The Relationship between the Testicular Blood Flow and the Semen Parameters of Rams during the Selected Periods of the Breeding and Non-Breeding Seasons
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Collection and Evaluation
2.3. Ultrasound Examination and Doppler Application
2.4. Statistical Analyses
3. Results
3.1. Routine Semen Evaluation Test
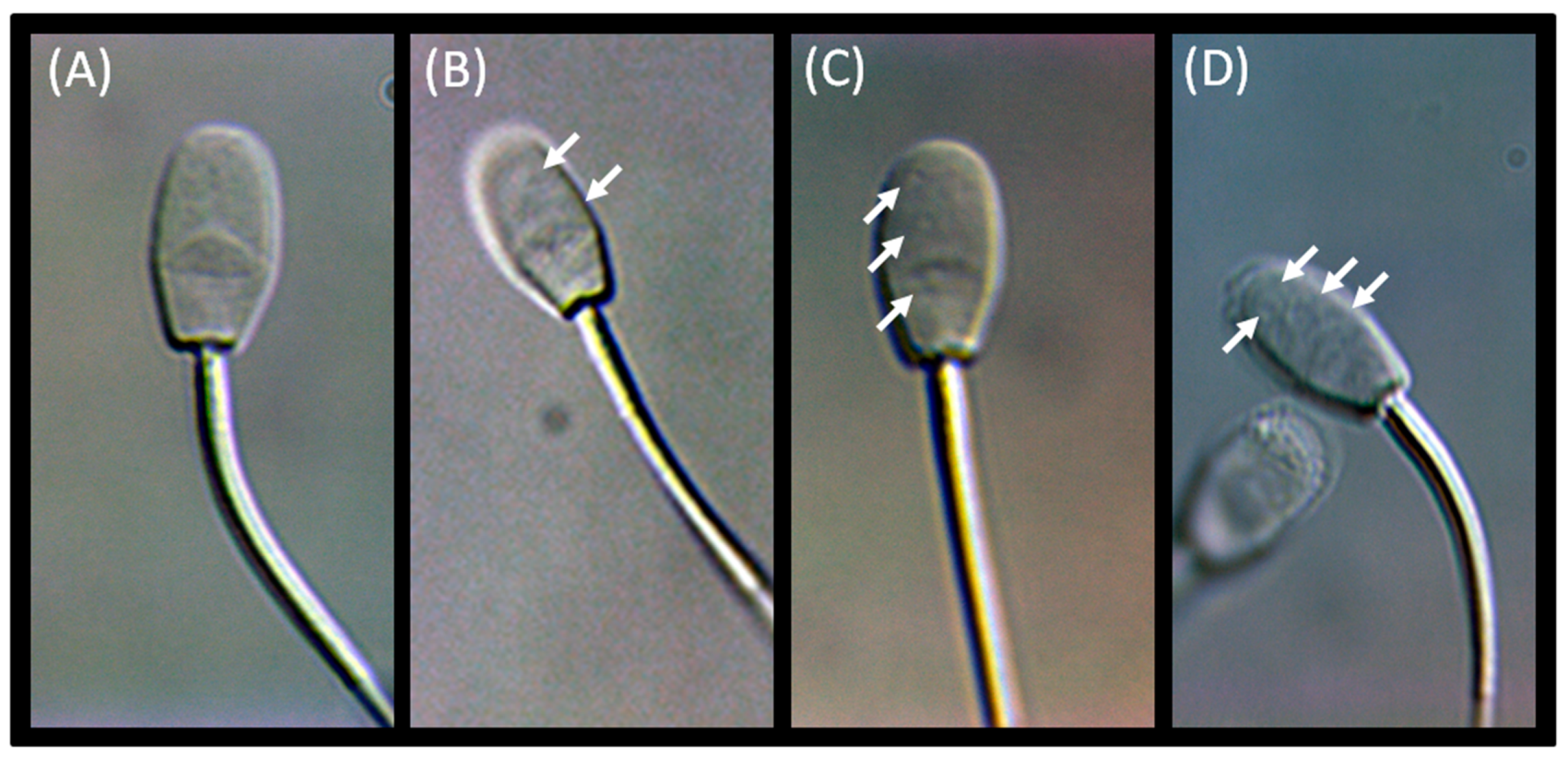
3.2. Advanced Semen Evaluation Tests
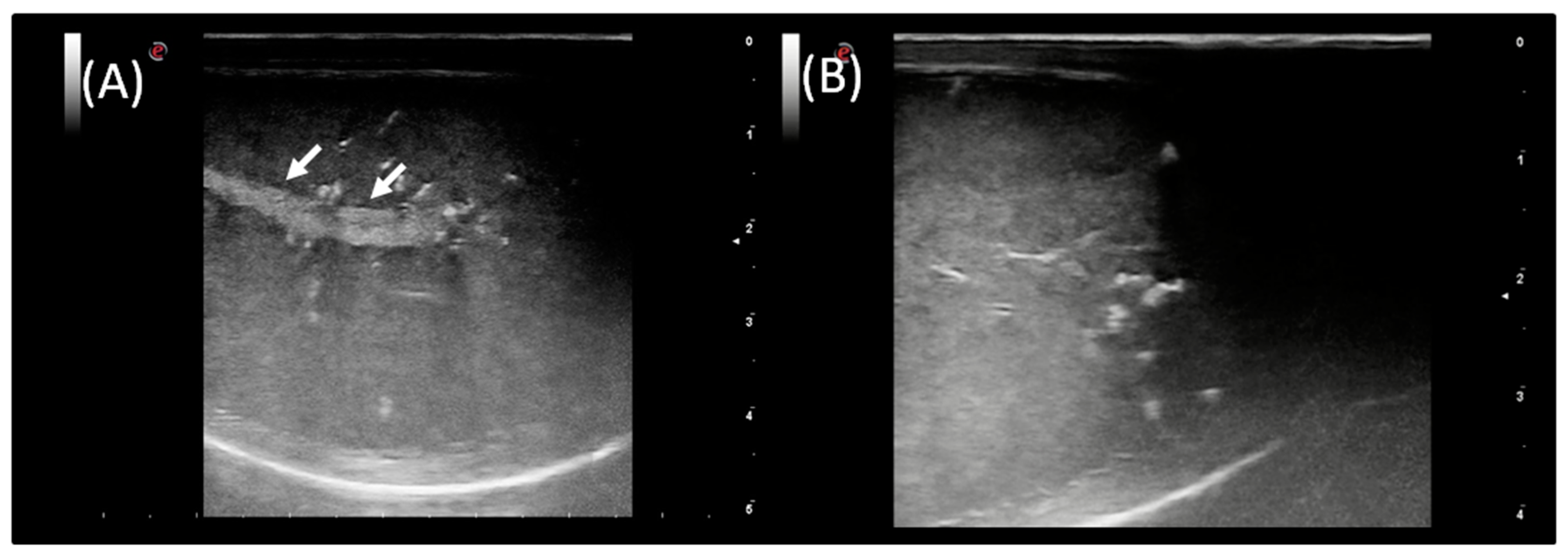
3.3. Ultrasound Examination and Doppler Application
3.4. Associations between the Semen Parameters and the Testicular Blood Flow
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosa, H.J.D.; Bryant, M.J. Seasonality of reproduction in sheep. Small Rumin. Res. 2003, 48, 155–171. [Google Scholar] [CrossRef]
- Maquivar, M.G.; Smith, S.M.; Busboom, J.R. Reproductive Management of Rams and Ram Lambs during the Pre-Breeding Season in US Sheep Farms. Animals 2021, 11, 2503. [Google Scholar] [CrossRef] [PubMed]
- Schurich, M.; Aigner, F.; Frauscher, F.; Pallwein, L. The role of ultra-sound in assessment of male fertility. Eur. J. Obstet. Gynecol. 2009, 144, S192–S198. [Google Scholar] [CrossRef]
- Medan, M.S.; El-Aty, A.M.A. Advances in ultrasonography and its applications in domestic ruminants and other farm animals reproduction. J. Adv. Res. 2010, 1, 123–128. [Google Scholar] [CrossRef][Green Version]
- Velasco, A.; Ruiz, S. New Approaches to Assess Fertility in Domestic Animals: Relationship between Arterial Blood Flow to the Testicles and Seminal Quality. Animals 2021, 11, 12. [Google Scholar] [CrossRef]
- Ahmadi, B.; Lau, C.P.S.; Giffin, J.; Santos, N.; Hahnel, A.; Raeside, J.; Christie, H.; Bartlewski, P. Suitability of epididymal and testicular ultrasonography and computerized image analysis for assessment of current and future semen quality in the ram. Exp. Biol. Med. 2012, 237, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, A.A.; Barth, A.D.; Brito, L.F. Relationship between semen quality and pixel–intensity of testicular ultrasonograms after scrotal insulation in beef bulls. Theriogenology 2005, 64, 408–415. [Google Scholar] [CrossRef]
- Moxon, R.; Bright, L.; Pritchard, B.; Bowen, I.M.; de Souza, M.B.; da Silva, L.D.M.; England, G.C. Digital image analysis of testicular and prostatic ultra-sonographic echogencity and heterogeneity in dogs and the relation to semen quality. Anim. Reprod. Sci. 2015, 160, 112–119. [Google Scholar] [CrossRef]
- Gacem, S.; Papas, M.; Catalan, J.; Miró, J. Examination of jackass (Equus asinus) accessory sex glands by B-mode ultrasound and of testicular artery blood flow by colour pulsed-wave Doppler ultrasound: Correlations with semen production. Reprod. Domest. Anim. 2020, 55, 181–188. [Google Scholar] [CrossRef]
- Pozor, M. Diagnostic applications of ultrasonography to stallion’s reproductive tract. Theriogenology 2005, 64, 505–509. [Google Scholar] [CrossRef]
