Simple Summary
This paper describes an unusually large and distinctive deep-sea red medusa with coiled tentacles in the family Atollidae. This family is monogeneric with ten presently accepted species in the genus Atolla. The new medusa is molecularly and morphologically distinct from the five species that we have been able to sample and morphologically distinct from all ten previously described species. We have also observed and collected samples from another two potentially new species. The ocean provides over 98% of the available living space on our planet and we still do not know who is living there or how they interact with one another. This paper adds to the increasing number of new deep-sea species being described as we increase our exploration, and as advances in undersea technology and genetic sequencing become more available.
Abstract
We have observed and collected unusual specimens of what we recognize as undescribed types of the genus Atolla over the past 15 years. Of these, there appear to be three potentially different types. One of these has now been genetically sequenced and compared both morphologically and molecularly with five other Atolla species that have been found in the eastern Pacific. This new variant is so morphologically distinct from other previously described Atolla species that we believe it can be described as a new species, Atolla reynoldsi sp. nov. This species along with two additional types may comprise a new genus. It is also clear that a more accurate and diagnostic morphological key for the genus Atolla needs to be developed. This paper will also provide some potential starting points for a new key to the genus.
1. Introduction
The subclass Coronamedusae Calder, 2009 [1] is within the class Scyphozoa Götte, 1887 [2] and contains one order (order Coronatae Vanhöffen, 1892 [3]). There are several families in the Coronatae including the family Atollidae Hickson, 1906 [4] which is monogeneric (Atolla Haeckel, 1880 [5]) with ten potential species [6], some of which are tentative. Atolla specimens have been found in every ocean basin in the world [7], but their contributions to the trophic ecology of pelagic ecosystems have been largely overlooked [8].
The well-documented species of Atolla are A. vanhoeffeni, A. chuni, and A. gigantea. Each of these have morphological characteristics that make them relatively easy to identify and are unique. Many of the other species can be separated into different taxa using the current taxonomic keys [8,9,10,11] but this paper shows that some of these morphological characteristics are not useful.
Atolla verrillii and A. valdiviae are both considered to be doubtful species [6] (perhaps both are A. wyvillei). Atolla tenella is described as having very distinctive pigmentation on the margin of the exumbrella but other than the illustration in the original description [12], we have not seen an image or mention of this pigmentation despite specific identifications in the literature [13], and Russell [11] considered the validity of the species as uncertain.
Atolla chuni was first described from two specimens collected south of the Cape of Good Hope [3] and is distinguished from other Atolla species by distinct papillae (‘pearls’) on the lappets. Larson reviewed additional descriptions of A. chuni, which he regarded as endemic to the Southern Ocean, and added observations from 1168 specimens [8].
There is still little known about the identity, behavior, or distribution of Atolla, one of the most common coronate scyphozoans in the deep ocean. In situ observations from crewed submersibles and remotely operated vehicles have revealed a number of observations on Atolla’s swimming and behavior. It is rare that a net tow or a trip to the deep does not reveal one or more Atolla. A four-year net study to examine one species (A. wyvillei) in the Bay of Biscay [14] found two distinct species (A. wyvillei and A. parva) with no apparent seasonal or depth differences. Hunt and Lindsay [15] discussed the potential for the hypertrophied tentacle that Atolla often exhibits for prey capture (also discussed in an unpublished report by Walker [16]). Direct observations using submersibles revealed that Atolla can capture prey including Nanomia (a phyosnect siphonophore) with this tentacle [17]. Additionally, Moore et al. [18] observed the large red caridean shrimp Notostomus robustus feeding on A. wyvillei—this feeding continued even after collection.
Thirty years of remotely operated vehicle observations with MBARI have revealed numerous observations of Atolla with trailing tentacles—so, when we find jellies that look like Atolla but are lacking the long trailing tentacle, it makes us stop and take a longer look. Over the past 15 years using a variety of ROVs, we have collected numerous specimens of three types of Atolla-like jellies that lack trailing tentacles. We have also collected other Atolla species and have found that existing species keys are often incomplete making it difficult to identify specimens to the species level [8,9,10,11]. Despite this, the new species described here is very distinctive and easy to differentiate from all other Atolla we have collected.
2. Materials and Methods
Specimens used in this study were collected using a diversity of means. Like many of the earlier scientific studies, some were collected using midwater trawls from the RV Western Flyer (Monterey Bay, Southern California, and the Gulf of California) as well as the RV Kilo Moana and the RV Ka’imikai-O-Kanaloa (In the Hawaiian Islands). The majority of the specimens were collected with the remotely operated vehicles ROV Tiburon, ROV Ventana, and ROV Doc Ricketts using the RV Point Lobos, RV Rachel Carson, and the RV Western Flyer in the Gulf of California, Southern California Bight, and Monterey Bay. Additional materials (Atolla tenella) were provided by Kevin Raskoff from the Arctic Ocean [13].
