Linear Discriminant Analysis for Investigating Differences in Upper Body Movement Symmetry in Horses before/after Diagnostic Analgesia in Relation to Expert Judgement
Abstract
:Simple Summary
Abstract
1. Introduction
- The accuracy of a data-driven categorization of the efficacy of diagnostic analgesia from quantitative gait changes would reflect that increased uncertainties exist when scoring hindlimb lameness (increased intra- and inter-observer variability for scoring hindlimb than for scoring forelimb lameness [27,28]) and would hence provide higher accuracy for categorizing forelimb and lower accuracy for hindlimb diagnostic analgesia.
- Quantitative distances between data clusters, as a measure of the data-driven ability to differentiate between categories of block efficacy, would vary across exercise conditions. For example, we expect lunge exercises with the blocked limb on the inside of the circle to provide higher distances between diagnostic analgesia categories, i.e., a better classification, due to the documented increase in movement asymmetry for that exercise condition in horses with pre-existing straight-line movement asymmetries [21,24].
- In analogy to visual observation, quantitative features derived from head movement would be most useful for differentiating between forelimb diagnostic analgesia efficacy categories, and pelvic movement asymmetry features would be most useful for differentiating between hindlimb categories.
2. Materials and Methods
2.1. Horses and Facilities
2.2. Instrumentation
2.3. Movement Symmetry Parameters
2.3.1. Sign Convention
2.3.2. Normalization
2.4. Exercise Conditions
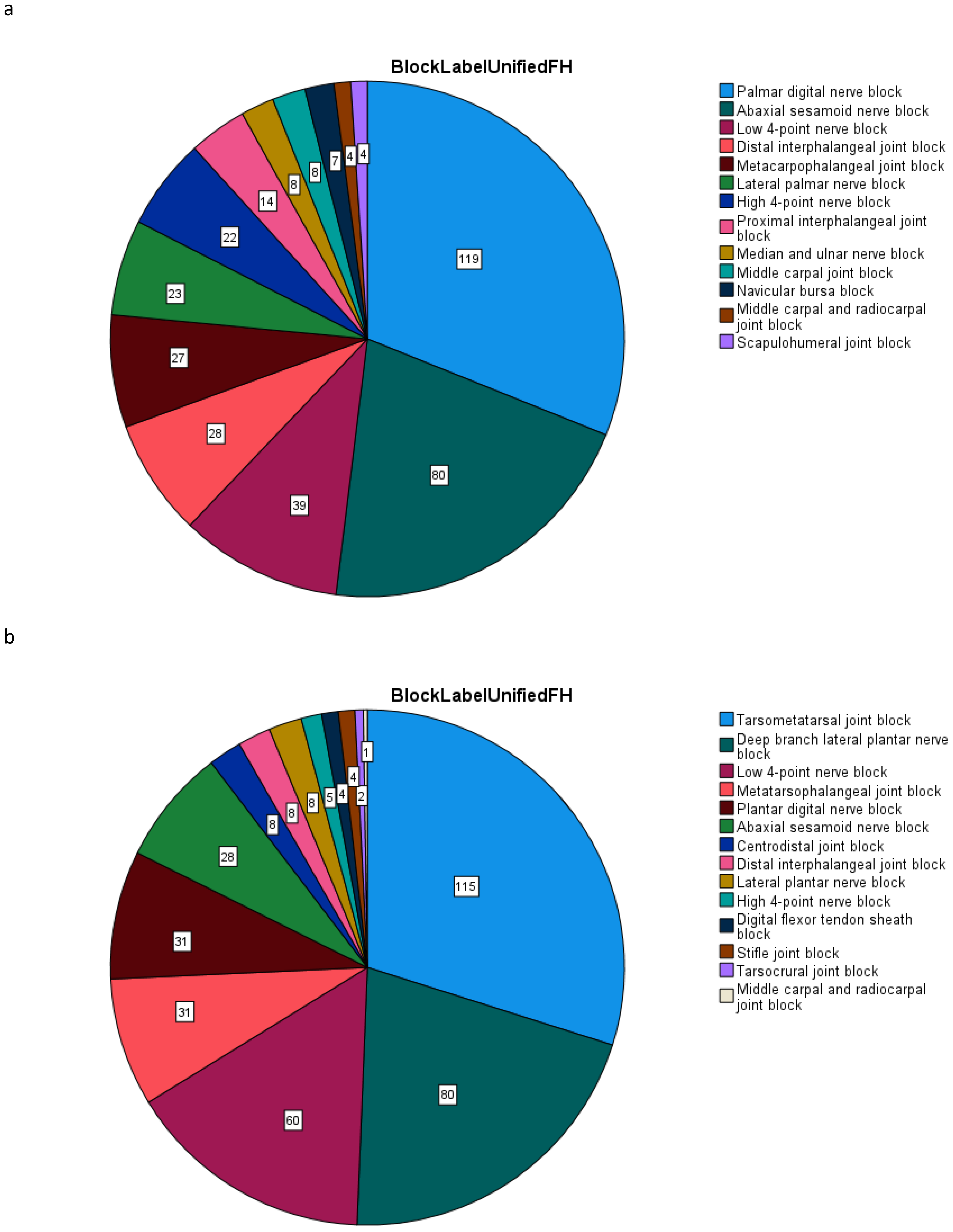
2.5. Block Efficacy
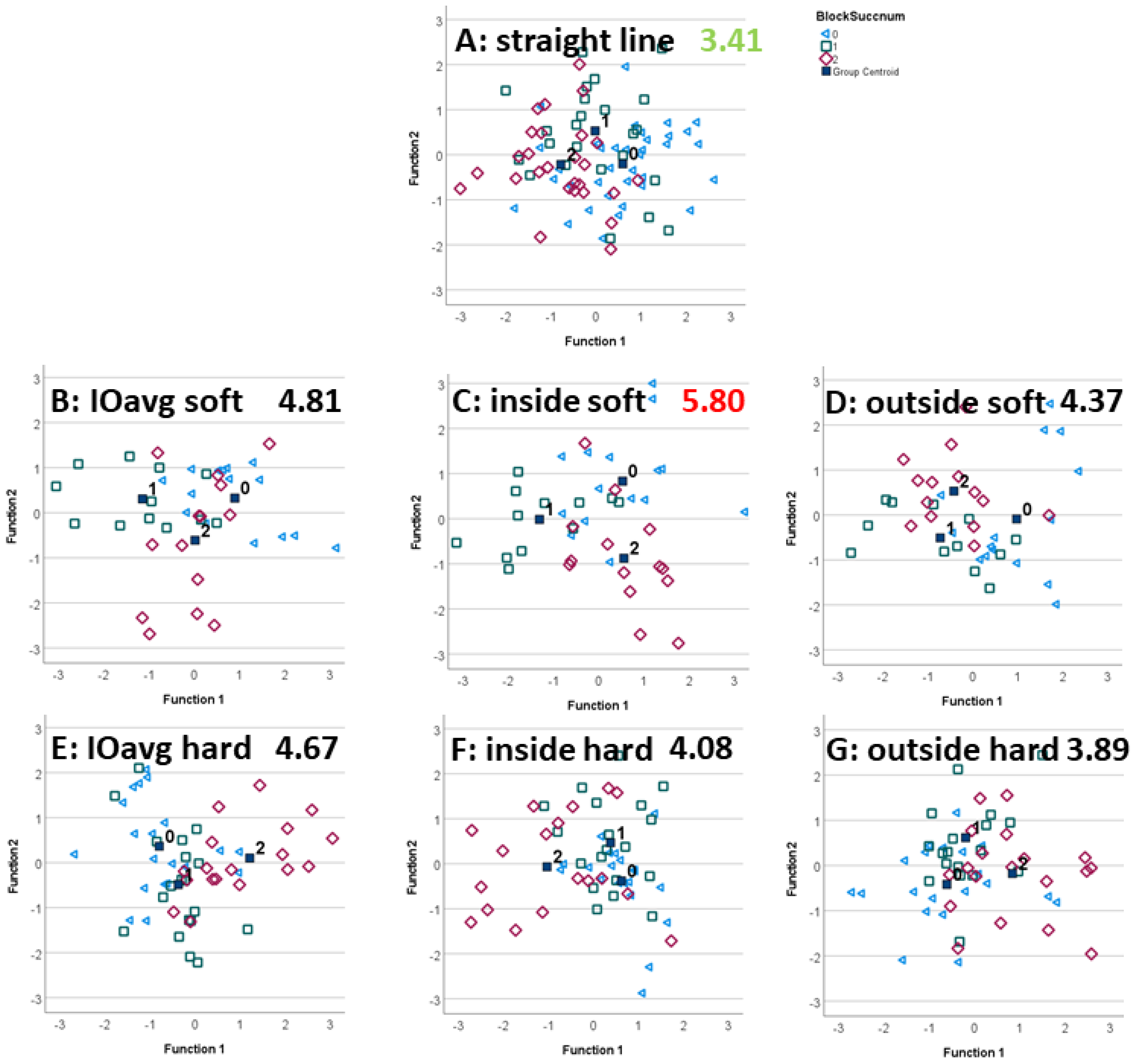
2.6. Linear Discriminant Analysis
2.6.1. Classification Accuracy
2.6.2. Variation between Exercises
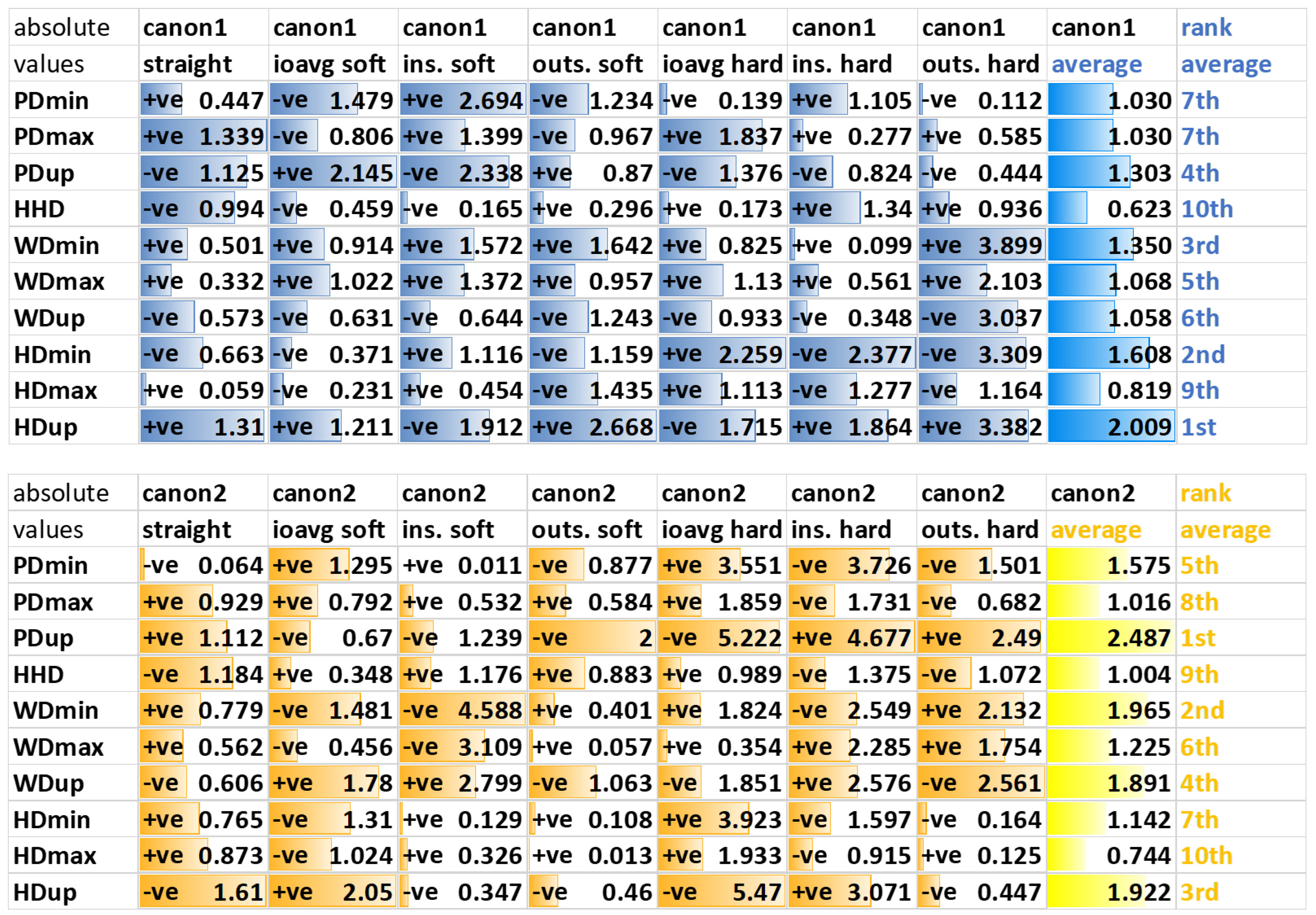
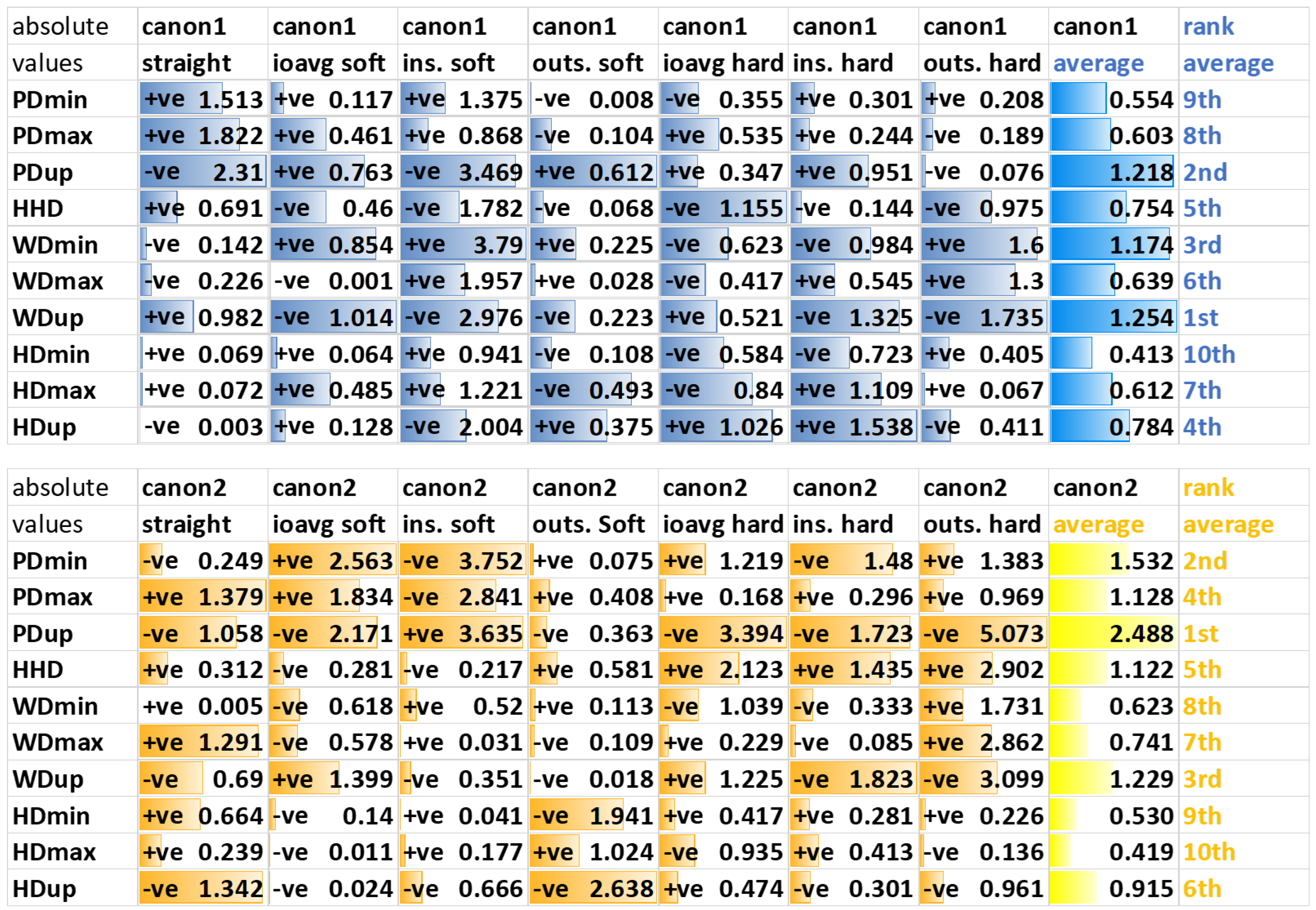
2.6.3. Movement Asymmetry Parameters
3. Results
3.1. Classification Accuracy
3.2. Exercise Condition
3.3. Movement Asymmetry Parameters
3.3.1. Forelimb Diagnostic Analgesia
3.3.2. Hindlimb Diagnostic Analgesia
4. Discussion
4.1. Classification Accuracy
4.2. Exercise Condition
4.2.1. Forelimb Diagnostic Analgesia
4.2.2. Hindlimb Diagnostic Analgesia
4.2.3. Average across Reins