- Batissaco, L.; Celeghini, E.C.C.; Pinaffi, F.L.V.; de Oliveira, B.M.M.; de Andrade, A.F.C.; Recalde, E.C.S.; Fernandes, C.B. Correlações entre a hemodinâmica testicular e as características espermáticas em carneiros. BJVRAS 2014, 50, 384. [Google Scholar] [CrossRef]
- Hedia, M.G.; El-Belely, M.S.; Ismail, S.T.; El-Maaty, A.M.A. Monthly changes in testicular blood flow dynamics and their association with testicular volume, plasma steroid hormones profile and semen characteristics in rams. Theriogenology 2019, 123, 68–73. [Google Scholar] [CrossRef]
- Carrillo, J.D.; Soler, M.; Lucas, X.; Agut, A. Colour and Pulsed Doppler Ultrasonographic Study of the Canine Testis. Reprod. Domest. Anim. 2012, 47, 655–659. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.B.; da Cunha Barbosa, C.; Pereira, B.S.; Monteiro, C.L.B.; Pinto, J.N.; Linhares, J.C.S.; da Silva, L.D.M. Doppler velocimetric parameters of the testicular artery in healthy dogs. Res. Vet. Sci. 2014, 96, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Carluccio, A.; Wegher, L.; Robbe, D.; Valorz, C.; Contri, A. Pulse wave Doppler ultrasound of testicular arteries and their relationship with semen characteristics in healthy bulls. J. Anim. Sci. Biotechnol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Pozor, M.A.; McDonnell, S.M. Color Doppler ultrasound evaluation of testicular blood flow in stallions. Theriogenology 2004, 61, 799–810. [Google Scholar] [CrossRef]
- Bergh, A.; Collin, O.; Lissbrant, E. Effects of Acute Graded Reductions in Testicular Blood Flow on Testicular Morphology in the Adult Rat. Biol. Reprod. 2001, 64, 13–20. [Google Scholar] [CrossRef]
- Biagiotti, G.; Cavallini, G.; Modenini, F.; Vitali, G.; Gianaroli, L. Spermatogenesis and spectral echo-colour Doppler traces from the main testicular artery. BJU Int. 2002, 90, 903–908. [Google Scholar] [CrossRef]
- Zelli, R.; Troisi, A.; Ngonput, A.E.; Cardinali, L.; Polisca, A. Evaluation of testicular artery blood flow by Doppler ultrasonography as a predictor of spermatogenesis in the dog. Res. Vet. Sci. 2013, 95, 632–637. [Google Scholar] [CrossRef]
- Ntemka, A.; Kiossis, E.; Boscos, C.; Theodoridis, A.; Kourousekos, G.; Tsakmakidis, I. Effects of testicular hemodynamic and echogenicity changes on ram semen characteristics. Reprod. Domest. Anim. 2018, 53, 50–55. [Google Scholar] [CrossRef]
- Kutzler, M.; Tyson, R.; Grimes, M.; Timm, K. Determination of Testicular Blood Flow in Camelids Using Vascular Casting and Color Pulsed-Wave Doppler Ultrasonography. Vet. Med. Int. 2011, 2011, 638602. [Google Scholar] [CrossRef] [PubMed]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm Morphology, Motility, and Concentration in Fertile and Infertile Men. NEJM 2001, 345, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Gouletsou, P.G.; Amiridis, G.S.; Cripps, P.J.; Lainas, T.; Deligiannis, K.; Saratsis, P.; Fthenakis, G.C. Ultrasonographic appearance of clinically healthy testicles and epididymides of rams. Theriogenology 2003, 59, 1959–1972. [Google Scholar] [CrossRef]