2.1. ROV Collections
We used three remotely operated vehicles (ROV Ventana, ROV Tiburon, and ROV Doc Ricketts) owned and operated by the Monterey Bay Aquarium Research Institute (MBARI) [19]. High-Definition video cameras were mounted on these vehicles and the video signal was conveyed to the surface support vessel specific for each ROV (ROV Ventana—R/V Point Lobos and R/V Rachel Carson; ROV Tiburon and ROV Doc Ricketts—R/V Western Flyer) through the ROV’s tether. At the surface, the video signal was viewed on a high-resolution monitor and was recorded on high-definition tape. More recent observations are with a 4K camera and digital recordings. Comments and descriptions of what is on the recordings are recorded on the audio track of the recording during the dive and can be accessed as needed by MBARI staff or collaborators. Additional environmental data (depth, location, temperature, dissolved oxygen, and salinity) during each dive are collected by instruments on the vehicle and the surface ship and integrated into an accessible and comprehensive relational database (http://dsg.mbari.org/dsg/home accessed on 7 March 2022) that is available to the public.
Specimens for this study were collected in 6.5 L ‘detritus’ samplers, designed for the gentle capture of delicate material in midwater [20], or in a ‘suction’ sampler consisting of a transparent funnel and two meters of flexible tubing leading back to 12 separate 6 L collection cylinders within the ROV tool sled. For the detritus samplers, the ROV pilot positioned the vehicle so that the open cylinder of the sampler enclosed the medusa, then the doors at either end were gently closed by hydraulic rams. For the suction sampler, the funnel, attached to a clear plexiglass tube and flexible tubing, could be extended in front of the ROV [20]. When a medusa was near the wide opening of the funnel, the suction pump was turned on and the animal was gently collected and deposited into a sample container, which was then replaced by an empty container.
Specimens were removed from the collection containers and photographed, if possible, prior to freezing portions or whole animals in liquid nitrogen for later molecular analysis. The remainder of each specimen was then preserved for morphological analysis. One specimen of the new species described in this paper was frozen, dried, and used for CHN elemental analysis as part of a different research project.
2.2. DNA Extraction and Amplification
Genomic DNA was isolated from frozen tissue samples using the Monarch Genomic DNA Purification Kit (New England Biolabs, Ipswich, MA, USA) or the DNeasy DNA Blood and Tissue kit (Qiagen, Germantown, MD, USA).
We amplified 18S rDNA gene fragments (1793 bp) with the MitchA and MitchB primers [21]. We had limited success amplifying a few species with universal COI Folmer primers [22]; therefore, we designed new primers based on successful amplifications and on published sequences. Primer sequences were anchored in more conserved areas and analyzed with PrimerQuest program, IDT, Coralville, Iowa, USA (www.idtdna.com/SciTool last accessed 12 December 2018). The COI fragments (697 bp) of the newly-described Atolla reynoldsi sp. nov. were amplified using forward primer Atollawhite_F2 (CGGGTCCAGTAATGGGAGAAG) and reverse primer AtollaGB_R2m1(TGAGCTCATACAACAAAACCAAG), and Atolla species B was amplified using forward primer Atollawhite_F2 and reverse primer AtollaGB_R3(CATATGATGRGCYCATACWAYAAAYCCT). All other Atolla and coronate species were amplified with the primers AtollaGB_F2 (CTGGRCCTTTAATGGGTGATG) and AtollaGB_R2(TGAGCTCATACAACAAARCCT). All fragments were amplified with Phusion High-Fidelity PCR Master Mix with HF buffer (New England BioLabs, Ipswich, MA, USA) in a Veriti PCR thermal cycler (Life Technologies, Carlsbad, CA, USA). PCR conditions were: 98 °C for 30 s; 35 cycles of 98 °C for 30 s, 48 °C for 10 s, and 72 °C for 10 s; and a final extension of 72 °C for 5 min. Gene fragments were sequenced bi-directionally with PCR primers and the BigDyeTerminator v3.1 (Life Technologies, Carlsbad, CA, USA) sequencing kit and analyzed on a 3500xL Genetic Analyzer (Life Technologies, Carlsbad, CA, USA).