4.3. Asymmetry Parameters
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassage, L., II; Ross, M.W. Diagnostic Analgesia. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Elsevier Health Sciences: London, UK, 2011; pp. 100–134. [Google Scholar]
- Ross, M.W.; Durham, M. Applied Anatomy of the Musculoskeletal System. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Elsevier Health Sciences: London, UK, 2011; pp. 88–99. [Google Scholar]
- Ross, M.W. Movement. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Elsevier Health Sciences: London, UK, 2011; pp. 64–79. [Google Scholar]
- Ross, M.W. Coexistent Lameness. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Elsevier Health Sciences: London, UK, 2011; ISBN 978-1-4160-6069-7. [Google Scholar]
- Hobbs, S.J.; Licka, T.; Polman, R. The Difference in Kinematics of Horses Walking, Trotting and Cantering on a Flat and Banked 10 m Circle. Equine Vet. J. 2011, 43, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Chateau, H.; Camus, M.; Holden-Douilly, L.; Falala, S.; Ravary, B.; Vergari, C.; Lepley, J.; Denoix, J.-M.; Pourcelot, P.; Crevier-Denoix, N. Kinetics of the Forelimb in Horses Circling on Different Ground Surfaces at the Trot. Vet. J. 2013, 198, e20–e26. [Google Scholar] [CrossRef] [PubMed]
- Greve, L.; Pfau, T.; Dyson, S. Alterations in Body Lean Angle in Lame Horses before and after Diagnostic Analgesia in Straight Lines in Hand and on the Lunge. Vet. J. 2018, 239, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barstow, A.; Bailey, J.; Campbell, J.; Harris, C.; Weller, R.; Pfau, T. Does ‘Hacking’ Surface Type Affect Equine Forelimb Foot Placement, Movement Symmetry or Hoof Impact Deceleration during Ridden Walk and Trot Exercise. Equine Vet. J. 2018, 51, 108–114. [Google Scholar] [CrossRef]
- Setterbo, J.J.; Garcia, T.C.; Campbell, I.P.; Reese, J.L.; Morgan, J.M.; Kim, S.Y.; Hubbard, M.; Stover, S.M. Hoof Accelerations and Ground Reaction Forces of Thoroughbred Racehorses Measured on Dirt, Synthetic, and Turf Track Surfaces. Am. J. Vet. Res. 2009, 70, 1220–1229. [Google Scholar] [CrossRef]
- Chateau, H.; Holden, L.; Robin, D.; Falala, S.; Pourcelot, P.; Estoup, P.; Denoix, J.-M.; Crevier-Denoix, N. Biomechanical Analysis of Hoof Landing and Stride Parameters in Harness Trotter Horses Running on Different Tracks of a Sand Beach (from Wet to Dry) and on an Asphalt Road. Equine Vet. J. 2010, 42 (Suppl. 3), 488–495. [Google Scholar] [CrossRef]