- Fréour, T.; Jean, M.; Mirallie, S.; Barriere, P. Computer-assisted sperm analysis parameters in young fertile sperm donors and relationship with age. Syst. Biol. Reprod. Med. 2012, 58, 102–106. [Google Scholar] [CrossRef]
- Boe-Hansen, G.B.; Satake, N. An update on boar semen assessments by flow cytometry and CASA. Theriogenology 2019, 137, 93–103. [Google Scholar] [CrossRef]
- Kozdrowski, R.; Dubiel, A.; Bielas, W.; Dziecioł, M. Two Protocols of Cryopreservation of Goat Semen with the Use of Computer-Assisted Semen Analysis System. Acta Vet. Brno 2007, 76, 601–604. [Google Scholar] [CrossRef]
- Balasuriya, A.; Speyer, B.; Serhal, P.; Doshi, A.; Harper, J.C. Sperm chromatin dispersion test in the assessment of DNA fragmentation and aneuploidy in human spermatozoa. Reprod. Biomed. Online 2011, 22, 428–436. [Google Scholar] [CrossRef]
- Liffner, S.; Pehrson, I.; García-Calvo, L.; Nedstrand, E.; Zalavary, S.; Hammar, M.; Rodríguez-Martínez, H.; Álvarez Rodríguez, M. Diagnostics of DNA fragmentation in human spermatozoa: Are sperm chromatin structure analysis and sperm chromatin dispersion tests (SCD-HaloSpermG2®) comparable? Andrologia 2019, 51, e13316. [Google Scholar] [CrossRef]
- Karaksy, A.O.E.; Amer, M.K.; Ahmed, H.E.D.H.; Zohdy, W.; Ahmed, A.R.; Ismail, N.N. Assessment of human sperm morphology: Comparison of motile sperm organelle morphology examination (MSOME) and light microscopy MSOME. Hum. Androl. 2014, 4, 75–84. [Google Scholar] [CrossRef]
- Gat, I.; Raviv, G.; Baum, M.; Orvieto, R. MSOME and IMSI: Reasonable rationale, selective clinical value. Clin. Exp. Obstet. Gynecol. 2019, 46, 679–689. [Google Scholar] [CrossRef]
- Tsakmakidis, I.A. Ram semen evaluation: Development and efficiency of modern techniques. Small Rumin. Res. 2010, 92, 126–130. [Google Scholar] [CrossRef]
- Larsen, L.; Scheike, T.; Jensen, T.K.; Bonde, J.P.; Ernst, E.; Hjollund, N.H.; Zhou, Y.; Skakkebaek, N.E.; Giwercman, A. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. Hum. Reprod. 2000, 15, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, J.; Iguer-Ouada, M.; Onclin, K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 2002, 57, 149–179. [Google Scholar] [CrossRef]
- Kumar, D.; Joshi, A.; Naqvi, S.M.K. Objective assessment of sperm motion characteristics of Malpura ram lambs raised under intensive management system in semiarid tropical environment. Trop. Anim. Health Prod. 2010, 42, 653–658. [Google Scholar] [CrossRef]
- Fetterolf, P.M.; Rogers, B.J. Prediction of human sperm penetrating ability using computerized motion parameters. Mol. Reprod. Dev. 1990, 27, 326–331. [Google Scholar] [CrossRef]
- Robayo, I.; Montenegro, V.; Valdés, C.; Cox, J.F. CASA Assessment of Kinematic Parameters of Ram Spermatozoa and their Relationship to Migration Efficiency in Ruminant Cervical Mucus. Reprod. Domest. Anim. 2008, 43, 393–399. [Google Scholar] [CrossRef]