2.3. DNA Analyses
Bi-directional sequences were assembled and edited sequence fragments with Geneious Prime (v.2022.0.1, https://www.geneious.com last updated 13 January 2022). We aligned data with MUSCLE and estimated the best substitution model with AIC [23] with ModelTest within Geneious Prime. We included all available data from GenBank for closely related species, including Periphylla periphylla, Paraphyllina sp., Periphyllopsis sp., Nausithoe sp., Atorella sp., species of Atolla, and Linuche as an outgroup (accession numbers included in the phylogenies). Mitochondrial data were translated with the invertebrate mitochondrial genetic code to detect the presence of stop codons or pseudogenes. We estimated Bayesian phylogenies for 18S rDNA and COI mtDNA separately with MrBayes (v.3.2.7a, [24,25]). Bayesian analyses included multiple runs that ranged from 5–108 generations where we sampled and printed every 1000 generations with six chains after we discarded the first 10% of data. We also estimated likelihood trees with the program IQtree 2 [26,27] with 1000 bootstrap replicates. We trimmed alignments to exclude missing data for likelihood analyses. Phylogenies were visualized with FigTree (v.1.4.4, http://tree.bio.ed.ac.uk/software/figtree/ accessed on 13 January 2022). Sequences were analyzed using the GTR + I + Γ selection model.
3. Results
3.1. Collection Information
3.1.1. Atolla Species Sequenced
We sequenced 34 Atolla specimens that were collected between 2005 and 2021 (Table 1). Species identification was based on some existing keys [8,9,10,11] but there is not a key that includes all ten potentially valid species [6]. During the last few years, it became apparent to us that identifying Atolla to the species level was not straight forward and that some of the traits used in the keys were suspect. We also observed three types of Atolla that were clearly different from the described species (Table 2, Table 3 and Table 4 and Figure 1, Figure 2 and Figure 3). All three types lacked the characteristic Atolla hyperextended trailing tentacle and presented with a Greek-cross gut morphology that was different from that seen in the other described species (see Section 3.2.3).

Table 1.
Physical measurements and accession numbers for the collected Atolla specimens used for sequencing COI and 18S rDNA.

Table 2.
Physical measurements and water parameters for the collected specimens of Atolla reynoldsi sp. nov.

Table 3.
Physical measurements and water parameters for the collected specimens of Atolla species A.

Table 4.
Physical measurements and water parameters for the collected specimens of Atolla species B.

Figure 1.
Images of Atolla reynoldsi sp. nov. from T960 on 4 April 2006. (a) Laboratory photo of Atolla reynoldsi sp. nov. (photo by Rob Sherlock). Diameter from margin to margin (excluding lappets) is 8.5 cm and tentacles were coiled in situ. (b) In situ image of Atolla reynoldsi sp. nov. The spikes and spike ridges on the lappets and the coiled tentacles are visible.

Figure 2.
(a) Laboratory photo of Atolla species A taken in the lab (photo by SHDH) of the specimen collected on 30 October 2021 (D1399). Diameter from margin to margin (excluding lappets) is 8.5 cm. (b) In situ image of Atolla species A (D1402) photographed on 14 November 2021 at a depth of 1913 m, 5.4 cm in diameter.

Figure 3.
(a) Laboratory photo of Atolla species B (photo by Rob Sherlock) of the specimen collected on 14 April 2007 (T1088). (b) In situ image of Atolla species B (T1088) photographed on 14 April 2007 at a depth of 2570 m.
3.1.2. Atolla reynoldsi sp. nov.
3.1.3. Atolla Species A
3.1.4. Atolla Species B
3.2. Morphological Distinctions
3.2.1. Pigmentation
Currently, only two of the described Atolla species have pigment spots as one of their diagnostic characters, whereas the new species has none. Atolla vanhoeffeni has eight very distinct pigment spots that were first identified by Vanhoffen [28] and used by Russell [29] to erect the new species A. vanhöffeni. These pigmentation spots are on the subumbrellar walls of the stomach where the gastric cavity begins to narrow (Figure 4a); they are not pores. Hartlaub [12] described another new species (A. tenella) that has two pigment spots on the margin and centered on the rhopaliar pedalia (Figure 4b). While the pigmentation for A. vanhoeffeni can be easily found on specimens, we have not observed or seen any photographs of the pigmentation for A. tenella. The specimens used for the original species description were small (5–10 mm), and since our specimens came from the same expedition as Raskoff et al. [13] and these were identified as A. tenella, we have kept that identification (note, a preserved sample from that expedition did not have pigment spots) and have tentatively identified many of our Hawaiian samples as A. aff. tenella? based on a close molecular similarity with the Arctic Ocean A. tenella (Appendix A Table A3).

Figure 4.
Line drawing showing pigmentation location and patterns for pigmentation observed on the (a) oral side of Atolla vanhoeffeni (modified from Russell 1957 [28]) and the (b) aboral side of A. tenella (modified from Hartlaub 1909 [12]). cm coronal muscle; go gonad; l lappet; rh rhopalium; te tentacle; rs radial septa; ps pigment spot; rp rhopaliar pedalia.