- Alexander, R.M.; Maloiy, G.M.O.; Hunter, B.; Jayes, A.S.; Nturibi, J. Mechanical Stresses during Fast Locomotion of Buffalo (Syncerus Caffer) and Elephant (Loxodonta Africana). J. Zool. Lond. 1979, 189, 135–144. [Google Scholar] [CrossRef]
- Witte, T.H.; Knill, K.; Wilson, A.M. Determination of Peak Vertical Ground Reaction Force from Duty Factor in the Horse (Equus Caballus). J. Exp. Biol. 2004, 207, 3639–3648. [Google Scholar] [CrossRef] [Green Version]
- Horan, K.; Coburn, J.; Kourdache, K.; Day, P.; Harborne, D.; Brinkley, L.; Carnall, H.; Hammond, L.; Peterson, M.; Millard, S.; et al. Influence of Speed, Ground Surface and Shoeing Condition on Hoof Breakover Duration in Galloping Thoroughbred Racehorses. Animals 2021, 11, 2588. [Google Scholar] [CrossRef]
- McCracken, M.J.; Kramer, J.; Keegan, K.G.; Lopes, M.; Wilson, D.A.; Reed, S.K.; LaCarrubba, A.; Rasch, M. Comparison of an Inertial Sensor System of Lameness Quantification with Subjective Lameness Evaluation. Equine Vet. J. 2012, 44, 652–656. [Google Scholar] [CrossRef]
- Keegan, K.G.; MacAllister, C.G.; Wilson, D.A.; Gedon, C.A.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F. Comparison of an Inertial Sensor System with a Stationary Force Plate for Evaluation of Horses with Bilateral Forelimb Lameness. Am. J. Vet. Res. 2012, 73, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.P.; Reed, S.K.; Schoonover, M.J.; Whitfield, C.T.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Keegan, K.G. Associations of Force Plate and Body-Mounted Inertial Sensor Measurements for Identification of Hind Limb Lameness in Horses. Am. J. Vet. Res. 2016, 77, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Arkell, M.; Archer, R.M.; Guitian, F.J.; May, S.A. Evidence of Bias Affecting the Interpretation of the Results of Local Anaesthetic Nerve Blocks When Assessing Lameness in Horses. Vet Rec. 2006, 159, 346–349. [Google Scholar] [CrossRef]
- Parkes, R.S.V.; Weller, R.; Groth, A.M.; May, S.; Pfau, T. Evidence of the Development of ‘Domain-Restricted’ Expertise in the Recognition of Asymmetric Motion Characteristics of Hindlimb Lameness in the Horse. Equine Vet. J. 2009, 41, 112–117. [Google Scholar] [CrossRef]