- Giwercman, A.; Lindstedt, L.; Larsson, M.; Bungum, M.; Spano, M.; Levine, R.J.; Rylander, L. Sperm chromatin structure assay as an independent predictor of fertility in vivo: A case-control study. Int. J. Androl. 2010, 33, e221–e227. [Google Scholar] [CrossRef]
- Karoui, S.; Díaz, C.; González-Marín, C.; Amenabar, M.E.; Serrano, M.; Ugarte, E.; Gosálvez, J.; Roy, R.; López-Fernández, C.; Carabaño, M.J. Is sperm DNA fragmentation a good marker for field AI bull fertility? J. Anim. Sci. 2012, 90, 2437–2449. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; del Rocío Fernández-Santos, M.; Domínguez-Rebolledo, A.; Esteso, M.C.; Garde, J.J. DNA Status on Thawed Semen from Fighting Bull: A Comparison Between the SCD and the SCSA Tests. Reprod. Domest. Anim. 2009, 44, 424–431. [Google Scholar] [CrossRef]
- López-Fernández, C.; Fernández, J.L.; Gosálbez, A.; Arroyo, F.; Vázquez, J.M.; Holt, W.V.; Gosálvez, J. Dynamics of sperm DNA fragmentation in domestic animals. Theriogenology 2008, 70, 898–908. [Google Scholar] [CrossRef]
- Olaciregui, M.; Luño, V.; Gonzalez, N.; Blas, I.D.; Gil, L. Freeze-dried dog sperm: Dynamics of DNA integrity. Cryobiology 2015, 71, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Gutiérrez, E.I.; Crespo, F.; Serres-Dalmau, C.; de las Rozas, A.L.G.; Dávila-Rodríguez, M.I.; López-Fernández, C.; Gósalvez, J. Assessment of Sperm DNA Fragmentation in Stallion (Equus caballus) and Donkey (Equus asinus) Using the Sperm Chromatin Dispersion Test. Reprod. Domest. Anim. 2009, 44, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.I.; Lombardo, D.; Arraztoa, C.C.; Giuliano, S.M.; Gambarotta, M.C.; Neild, D.M. Evaluation of DNA fragmentation in llama (Lama glama) sperm using the sperm chromatin dispersion test. Anim. Reprod. Sci. 2012, 131, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bartoov, B.; Berkovitz, A.; Eltes, F. Selection of Spermatozoa with Normal Nuclei to Improve the Pregnancy Rate with Intracytoplasmic Sperm Injection. NEJM 2001, 345, 1067–1068. [Google Scholar] [CrossRef]
- Berkovitz, A.; Eltes, F.; Ellenbogen, A.; Peer, S.; Feldberg, D.; Bartoov, B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum. Reprod. 2006, 21, 1787–1790. [Google Scholar] [CrossRef]
- Axnér, E.; Forsberg, C.L. Sperm Morphology in the Domestic Cat, and its Relation with Fertility: A Retrospective Study. Reprod. Domest. Anim. 2007, 42, 282–291. [Google Scholar] [CrossRef]
- Nagy, S.; Johannisson, A.; Wahlsten, T.; Ijäs, R.; Andersson, M.; Rodriguez-Martinez, H. Sperm chromatin structure and sperm morphology: Their association with fertility in AI-dairy Ayrshire sires. Theriogenology 2013, 79, 1153–1161. [Google Scholar] [CrossRef]
- Gravance, C.G.; Champion, Z.J.; Casey, P.J. Computer-assisted sperm head morphometry analysis (ASMA) of cryopreserved ram spermatozoa. Theriogenology 1998, 49, 1219–1230. [Google Scholar] [CrossRef]