3.2.2. Papillae
Atolla chuni is the only described species of Atolla with protrusions labeled as warts on the exumbrellar surface [8]. A. chuni also has paired warts (sw) on top of the radial septa (Figure 5a) and another 7–9 warts (with one in the center and the others in two lateral rows) on the rhopaliar pedalia (Figure 5a). We are using the term papillae rather than warts for A. reynoldsi sp. nov. for the solitary protrusions as papillae is more commonly used in the literature for cnidarians. We are also using the term spikes to reflect the protrusions on ridges (for Atolla reynoldsii sp. nov.) as they have a variety of morphologies (Figure 5b–d) and are not simple rounded warts as in A. chuni. We have not included A. chuni in our analysis as we have never found a specimen that meets this description. Atolla reynoldsi sp. nov. has distinct ridges (~7) on each side of the rhopaliar pedalia that have spikes that are rounded close to the margin but pointed closer to the end of the pedalia. There are four solitary papillae closest to the body disc and no papillae over the radial septa. Atolla sp. A lacks papillae or spikes on ridges while Atolla sp. B has papillae (small and lined up in two rows like those of A. chuni) on the rhopalia but not over the septa. There are no spiked ridges in Atolla sp. B.
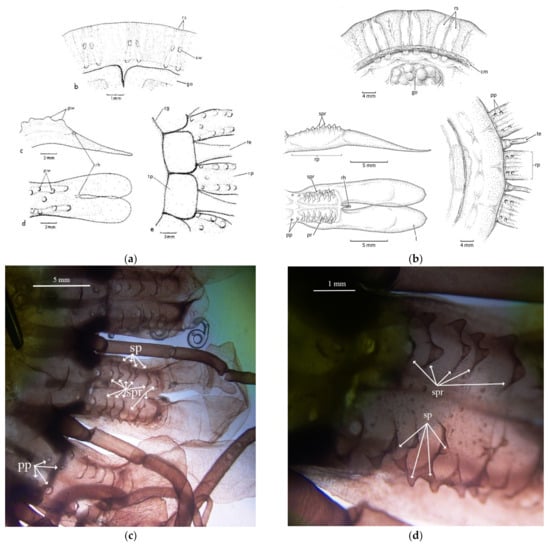
Figure 5.
(a) Line drawing showing the pedaliar wart (pw) and septal wart (sw) pattern for Atolla chuni (from Larson [3] and used with permission under license number 5203151138336) and (b) the spikes on the rhopaliar pedalia for Atolla reynoldsi sp. nov. (drawn from photographs). Dissection microscope images (c) 7.5× and (d) 30× of the papillae (pp), spikes (sp), and the spike ridges (spr) of Atolla reynoldsi sp. nov (D1369D1). There were no septal warts observed. l lappet; rp rhopaliar pedalia; rh rhopalium; cm coronal muscle; te tentacle; go gonad; rs radial septa; pw pedaliar wart; sw septal wart; pp papillae; sp spike; spr spiked ridge.
3.2.3. Stomach Morphology
There have been two basic stomach patterns (Figure 4b and Figure 6a) described for species within the genus Atolla [28]. Atolla vanhoeffeni presents a simple cross-shaped pattern (Figure 6a) while the basal stomach pattern for the other Atolla species is more similar to a four-leaf clover (Figure 6b). All three new Atolla types observed in Monterey are not only larger than the other described species (with the exception of Atolla gigantea), but also exhibit a different stomach pattern. We have termed this new morphology a Greek-cross shape (similar to Maltese cross shape with oval arms) and it presents as a thin base that then expands into a vase shape and ends with a shallow indentation near the edge of Atolla reynoldsi sp. nov. (Figure 6c) and Atolla sp. B, or a much more globular shape with very deep indentations near the margin edge for Atolla sp. A. (Figure 6d). Both Atolla reynoldsi sp. nov. and Atolla species B have this Greek-cross stomach pattern with smooth edges (Figure 6c) while Atolla species A has the Greek-cross stomach pattern with evaginations along the stomach and invaginations close to the center of the medusa and evaginations at the margin near the coronal muscle (Figure 6d).

Figure 6.
Line drawings showing the stomach patterns for (a) Atolla vanhoeffeni, (b) Atolla wyvillei, (c) Atolla reynoldsi sp. nov.—(D1369) in situ, 8.5 cm in diameter showing four narrow bases at the center of the medusa that expand into a vase-like shape before ending with a shallow indentation. (d) Atolla sp. A (D1399) in lab, 8.5 cm in diameter showing a much more rounded expansion with a much deeper indentation and the edges of the rounded expansion show invaginations near the center of the stomach and evaginations around the margin near the coronal muscle (cm). go gonad; l lappet; rh rhopalium; te tentacle; rs radial septa; ps pigment spot; rp rhopaliar pedalia.