- Hardeman, A.M.; Serra Bragança, F.M.; Swagemakers, J.H.; Weeren, P.R.; Roepstorff, L. Variation in Gait Parameters Used for Objective Lameness Assessment in Sound Horses at the Trot on the Straight Line and the Lunge. Equine Vet. J. 2019, 51, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfau, T.; Stubbs, N.C.; Kaiser, L.J.; Brown, L.E.A.; Clayton, H.M. Effect of Trotting Speed and Circle Radius on Movement Symmetry in Horses during Lunging on a Soft Surface. Am. J. Vet. Res. 2012, 73, 1890–1899. [Google Scholar] [CrossRef]
- Pfau, T.; Jennings, C.; Mitchell, H.; Olsen, E.; Walker, A.; Egenvall, A.; Tröster, S.; Weller, R.; Rhodin, M. Lungeing on Hard and Soft Surfaces: Movement Symmetry of Trotting Horses Considered Sound by Their Owners. Equine Vet. J. 2016, 48, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Rhodin, M.; Pfau, T.; Roepstorff, L. Egenvall, a Effect of Lungeing on Head and Pelvic Movement Asymmetry in Horses with Induced Lameness. Vet. J. 2013, 198, e39–e45. [Google Scholar] [CrossRef] [Green Version]
- Rhodin, M.; Roepstorff, L.; French, A.; Keegan, K.G.; Pfau, T.; Egenvall, A. Head and Pelvic Movement Asymmetry during Lungeing in Horses with Symmetrical Movement on the Straight. Equine Vet. J. 2015, 48, 315–320. [Google Scholar] [CrossRef]
- Rhodin, M.; Egenvall, A.; Andersen, P.H.; Pfau, T. Head and Pelvic Movement Asymmetries at Trot in Riding Horses in Training and Perceived as Free from Lameness by the Owner. PLoS ONE 2017, 12, e0176253. [Google Scholar] [CrossRef]
- Hawkins, A.; O’Leary, L.; Bolt, D.; Fiske-Jackson, A.; Berner, D.; Smith, R. Retrospective Analysis of Oblique and Straight Distal Sesamoidean Ligament Desmitis in 52 Horses. Equine Vet. J. 2021, 54, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Robilliard, J.J.; Weller, R.; Jespers, K.; Eliashar, E.; Wilson, A.M. Assessment of Mild Hindlimb Lameness during over Ground Locomotion Using Linear Discriminant Analysis of Inertial Sensor Data. Equine Vet. J. 2007, 39, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.G.; Wilson, D.A.; Wilson, D.J.; Smith, B.; Gaughan, E.M.; Pleasant, R.S.; Lillich, J.D.; Kramer, J.; Howard, R.D.; Bacon-Miller, C. Evaluation of Mild Lameness in Horses Trotting on a Treadmill by Clinicians and Interns or Residents and Correlation of Their Assessments with Kinematic Gait Analysis. Am. J. Vet. Res. 1998, 59, 1370–1377. [Google Scholar]