- Maroto-Morales, A.; Ramón, M.; García-Álvarez, O.; Soler, A.J.; Esteso, M.C.; Martínez-Pastor, F.; Pérez-Guzmán, M.D.; Garde, J.J. Characterization of ram (Ovis aries) sperm head morphometry using the Sperm-Class Analyzer. Theriogenology 2010, 73, 437–448. [Google Scholar] [CrossRef]
- Vanderzwalmen, P.; Hiemer, A.; Rubner, P.; Bach, M.; Neyer, A.; Stecher, A.; Uher, P.; Zintz, M.; Lejeune, B.; Vanderzwalmen, S.; et al. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod. Biomed. Online 2008, 17, 617–627. [Google Scholar] [CrossRef]
- Milczewski, V.; Chahad-Ehlers, S.; Spercoski, K.M.; Morais, R.N.; Soccol, V.T. Quantifying the effect of seasonality on testicular function of Suffolk ram in lower latitude. Small Rumin. Res. 2015, 124, 68–75. [Google Scholar] [CrossRef]
- Mroczkowski, S.; Piwczynski, D.; Baranowski, A. Trends in Polish Merino sheep breeding over 1975–1996. Zesz. Naukowe Akad. Tech-Rol. Bydg. Zootech. 2000, 31, 13–18. [Google Scholar]
- Gaglio, G.; Poglayen, G.; Capelli, G.; Gruner, L.; Mara, L.; Giannetto, S.; Scala, A. Influence of gastrointestinal trichostrongylidosis on ram fertility. Pol. J. Vet. Sci. 2010, 13, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, Y.; Al-Mutary, M.G.; Al-Ghadi, M.; Alfuraiji, M.M.; Al-himaidi, A.R.; Ammari, A. Seasonal variations in scrotal circumference and semen characteristics of Naimi and Najdi rams in Saudi Arabia. S. Afr. J. Anim. Sci. 2017, 47, 454. [Google Scholar] [CrossRef]
- Belkadi, S.; Safsaf, B.; Heleili, N.; Tlidjane, M.; Belkacem, L.; Oucheriah, Y. Seasonal influence on sperm parameters, scrotal measurements, and serum testosterone in Ouled Djellal breed rams in Algeria. Vet. World 2017, 10, 1486–1492. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Varsakeli, S.; Alexopoulos, C.; Amarantidis, I. Seasonal variation in semen characteristics of Chios and Friesian rams in Greece. Small Rumin. Res. 2000, 37, 125–130. [Google Scholar] [CrossRef]
- Budai, C.; Oláh, J.; Egerszegi, I.; Jávor, A.; Kovacs, A. Seasonal variations in somereproductive parameters of Dorper Rams in Hungary. Acta Agrar. Debr. 2013, 53, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rabadán, P.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; del Olmo, E.; Pérez-Guzmán, M.D.; Bisbal, A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of Blanca-Celtibérica buck ejaculates. Anim. Reprod. Sci. 2012, 132, 88–95. [Google Scholar] [CrossRef]
- Foxcroft, G.R.; Dyck, M.K.; Ruiz-Sanchez, A.; Novak, S.; Dixon, W.T. Identifying useable semen. Theriogenology 2008, 70, 1324–1336. [Google Scholar] [CrossRef]
- Vicente-Fiel, S.; Palacín, I.; Santolaria, P.; Fantova, E.; Quintín-Casorrán, F.J.; Sevilla-Mur, E.; Yániz, J.L. In vitro assessment of sperm quality from rams of high and low field fertility. Anim. Reprod. Sci. 2014, 146, 15–20. [Google Scholar] [CrossRef]