3.2.4. Radial Septa
Radial septa are easily observed both in situ and in preserved specimens and have been used as a diagnostic character in keys for the genus Atolla. Our observations have revealed that preservation has an impact on the morphology of the septa. Specifically, for A. gigantea, the septa are clearly divergent when examining in situ frame grabs (Figure 7a) but appear to be straight when looking at preserved specimens (Figure 7b). This could be simply due to contraction of the bell, but it still makes the use of this morphology suspect for taxonomy as the amount of contraction would likely vary with fixative type and concentration.

Figure 7.
(a) In situ image of Atolla gigantea (D915) showing what appears to be divergent septa and (b) a photograph of the same specimen preserved in 5% formalin showing what appear to be straight septa. This specimen was 7.6 cm in diameter.
3.2.5. Tentacles
The appearance of a hypertrophied tentacle is generally used as a diagnostic character for the genus Atolla. The number of tentacles has been used as another diagnostic but there appears to be a great deal of variability in the number for each species. At this point, there does not seem to be enough confidence to use tentacle number as a diagnostic nor (if these three new species are to be kept in the genus Atolla) can the hypertrophied tentacle be used. Nine of the ten specimens of Atolla reynoldsi sp. nov. observed had coiled tentacles; the tenth was able to coil some of the tentacles but then released the coil to display tentacles more similar to other Atolla species.
3.3. Molecular Results
We sequenced three individuals of A. reynoldsi sp. nov. for the COI mtDNA and 18S rDNA fragments in addition to 30 new sequences of close relatives for statistical analyses. (Table 1, GenBank accession #’s OM214492-OM214523 and OM260056-ON260088). We sequenced nine other coronate species for 18s rDNA and one for CO1 mtDNA (Table 5, GenBank accession numbers OM201135-OM201143 and OM237455).

Table 5.
Coronate genera sequenced for rooting in the 18S rDNA molecular tree.
Sequencing efforts for Atolla sp. A are ongoing: we have gotten some preliminary 18S rDNA sequences but they are not included in Figure 8 as they are only ~300 bp long and identical to the sequence obtained for Atolla sp. B. The alignments of 18S rDNA were conserved among Atolla, Periphylla, Periphylopsis, Linuche, and Nausithoe species and resulted in very few mutations. As a result, phylogenies were mostly unresolved, especially within genera. However, Atolla reynoldsi sp. nov. was distinct from all other Atolla species (Figure 8).

Figure 8.
Bayesian and Likelihood estimates of phylogenetic trees for coronate jellies with an 1826 base pairs (bp) alignment of the 18S rDNA fragment with a GTR + I + Γ selection model and an 892 bp alignment of the COI mtDNA fragment with a GTR + Ι + Γ selection model. Posterior probabilities and bootstrap results displayed as triangles (see legend). Support of nodes with below threshold value not shown.
The COI mtDNA alignments were more informative and provided delineation among and even within species from distinct localities with full Bayesian and likelihood support (Figure 8). Atolla reynoldsi sp. nov. differed from its closest relative, another undescribed type of Atolla sp. ‘B’ by about ~22% for the GTR + I + Γ selection model. This differentiation was far greater than among many other described species of Atolla (Figure 8). We do not yet have COI mt DNA for Atolla sp. A.
4. Discussion
4.1. Systematics
Class Scyphozoa Götte, 1887 [1]
Subclass Coronamedusae Calder, 2009 [2]
Order Coronatae Vanhöffen, 1892 [3]
Family Atollidae Hickson, 1906 [4]
Genus Atolla Haeckel, 1880 [5]
Atolla reynoldsi sp. nov.
Diagnosis: Atolla reynoldsi sp. nov. can have from 26–39 tentacles and rhopalia. The overall shape is flattened although the center zone is a rounded dome, albeit not very tall (Figure 1). The tentacles in situ are usually coiled and a hypertrophied tentacle has not been observed. There are ~nine lateral ridges along the pedalia that have some spikes of various heights (Figure 5b–d). The gut has a distinctive Greek-cross morphology (Figure 6c). Diagnostic characters separating this new species from extant Atolla species include the spiked ridges and papillae on the exumbrellar surface of the rhopaliar pedalia, the ability to coil the tentacles, the Greek-cross gut morphology, and the lack of a hyptertrophied tentacle. The gonads are oval when immature but become large and horseshoe-shaped when mature. The radial septa are straight or slightly divergent and extend beyond the coronal muscle.
Type material: The type specimen was collected on 30 June 2021 at 3189 m depth, at 35°29′58.0776″ N and 123°59′55.536″ W in Monterey Bay, California. The holotype specimen and three paratype specimens have been deposited at the California Academy of Sciences (Holotype: CASIZ no. 233651; Paratypes: CASIZ 233650, CASIZ 233652, and CASIZ 233653). Two additional paratypes are housed at the Monterey Bay Aquarium Research Institute (MBARI). A total of ten specimens have been collected between April 2006 and June 2021 (Table 1) in Monterey Bay (eastern North Pacific Ocean) at depths between 1013 and 3189 m.
