cell expression of proliferating cell nuclear antigen. Biol. Reprod. 1998, 59, 444–450. [Google Scholar] [CrossRef][Green Version]
- Li, A.; Felix, J.C.; Minoo, P.; Amezcua, C.A.; Jain, J.K. Effect of mifepristone on proliferation and apoptosis of Ishikawa endometrial adenocarcinoma cells. Fertil. Steril. 2005, 84, 202–211. [Google Scholar] [CrossRef]
- Giulianelli, S.; Molinolo, A.; Lanari, C. Targeting Progesterone Receptors in Breast Cancer. Vitam. Horm. 2013, 93, 161–184. [Google Scholar] [CrossRef]
- Khaled, Z.; Serdar, E.B. Encyclopedia of Hormones; Elsevier: Amsterdam, The Netherlands, 2003; pp. 512–522. [Google Scholar]
- Rollon, E.; Millan, Y.; de las Mulas, J.M. Effects of aglepristone, a progesterone receptor antagonist, in a dog with a vaginal fibroma. J. Small Anim. Pract. 2008, 49, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Guil-Luna, S.; Millan, Y.; De Andres, J.; Rollon, E.; Domingo, V.; Garcia-Macias, J.; Sanchez-Cespedes, R.; Martin de Las Mulas, J. Prognostic impact of neoadjuvant aglepristone treatment in clinicopathological parameters of progesterone receptor-positive canine mammary carcinomas. Vet. Comp. Oncol. 2017, 15, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Pieczewska, B.; Glinska-Suchocka, K.; Nizanski, W.; Dzieciol, M. Decreased Size of Mammary Tumors Caused by Preoperative Treatment with Aglepristone in Female Domestic Dogs (Canis familiaris) Do Not Influence the Density of the Benign Neoplastic Tissue Measured Using Shear Wave Elastography Technique. Animals 2021, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.P.; Kazemian, A.; Klisch, K.; Gysin, T.; Tavares Pereira, M.; Gram, A. Canine Endotheliochorial Placenta: Morpho-Functional Aspects. Adv. Anat. Embryol. Cell Biol. 2021, 234, 155–179. [Google Scholar] [CrossRef]
- Kowalewski, M.P. Endocrine and molecular control of luteal and placental function in dogs: A review. Reprod. Domest. Anim. 2012, 47 (Suppl. S6), 19–24. [Google Scholar] [CrossRef]
- Nohr, B.; Hoffmann, B.; Steinetz, B.E. Investigation of the endocrine control of parturition in the dog by application of an antigestagen. J. Reprod Fertil. Suppl. 1993, 47, 542–543. [Google Scholar]
- Baan, M.; Taverne, M.A.; de Gier, J.; Kooistra, H.S.; Kindahl, H.; Dieleman, S.J.; Okkens, A.C. Hormonal changes in spontaneous and aglepristone-induced parturition in dogs. Theriogenology 2008, 69, 399–407. [Google Scholar] [CrossRef]
| Primer Gene Name | Accession Numbers | Primer Sequence | Product Length (bp) | |
|---|---|---|---|---|
| PTGES | NM_001122854 | Forward | 5′-GTC CTG GCG CTG GTG AGT-3′ | 89 |
| Reverse | 5′-ATG ACA GCC ACC ACG TAC ATC-3′ | |||
| TaqMan probe | 5′-TCC CAG CCT TCC TGC TCT GCA GC-3′ | |||
| PRLR | HQ267784 | Forward | 5′-GGA TCT TTG CCG TTC TTT-3′ | 92 |
| Reverse | 5′-AAG GAT GCA GGT CAC CAT GCT AT-3′ | |||
| TaqMan probe | 5′-ATT ATG GTC GTA GCA GTG GCT TTG AAA GGC-3′ | |||
| PGR | NM_001003074 | Forward | 5′-CGA GTC ATT ACC TCA GAA GAT TTG TTT-3′ | 113 |
| Reverse | 5′-CTT CCA TTG CCC TTT TAA AGA AGA-3′ | |||
| TaqMan probe | 5′-AAG CAT CAG GCT GTC ATT ATG GTG TCC TAA CTT-3′ | |||
| ECM1 | XM_845921.4 | Forward | 5′-CAG TCT GGC TTC TCC CAC CTT A-3′ | 99 |