- Malama, E.; Bollwein, H.; Taitzoglou, I.A.; Theodosiou, T.; Boscos, C.M.; Kiossis, E. Chromatin integrity of ram spermatozoa. Relationships to annual fluctuations of scrotal surface temperature and temperature-humidity index. Theriogenology 2013, 80, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.B.R.; De Andrade, A.F.C.; De Arruda, R.P.; Batissaco, L.; Florez-Rodriguez, S.A.; De Oliveira, B.M.M.; Torres, M.A.; Lançoni, R.; Ravagnani, G.M.; Filho, R.R.P.; et al. Recovery of normal testicular temperature after scrotal heat stress in rams assessed by infrared thermography and its effects on seminal characteristics and testosterone blood serum concentration. Theriogenology 2016, 86, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Menegassi, S.R.O.; Barcellos, J.O.J.; Dias, E.A.; Koetz, C.; Pereira, G.R.; Peripolli, V.; McManus, C.; Canozzi, M.E.A.; Lopes, F.G. Scrotal infrared digital thermography as a predictor of seasonal effects on sperm traits in Braford bulls. Int. J. Biometeorol. 2015, 59, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, P.; Vicente-Fiel, S.; Palacín, I.; Fantova, E.; Blasco, M.E.; Silvestre, M.A.; Yániz, J.L. Predictive capacity of sperm quality parameters and sperm subpopulations on field fertility after artificial insemination in sheep. Anim. Reprod. Sci. 2015, 163, 82–88. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; Karagiannidis, A.; Alexopoulos, C.; Brozos, C.; Saratsis, P.; Kyriakis, S. Clinical and epidemiological findings during ram examination in 47 flocks in southern Greece. Prev. Vet. Med. 2001, 52, 43–52. [Google Scholar] [CrossRef]
- Carvajal-Serna, M.; Miguel-Jimenez, S.; Perez-Pe, R.; Casao, A. Testicular Ultrasound Analysis as a Predictive Tool of Ram Sperm Quality. Biology 2022, 11, 261. [Google Scholar] [CrossRef]
- Jee, W.H.; Choe, B.Y.; Byun, J.Y.; Shinn, K.S.; Hwang, T.K. Resistive index of the intrascrotal artery in scrotal inflammatory disease. Acta Radiol. 1997, 38, 1026–1030. [Google Scholar] [CrossRef]
- Wielgoś, M.; Bablok, L.; Fracki, S.; Marianowski, L. Doppler flowmetry measurements in testicular artery of aging men. Ginekol. Pol. 1998, 69, 537–540. [Google Scholar]
- Pinggera, G.M.; Mitterberger, M.; Bartsch, G.; Strasser, H.; Gradl, J.; Aigner, F.; Pallwein, L.; Frauscher, F. Assessment of the intratesticular resistive index by colour Doppler ultrasonography measurements as a predictor of spermatogenesis. BJU Int. 2008, 101, 722–726. [Google Scholar] [CrossRef]
- Pozor, M.A.; Nolin, M.; Roser, J.; Runyon, S.; Macpherson, M.L.; Kelleman, A. Doppler indices of vascular impedance as indicators of testicular dysfunction in stallions. J. Equine Vet. Sci. 2014, 34, 38–39. [Google Scholar] [CrossRef]
- Gloria, A.; Francesco, L.D.; Marruchella, G.; Robbe, D.; Contri, A. Pulse-wave Doppler pulsatility and resistive indexes of the testicular artery increase in canine testis with abnormal spermatogenesis. Theriogenology 2020, 158, 454–460. [Google Scholar] [CrossRef] [PubMed]