Etymology: Named after the first volunteer at the Monterey Bay Aquarium (Jeff Reynolds) who guarded a beached whale on Del Monte Beach overnight so that the Aquarium could retrieve it and prepare it for eventual overhead display.
Systematic remarks: The order Coronatae is identified by the separation of the exumbrella into two concentric zones by a circular coronal groove. The central zone is a circular disc or dome while the marginal zone is divided by radiating grooves into thickened pedalia, with peripheral lappets. The presence of more than eight rhopalia place it into the family Atollidae, which is currently monogeneric.
The previously described number of rhopalia in the genus Atolla is 16–32. However, Atolla reynoldsi sp. nov. has up to 39 rhopalia and the Atolla sp. A and Atolla sp. B have up to 64 rhopalia. While it is possible that these types with 32 or more rhopalia might be a new genus, we are not comfortable at this time in making this recommendation as we have not examined all 10 putative species or completed the molecular analysis for Atolla sp. A and Atolla sp. B. Therefore, we recommend that the new diagnosis for the family Atollidae be modified to include up to 64 tentacles and rhopalia. We are in the process of writing up new species descriptions for Atolla sp. A and Atolla sp. B as soon as we complete our molecular analysis of these two types.
4.2. Molecular Analysis
The 18S rDNA fragment was highly conserved and resulting phylogenies were paraphyletic among Atolla, Periphylla periphylla, Paraphyllina, and Periphyllopsis species. Despite unresolved polytomies, the 18S fragment clearly delineated between Atolla reynoldsi sp. nov. and Atolla sp. B, while other species of Atolla had identical residues.
The COI mtDNA locus provided better resolution and stronger support for the delineation of species and for the inclusion of A. reynoldsi sp. nov. into the Atolla genus. Atolla reynoldsi sp. nov. differed from its closest relatives, two undescribed species of Atolla (sp. A and B) by about ~22% for the GTR + I + I selection model. This differentiation was far greater than many other described species of Atolla but they were still closer to Atolla than other genera in the order (Figure 8). Atolla reynoldsi sp. nov. and Atolla spps. A and B were more distantly related to other Atolla species, although there was full likelihood and Bayesian support for their inclusion into the Atolla genus.
The remainder of Atolla species were more closely related and their interrelationships were less clearly resolved. Atolla tenella from the Arctic region (as identified by Raskoff et al. [13] and what we identified as A. aff. tenella from Hawaii (based on having 30 tentacles) were distinct from each other, and neither had the pigment spots that were indicative of the species in the original description [12]. Published COI sequences for A. wyvillei from the North Atlantic also differed from the COI fragment of A. aff. wyvillei from the Gulf of California and Southern California. Atolla gigantea, A. vanhoeffeni, and A. parva were more easily identified morphologically and sequence data were congruent with morphology.
5. Conclusions
Our investigations reveal that there are types of Atolla-like coronates that do not fall within the current taxonomic descriptions of the family Atollidae or the genus Atolla. Until more information can be gathered, we are proposing that Atolla reynoldsi sp. nov. remain within Atolla (along with the other two potential new types A and B) but that more work needs to be completed to clarify their placement within the coronates. We do plan on continuing this work and describing these two new types.
Despite the lack of an adequate key, it is clear that that Atolla reynoldsi sp. nov. is molecularly distinct from the Atolla species that we have been able to collect and that it is morphologically distinct from all ten described Atolla species (although sharing the presence of papillae with A. chuni).
The two additional types (Atolla species A and Atolla species B) may likewise be new species but we do not have enough samples at this time to make that claim. All three types (Atolla reynoldsi sp. nov., Atolla species A, and Atolla species B) may need to be placed into a new genus due to their distinct stomach morphology and the lack of a trailing tentacle, but until further work is completed, we recommend that they remain within the genus Atolla and that the family description (Atollidae) be modified to include 16–62 rhopalia rather than 16–32 rhopalia.
Current keys for Atolla species need to be revised as there are no keys that include all ten species, the number of tentacles is more variable than original authors had noted, and the use of radial septa in the keys is problematic, as determining if they are divergent or straight is somewhat subjective and can be changed by preservation. We recommend that additional examination of all ten described species be completed, ideally with specimens from the type localities, and that a more accurate key be developed for the described species. A table listing diagnostic traits for species included in this analysis is provided as an appendix (Table 2).