- Keegan, K.G.; Dent, E.V.; Wilson, D.A.; Janicek, J.; Kramer, J.; Lacarrubba, A.; Walsh, D.M.; Cassells, M.W.; Esther, T.M.; Schiltz, P.; et al. Repeatability of Subjective Evaluation of Lameness in Horses. Equine Vet. J. 2010, 42, 92–97. [Google Scholar] [CrossRef]
- Pfau, T.; Reilly, P. How Low Can We Go? Influence of Sample Rate on Equine Pelvic Displacement Calculated from Inertial Sensor Data. Equine Vet. J. 2020, 53, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Witte, T.H.; Wilson, A.M. A Method for Deriving Displacement Data during Cyclical Movement Using an Inertial Sensor. J. Exp. Biol. 2005, 208, 2503–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, S.M.; Koch, T.O.; Pfau, T. Inertial Sensors for Assessment of Back Movement in Horses during Locomotion over Ground. Equine Vet. J. 2010, 42, 417–424. [Google Scholar] [CrossRef]
- Starke, S.D.; Witte, T.H.; May, S.A.; Pfau, T. Accuracy and Precision of Hind Limb Foot Contact Timings of Horses Determined Using a Pelvis-Mounted Inertial Measurement Unit. J. Biomech. 2012, 45, 1522–1528. [Google Scholar] [CrossRef]
- Starke, S.D.; Willems, E.; May, S.A.; Pfau, T. Vertical Head and Trunk Movement Adaptations of Sound Horses Trotting in a Circle on a Hard Surface. Vet. J. 2012, 193, 73–80. [Google Scholar] [CrossRef]
- Haeb-Umbach, R.; Ney, H. Linear Discriminant Analysis for Improved Large Vocabulary Continuous Speech Recognition. In Proceedings of the ICASSP 1992, San Francisco, CA, USA, 23–26 March 1992; IEEE Computer Society: Washington, DC, USA, 1992. [Google Scholar]
- Pylkkonen, J. LDA Based Feature Estimation Methods for LVCSR. In Proceedings of the INTERSPEECH 2006-ICSLP, Pittsburgh, PA, USA, 17 September 2006. [Google Scholar]
- Pfau, T.; Persson-Sjodin, E.; Gardner, H.; Orssten, O.; Hernlund, E.; Rhodin, M. Effect of Speed and Surface Type on Individual Rein and Combined Left–Right Circle Movement Asymmetry in Horses on the Lunge. Front. Vet. Sci. 2021, 8, 692031. [Google Scholar] [CrossRef]