| Breeding Season | p Value | |||
|---|---|---|---|---|
| BBS | BS | ABS | ||
| Ejaculate volume (mL) | 0.48 ± 0.22 a (45.9) | 0.56 ± 0.39 a (68.9) | 0.44 ± 0.27 a (61.5) | 0.630 |
| Sperm concentration (×109/mL) | 1.43 ± 0.54 a (37.9) | 3.21 ± 0.75 b (23.5) | 1.97 ± 0.93 a (47.2) | <0.0001 |
| Total sperm number per ejaculate (×109) | 0.66 ± 0.32 a (47.8) | 1.88 ± 1.42 b (75.7) | 0.84 ± 0.73 a (86.9) | 0.008 |
| Forward motility (%) | 54.1 ± 14.2 a (26.2) | 72.0 ± 17.1 b (23.7) | 67.6 ± 12.9 ab (19.1) | 0.017 |
| Sperm vitality (%) | 66.7 ± 13.6 a (20.5) | 77.4 ± 15.2 a (19.64) | 76.2 ± 21.0 a (27.5) | 0.104 |
| Sperm abnormalities (%) | 18.5 ± 9.3 a (49.9) | 7.3 ± 6.8 b (93.6) | 11.3 ± 6.5 ab (57.7) | 0.005 |
| Breeding Season | p Value | ||||
|---|---|---|---|---|---|
| BBS | BS | ABS | |||
| VCL (µm/s) | SD | 22.9 ± 9.32 a (40.6) | 26.6 ± 15.5 a (58.0) | 40.1 ± 14.6 b (36.5) | 0.015 |
| MD | 44.1 ± 20.4 a (46.2) | 53.5 ± 15.9 a (29.7) | 63.3 ± 4.5 b (7.0) | 0.003 | |
| RD | 120.3 ± 41.3 a (34.3) | 110.1 ± 15.7 a (14.2) | 114.8 ± 12.9 a (31.5) | 0.664 | |
| VSL (µm/s) | SD | 9.2 ± 4.2 a (46.1) | 10.1 ± 6.7 a (66.5) | 14.0 ± 5.9 a (42.7) | 0.201 |
| MD | 30.3 ± 29.8 a (98.4) | 26.5 ± 13.9 a (52.5) | 25.9 ± 9.2 a (35.5) | 0.831 | |
| RD | 81.1 ± 46.5 a (57.4) | 72.9 ± 24.9 a (34.2) | 58.9 ± 13.4 a (35.5) | 0.355 | |
| VAP (µm/s) | SD | 14.2 ± 5.9 a (42.1) | 18.8 ± 13.6 a (72.4) | 24.1 ± 9.3 a (38.5) | 0.098 |
| MD | 32.2 ± 15.1 a (46.9) | 36.4 ± 15.0 a (41.4) | 39.8 ± 7.9 a (19.9) | 0.489 | |
| RD | 94.0 ± 48.6 a (51.7) | 87.0 ± 20.1 a (23.1) | 80.5 ± 15.9 a (19.7) | 0.773 | |
| LIN (µm/s) | SD | 38.0 ± 19.6 a (51.5) | 37.1 ± 16.3 a (44.0) | 34.9 ± 8.2 a (23.5) | 0.837 |
| MD | 46.2 ± 23.1 a (49.9) | 46.5 ± 20.3 a (43.6) | 41.0 ± 14.6 a (35.7) | 0.505 | |
| RD | 64.2 ± 22.8 a (35.5) | 65.3 ± 18.4 a (28.1) | 51.4 ± 18.5 a (36.1) | 0.221 | |
| STR (µm/s) | SD | 59.4 ± 24.1 a (40.5) | 63.1 ± 13.7 a (21.7) | 57.8 ± 7.7 a (13.3) | 0.630 |
| MD | 64.1 ± 28.2 a (44.0) | 68.0 ± 18.6 a (27.3) | 63.8 ± 10.4 a (16.3) | 0.382 | |
| RD | 79.6 ± 16.8 a (21.2) | 81.7 ± 13.5 a (16.7) | 71.3 ± 13.4 a (18.9) | 0.152 | |
| WOB (µm/s) | SD | 57.1 ± 20.9 a (36.6) | 57.3 ± 15.3 a (26.7) | 59.9 ± 7.5 a (12.6) | 0.692 |
| MD | 68.5 ± 24.8 a (36.3) | 65.1 ± 19.9 a (26.1) | 62.8 ± 11.7 a (18.6) | 0.126 | |
| RD | 77.9 ± 17.6 a (22.6) | 78.2 ± 12.0 a (15.3) | 70.1 ± 12.8 a (18.2) | 0.240 | |
| Breeding Season | p Value | |||
|---|---|---|---|---|
| BBS | BS | ABS | ||
| DFI (%) | 3.26 ± 0.64 a (19.7) | 1.62 ± 0.53 b (32.6) | 2.13 ± 1.06 b (49.8) | <0.0001 |
| Grade I (%) | 91.6 ± 2.48 a (2.7) | 96.9 ± 2.36 b (2.4) | 96.2 ± 2.25 b (2.3) | <0.0001 |
| Grade II (%) | 4.21 ± 0.96 a (22.8) | 2.10 ± 1.07 b (51.3) | 2.81 ± 1.53 b (54.5) | 0.0006 |
| Grade III (%) | 2.51 ± 1.47 a (58.7) | 0.82 ± 0.77 b (92.1) | 0.90 ± 0.88 b (97.6) | 0.004 |