Erection of a better dichotomous key for the genus will require better identification of the putative existing species so we suggest that specimens from the original locations be obtained and photographed/sequenced in order to create a more accurate key. It is possible that some of the described species are not valid species and will need to be placed into an existing species. Atolla vanhoeffeni can be clearly distinguished morphologically from all other extant species (based on the pigment spots) and it also groups as a separate species molecularly. While A. tenella may also have pigmentation spots, we have been unable to find any photographic evidence and the validity of this species should be examined as it might just be A. wyvillei [11]. The use of radial septa orientation (divergent or straight) is problematic as fixation causes this to change. Atolla wyvillei is supposed to have septa that pass the coronal muscle but we have found that other specimens that classify as A. reynoldsi sp. nov., A. parva, and A gigantea also have septa that extend beyond the coronal muscle. Atolla chuni is known to have papillae or warts and these have been well documented by Larson and are quite different from those of A. reynoldsi sp. nov., but we were not able to find A. chuni specimens to sequence.
Supplementary Materials
The following videos are available online at https://www.mdpi.com/article/10.3390/ani12060742/s1, Video S1: Atolla reynoldsi sp. nov. D0087, 20 October 2009. Video S2: Atolla reynoldsi sp. nov. D0546, 9 November 2013. Video S3: Atolla reynoldsi sp. nov. T964, 7 April 2006. Video S4: Atolla gigantea D0315, 11/5/2011. Video S5: Atolla species A D1399, 30 October 2021. Video S6: Atolla species A D1402 14 November 2021. Video S7: Atolla species B D991 6 December 2017. Video S8: Atolla species B D613 20 May 2014.
Author Contributions
G.I.M. provided the initial impetus for this manuscript with substantial input from B.H.R. and S.H.D.H. L.M.C. provided the molecular sequencing of the specimens and L.M.C., S.B.J. and S.H.D.H. provided analysis of the resulting molecular trees. S.B.J. created Figure 8 and Appendix A Table A3; S.H.D.H. created Appendix A Table A2. All five authors contributed to the final manuscript creation, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this work was provided by the David and Lucile Packard Foundation through a grant to MBARI and by a grant from NSF Division of Environmental Biology (DEB-1542679) to SHD Haddock for the work in Hawaii. Specimens of Atolla tenella were provided by Kevin Raskoff with funding support to Russell Hopcroft under NOAA’s Office of Exploration grant #NA5OAR4601079 and additional support from the Census of Marine Life.
Institutional Review Board Statement
The work did not require Institutional Review Board approval as the study focused on the description of a new invertebrate species and used other related species that are not identified as endangered or threatened. No vertebrates or cephalopods were involved, so those relevant animal care procedures were not invoked.
Data Availability Statement
COI and 18S sequence fragments have been deposited in GenBank (2022) with accession numbers OM260056-OM260088, OM214492-OM214523, OM201135-OM201143, OM237455, and OM202513.
Acknowledgments
Thank you to the researchers that provided samples and discussions (Kevin Raskoff, Kim Reisenbichler, and Rob Sherlock). The crews of the RV Point Lobos, RV Rachel Carson, and the RV Western Flyer provided essential support for the ROV operations and specimen collections could not have been accomplished without the ROV pilots for the ROV Tiburon, ROV Ventana, and the ROV Doc Ricketts. Nicholas Bezio created Figure 4a,b, Figure 5b and Figure 6a,b; Claudia Mills and Gustav Paulay provided taxonomic assistance and comments from three anonymous referees have enhanced the final paper.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Water parameters and collection information for sequenced Atolla and other coronate specimens. Sample ID refers to the remotely operated vehicle and the dive number (V for ROV Ventana, T for ROV Tiburon, or D for ROV Doc Ricketts), blue-water SCUBA dive (BW) or trawls (T) aboard the RV Kilo Moana (KM), the RV Ka’imikai-O-Kanaloa (KOK), or the RV Western Flyer (WF). Two specimens of Atolla tenella collected in the Arctic were provided by Kevin Raskoff (KR). Some information was not available (NA).
Table A1.
Water parameters and collection information for sequenced Atolla and other coronate specimens. Sample ID refers to the remotely operated vehicle and the dive number (V for ROV Ventana, T for ROV Tiburon, or D for ROV Doc Ricketts), blue-water SCUBA dive (BW) or trawls (T) aboard the RV Kilo Moana (KM), the RV Ka’imikai-O-Kanaloa (KOK), or the RV Western Flyer (WF). Two specimens of Atolla tenella collected in the Arctic were provided by Kevin Raskoff (KR). Some information was not available (NA).