- Hammarberg, M.; Egenvall, A.; Pfau, T.; Rhodin, M. Rater Agreement of Visual Lameness Assessment in Horses during Lungeing. Equine Vet. J. 2016, 48, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepulveda Caviedes, M.F.; Forbes, B.S.; Pfau, T. Repeatability of Gait Analysis Measurements in Thoroughbreds in Training. Equine Vet. J. 2018, 50, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starke, S.; Raistrick, K.; May, S.; Pfau, T. The Effect of Trotting Speed on the Evaluation of Subtle Lameness in Horses. Vet. J. 2013, 197, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Persson-Sjodin, E.; Serra Braganca, F.; Pfau, T.; Egenvall, A.; Weishaupt, M.; Rhodin, M. Movement Symmetry of the Withers Can Be Used to Discriminate Primary Forelimb Lameness from Compensatory Forelimb Asymmetry in Horses with Induced Lameness. Equine Vet. J. 2016, 48, 32. [Google Scholar]
- Rhodin, M.; Persson-Sjodin, E.; Egenvall, A.; Serra Braganca, F.; Pfau, T.; Roepstorff, L.; Weishaupt, M.; Thomsen, M.H.; van Weeren, P.R.; Hernlund, E.; et al. Vertical Movement Symmetry of the Withers in Horses with Induced Forelimb and Hindlimb Lameness at Trot. Equine Vet. J. 2018, 50, 818–824. [Google Scholar] [CrossRef]
- Pfau, T.; Noordwijk, K.; Caviedes, M.F.S. Head, Withers and Pelvic Movement Asymmetry and Their Relative Timing in Trot in Racing Thoroughbreds in Training. Equine Vet. J. 2018, 50, 117–124. [Google Scholar] [CrossRef]
- Buchner, H.H.; Savelberg, H.H.; Schamhardt, H.C.; Barneveld, A. Head and Trunk Movement Adaptations in Horses with Experimentally Induced Fore- or Hindlimb Lameness. Equine Vet. J. 1996, 28, 71–76. [Google Scholar] [CrossRef]
- May, S.A.; Wyn-Jones, G. Identification of Hindleg Lameness. Equine Vet. J. 1987, 19, 185–188. [Google Scholar] [CrossRef]
- Maliye, S.; Marshall, J.F. Objective Assessment of the Compensatory Effect of Clinical Hind Limb Lameness in Horses: 37 Cases (2011–2014). Am. J. Vet. Res. 2016, 249, 940–944. [Google Scholar] [CrossRef]
- Maliye, S.; Voute, L.C.; Marshall, J.F. Naturally-Occurring Forelimb Lameness in the Horse Results in Significant Compensatory Load Redistribution during Trotting. Vet. J. 2015, 204, 208–213. [Google Scholar] [CrossRef]