| Grade IV (%) | 1.66 ± 0.94 a (56.5) | 0.19 ± 0.10 b (98.8) | 0.11 ± 0.10 b (98.8) | <0.0001 |
| Breeding Season | p Value | |||
|---|---|---|---|---|
| BBS | BS | ABS | ||
| PSV (cm/s) | 37.9 ± 11.0 a (28.8) | 27.3 ± 6.51 b (23.8) | 31.0 ± 6.65 b (21.5) | <0.0001 |
| EDV (cm/s) | 7.16 ± 2.95 a (41.2) | 9.90 ± 2.88 b (29.1) | 7.52 ± 2.22 a (28.6) | <0.0001 |
| RI | 0.81 ± 0.06 a (7.5) | 0.61 ± 0.09 b (14.6) | 0.75 ± 0.05 c (6.3) | <0.0001 |
| PI | 1.82 ± 0.35 a (19.5) | 1.03 ± 0.26 b (25.3) | 1.58 ± 0.25 c (15.8) | <0.0001 |
| Blood Flow | Semen Parameters | Breeding Season | ||
|---|---|---|---|---|
| BBS | BS | ABS | ||
| PSV | Ejaculate volume | 0.61; p = 0.032 | 0.76; p = 0.009 | |
| PSV | Sperm vitality | −0.64; p = 0.042 | −0.70; p = 0.019 | |
| PSV | MSOME Grade IV | −0.40; p = 0.024 | ||
| EDV | MSOME Grade IV | −0.38; p = 0.038 | ||
| EDV | Total sperm number per ejaculate | 0.76; p < 0.0001 | 0.54; p = 0.046 | 0.88; p = 0.0007 |
| EDV | Sperm abnormalities | 0.73; p = 0.030 | 0.44; p = 0.0003 | 0.48; p = 0.020 |
| RI | Sperm concentration | −0.61; p = 0.002 | −0.68; p = 0.024 | |
| RI | CASA VCL SD | −0.65; p = 0.023 | −0.63; p = 0.039 | |
| RI | CASA LIN RD | −0.69; p = 0.024 | ||
| RI | CASA STR RD | −0.67; p = 0.029 | ||
| RI | CASA WOB RD | −0.66; p = 0.030 | ||
| RI | MSOME Grade IV | 0.61; p = 0.034 | 0.60; p = 0.036 | |
| PI | Sperm concentration | −0.68; p = 0.001 | −0.66; p = 0.027 | |
| PI | Forward motility | −0.74; p < 0.0001 | −0.70; p = 0.016 | |
| PI | MSOME Grade IV | 0.52; p = 0.024 | 0.46; p = 0.018 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, N.; Faundez, R.; Borzyszkowski, K.; Dąbrowski, S.; Jasiński, T.; Domino, M. The Relationship between the Testicular Blood Flow and the Semen Parameters of Rams during the Selected Periods of the Breeding and Non-Breeding Seasons. Animals 2022, 12, 760. https://doi.org/10.3390/ani12060760
Kozłowska N, Faundez R, Borzyszkowski K, Dąbrowski S, Jasiński T, Domino M. The Relationship between the Testicular Blood Flow and the Semen Parameters of Rams during the Selected Periods of the Breeding and Non-Breeding Seasons. Animals. 2022; 12(6):760. https://doi.org/10.3390/ani12060760
Chicago/Turabian StyleKozłowska, Natalia, Ricardo Faundez, Kamil Borzyszkowski, Sebastian Dąbrowski, Tomasz Jasiński, and Małgorzata Domino. 2022. "The Relationship between the Testicular Blood Flow and the Semen Parameters of Rams during the Selected Periods of the Breeding and Non-Breeding Seasons" Animals 12, no. 6: 760. https://doi.org/10.3390/ani12060760
APA StyleKozłowska, N., Faundez, R., Borzyszkowski, K., Dąbrowski, S., Jasiński, T., & Domino, M. (2022). The Relationship between the Testicular Blood Flow and the Semen Parameters of Rams during the Selected Periods of the Breeding and Non-Breeding Seasons. Animals, 12(6), 760. https://doi.org/10.3390/ani12060760