| Reverse | 5′-GCG GTT TGT GTG GCT GTG A-3′ | |||
| TaqMan probe | 5′-AGA CTA GAT ATT CCC GCT GCT GCC GCT-3′ | |||
| CX43 | AY462223 | Forward | 5-AAA AGA GAA CCC TGC CCT CAT C-3 | 91 |
| Reverse | 5-AGG ACA CGA CCA GCA TGA AGA-3 | |||
| TaqMan probe | 5-ACT GCT TCC TCT CTC GCC CCA CG-3 | |||
| IGF1 | NM_001313855 | Applied Biosystems, prod nr. Cf02627846_m1 | 104 | |
| IGF2 | NM_001195403 | Applied Biosystems, prod nr. Cf02647136_m1 | 126 | |
| COL4 | XM_022408226 | Applied Biosystems, prod nr. Cf02696157_mH | 82 | |
| KDM4A | XM_005629106 | Applied Biosystems, prod nr. Cf02708629_m1 | 96 | |
| EIF4H | XM_014114129 | Applied Biosystems, prod nr. Cf02713640_m1 | 136 | |
| PTK2 | XM_005627993 | Applied Biosystems, prod nr. Cf02684608_m1 | 104 | |
| Antibody | Company | Reference Number | Host | Dilution |
|---|---|---|---|---|
| ECM1 | Proteintech | 11521-1-AP | Rabbit polyclonal | IF 1:100 |
| COL4 | Abcam | ab6586 | Rabbit polyclonal | IF 1:300 |
| CX43 | Abcam | ab11370 | Rabbit polyclonal | IF 1:400 |
| SV40Tag | Abcam | ab16879 | Mouse monoclonal | IF 1:500 |
| Vimentin | Abcam | ab92574 | Rabbit monoclonal | IF 1:500 |
| WB 1:400 | ||||
| Alexa fluor 488 goat anti-mouse IgG (H+L) | Invitrogen | A11029 | Goat | IF 1:100 |
| Alexa fluor 594 goat anti-rabbit IgG (H+L) | Invitrogen | A11037 | Goat | IF 1:100 |
| PGR | IOPath | IM1408-Clone PR10A9 | Mouse monoclonal | WB 1:300 |
| PCNA | Abcam | ab29 | Mouse monoclonal | WB 1:1000 |
| a-CASP3 | BD Biosciences | 559565 | Rabbit monoclonal | WB 1:200 |
| α-SMA | DAKO | GA61161-2 | Mouse anti-human | WB 1:500 |
| ACTB | Santa Cruz Biotechnology | sc-69879 | Mouse monoclonal | WB 1:1000 |
| Goat anti-mouse HRP-labelled secondary IgG | Promega | W402B | Goat anti-mouse IgG | WB 1:15,000 |
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, HRP | Thermo Fisher Scientific | 31460 | Goat anti-rabbit IgG | WB 1:15,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazemian, A.; Tavares Pereira, M.; Hoffmann, B.; Kowalewski, M.P. Antigestagens Mediate the Expression of Decidualization Markers, Extracellular Matrix Factors and Connexin 43 in Decidualized Dog Uterine Stromal (DUS) Cells. Animals 2022, 12, 798. https://doi.org/10.3390/ani12070798
Kazemian A, Tavares Pereira M, Hoffmann B, Kowalewski MP. Antigestagens Mediate the Expression of Decidualization Markers, Extracellular Matrix Factors and Connexin 43 in Decidualized Dog Uterine Stromal (DUS) Cells. Animals. 2022; 12(7):798. https://doi.org/10.3390/ani12070798
Chicago/Turabian StyleKazemian, Ali, Miguel Tavares Pereira, Bernd Hoffmann, and Mariusz P. Kowalewski. 2022. "Antigestagens Mediate the Expression of Decidualization Markers, Extracellular Matrix Factors and Connexin 43 in Decidualized Dog Uterine Stromal (DUS) Cells" Animals 12, no. 7: 798. https://doi.org/10.3390/ani12070798
APA StyleKazemian, A., Tavares Pereira, M., Hoffmann, B., & Kowalewski, M. P. (2022). Antigestagens Mediate the Expression of Decidualization Markers, Extracellular Matrix Factors and Connexin 43 in Decidualized Dog Uterine Stromal (DUS) Cells. Animals, 12(7), 798. https://doi.org/10.3390/ani12070798