- Uhlir, C.; Licka, T.; Kübber, P.; Peham, C.; Scheidl, M.; Girtler, D. Compensatory Movements of Horses with a Stance Phase Lameness. Equine Vet. J. 1997, 23, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, M.A.; Wiestner, T.; Hogg, H.P.; Jordan, P.; Auer, J.A. Compensatory Load Redistribution of Horses with Induced Weightbearing Hindlimb Lameness Trotting on a Treadmill. Equine Vet. J. 2004, 36, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, M.A.; Wiestner, T.; Hogg, H.P.; Jordan, P.; Auer, J.A. Compensatory Load Redistribution of Horses with Induced Weight-Bearing Forelimb Lameness Trotting on a Treadmill. Vet. J. 2006, 171, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Boultbee, H.; Davis, H.; Walker, A.; Rhodin, M. Agreement between Two Inertial Sensor Gait Analysis Systems for Lameness Examinations in Horses. Equine Vet. Educ. 2016, 28, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Pfau, T.; Caviedes, M.F.S.; Mccarthy, R.; Cheetham, L.; Forbes, B.; Rhodin, M. Comparison of Visual Lameness Scores to Gait Asymmetry in Racing Thoroughbreds during Trot In-Hand. Equine Vet. Educ. 2018, 32, 191–198. [Google Scholar] [CrossRef]
- Hewetson, M.; Christley, R.M.; Hunt, I.D.; Voute, L.C. Investigations of the Reliability of Observational Gait Analysis for the Assessment of Lameness in Horses. Vet. Rec. 2006, 158, 852–857. [Google Scholar] [CrossRef]
- Rey, A.; Goldstein, R.M.; Perruchet, P. Does Unconscious Thought Improve Complex Decision Making? Psychol. Res. 2009, 73, 372–379. [Google Scholar] [CrossRef]

| Fore/ Hind | Direction | Surface | Reclass. (%) | Leave-1-Out (%) | Abs Diff. (%) | N |
|---|---|---|---|---|---|---|
| F | str | Hard | 53.5 | 39.5 | 14 | 86 |
| F | Avg | All | 63.6 | 52.5 | 11.1 | 99 |
| Soft | 63.5 | 46.2 | 17.3 | 52 | ||
| Hard | 80.9 | 51.1 | 29.8 | 47 | ||
| F | Inside | All | 63.6 | 50.5 | 13.1 | 99 |
| Soft | 57.7 | 40.4 | 17.3 | 52 | ||
| Hard | 68.1 | 57.4 | 10.7 | 47 | ||
| F | Outside | All | 61.6 | 52.5 | 9.1 | 99 |
| Soft | 63.5 | 55.8 | 7.7 | 52 | ||
| Hard | 72.3 | 38.3 | 34 | 47 | ||
| F | weighted total | 60.82 | 49.06 | 383 | ||
| H | Str | Hard | 60.2 | 51.1 | 9.1 | 88 |
| H | Avg | All | 60.6 | 49.5 | 11.1 | 99 |
| Soft | 70.7 | 46.3 | 24.4 | 41 | ||
| hard | 60.3 | 48.3 | 12 | 58 | ||
| H | Inside | all | 61.6 | 44.4 | 17.2 | 99 |
| soft | 78 | 56.1 | 21.9 | 41 | ||
| hard | 62.1 | 44.8 | 17.3 | 58 | ||
| H | Outside | all | 51.5 | 38.4 | 13.1 | 99 |
| soft | 68.3 | 34.1 | 34.2 | 41 | ||
| hard | 58.6 | 39.7 | 18.9 | 58 | ||
| H | weighted total | 58.43 | 45.70 | 385 |
| Blocked Limb | Direction | Surface | neg2part | part2pos | neg2pos | Total | Highest Indiv. |
|---|---|---|---|---|---|---|---|
| F | str | hard | 0.65 | 0.62 | 0.96 | 2.23 | |
| avg | Soft | 1.64 | 1.14 | 1.49 | 4.27 | ||
| Hard | 1.30 | 2.34 | 1.91 | 5.56 | |||
| inside | Soft | 1.23 | 0.67 | 1.31 | 3.21 | ||
| Hard | 1.36 | 2.48 | 2.29 | 6.13 | part2pos, neg2pos | ||
| outside | Soft | 1.39 | 1.68 | 1.33 | 4.39 | ||
| Hard | 1.80 | 2.03 | 1.10 | 4.93 | neg2part | ||
| H | str | Hard | 0.96 | 1.07 | 1.38 | 3.41 | |
| avg | Soft | 2.05 | 1.48 | 1.28 | 4.81 | neg2part | |
| Hard | 0.95 | 1.69 | 2.03 | 4.67 | neg2pos | ||
| inside | Soft | 2.03 | 2.06 | 1.71 | 5.80 | part2pos | |
| Hard | 0.88 | 1.51 | 1.69 | 4.08 | |||
| outside | Soft | 1.75 | 1.08 | 1.53 | 4.37 | ||
| Hard | 1.11 | 1.30 | 1.47 | 3.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfau, T.; Bolt, D.M.; Fiske-Jackson, A.; Gerdes, C.; Hoenecke, K.; Lynch, L.; Perrier, M.; Smith, R.K.W. Linear Discriminant Analysis for Investigating Differences in Upper Body Movement Symmetry in Horses before/after Diagnostic Analgesia in Relation to Expert Judgement. Animals 2022, 12, 762. https://doi.org/10.3390/ani12060762
Pfau T, Bolt DM, Fiske-Jackson A, Gerdes C, Hoenecke K, Lynch L, Perrier M, Smith RKW. Linear Discriminant Analysis for Investigating Differences in Upper Body Movement Symmetry in Horses before/after Diagnostic Analgesia in Relation to Expert Judgement. Animals. 2022; 12(6):762. https://doi.org/10.3390/ani12060762
Chicago/Turabian StylePfau, Thilo, David M. Bolt, Andrew Fiske-Jackson, Carolin Gerdes, Karl Hoenecke, Lucy Lynch, Melanie Perrier, and Roger K. W. Smith. 2022. "Linear Discriminant Analysis for Investigating Differences in Upper Body Movement Symmetry in Horses before/after Diagnostic Analgesia in Relation to Expert Judgement" Animals 12, no. 6: 762. https://doi.org/10.3390/ani12060762
APA StylePfau, T., Bolt, D. M., Fiske-Jackson, A., Gerdes, C., Hoenecke, K., Lynch, L., Perrier, M., & Smith, R. K. W. (2022). Linear Discriminant Analysis for Investigating Differences in Upper Body Movement Symmetry in Horses before/after Diagnostic Analgesia in Relation to Expert Judgement. Animals, 12(6), 762. https://doi.org/10.3390/ani12060762