| Species ID | Date | Sample ID | Depth m | Temp °C | Sal PSU | Oxy ml/L | Lat Decimal | Long Decimal |
|---|---|---|---|---|---|---|---|---|
| A. gigantea | 10 August 2015 | D0791D5 | 1112 | 3.809 | 34.457 | 0.373 | 36.531771 | −122.507713 |
| A. gigantea | 14 December 2016 | D0915D5 | 1016 | 3.852 | 34.467 | 0.411 | 36.260438 | −122.593946 |
| A. gigantea | 10 December 2017 | D0995D10 | 897 | 4.199 | 34.427 | 0.297 | 36.748584 | −122.103112 |
| A. gigantea | 19 March 2017 | KOK- T06 | NA | NA | NA | NA | 19.275 | 156.133333 |
| A. gigantea | 30 May 2021 | D1337sss3 | 1253 | 4.278 | 34.464 | 0.332 | 33.850068 | −119.850222 |
| A. gigantea | 30 May 2021 | D1337D5 | 936 | 4.436 | 34.45 | 0.321 | 33.850169 | −119.651757 |
| A. parva | 20 March 2017 | KOK-T7/8 | NA | NA | NA | NA | 19.483333 | 156.13333 |
| A.parva | 21 March 2017 | KOK17-T11 | NA | NA | NA | NA | 19.666667 | 156.13333 |
| A. parva | 6 November 2018 | KM-T02.17 | NA | NA | NA | NA | 19.426389 | 156.408611 |
| A. parva | 7 November 2018 | KM18 T05 | NA | NA | NA | NA | 19.318333 | 156.185833 |
| A. tenella | 17 July 2005 | KR-17 | NA | NA | NA | NA | Arctic | |
| A. tenella | 25 July 2005 | KR-25 | NA | NA | NA | NA | Arctic | |
| A. aff. tenella | 2 March 2015 | D718D7 | 1696 | 2.633 | 34.615 | 1.212 | 24.412664 | −109.095732 |
| A. aff. tenella | 20 March 2017 | KOK17-T09 | NA | NA | NA | NA | 19.483333 | 156.13333 |
| A. aff. tenella | 21 March 2017 | KOK17-T11 | NA | NA | NA | NA | 19.666667 | 156.13333 |
| A. aff. tenella | 6 November 2018 | KM- T02.18 | NA | NA | NA | NA | 19.426389 | 156.408611 |
| A. aff. tenella | 6 November 2018 | KM-T02.19 | NA | NA | NA | NA | 19.426389 | 156.408611 |
| A. tenella | 7 November 2018 | KM18 T04-13 | 2500 | NA | NA | NA | 19.318333 | 156.185833 |
| A. vanhoeffeni | 22 October 2012 | WF trawl | NA | NA | NA | NA | 36.699558 | −122.049488 |
| A. vanhoeffeni | 24 April 2013 | V3709ss2 | 512 | 6.002 | 34.251 | 0.343 | 36.700040 | −122.048422 |
| A. vanhoeffeni | 27 May 2015 | V3828D1 | 424 | 6.876 | 34.179 | 0.924 | 36.703304 | −122.052176 |
| A. vanhoeffeni | 31 January 2020 | WF-D1243trawl | NA | NA | NA | NA | 36.16666 | −119.25 |
| A. vanhoeffeni | 31 January 2020 | WF-D1243trawl | NA | NA | NA | NA | 36.16666 | −119.25 |
| A. aff. wyvillei | 18 March 2010 | V3540ss4 | 626 | 5.077 | 34.319 | 0.195 | 36.705400 | −122.053820 |
| A. aff. wyvillei | 22 February 2015 | D710ss11 | 746 | 6.020 | 34.509 | 0.023 | 24.277515 | −109.360873 |
| A. aff. wyvillei | 24 February 2015 | D712ss9 | 706 | 5.997 | 34.516 | 0.032 | 25.430911 | −109.835949 |
| A. aff. wyvillei | 25 February 2015 | D713ss7 | 774 | 6.002 | 34.516 | 0.032 | 25.446143 | −109.848168 |
| A. aff. wyvillei | 9 March 2015 | D723ss9 | 697 | 5.956 | 34.516 | 0.034 | 25.442727 | −109.852024 |
| A. aff. wyvillei | 30 May 2021 | D1337ss4 | 985 | 4.436 | 34.45 | 0.321 | 33.850169 | −119.651757 |
| A reynoldsi sp. nov. | 5 December 2015 | D0830D9 | 1013 | 3.861 | 34.436 | 0.348 | 36.688186 | −122.118768 |
| A reynoldsi sp. nov. | 6 December 2017 | D0991ss3 | 1878 | 2.229 | 34.602 | 1.373 | 36.548736 | −122.541753 |
| A reynoldsi sp. nov. | 30 July 2021 | D1369D1 | 3189 | 1.576 | 34.665 | 2.470 | 35.499466 | −123.99876 |
| Atolla type A | 30 October 2021 | D1399-ss3 | 1253 | 3.264 | 34.515 | 0.764 | 36.7009226 | −122.067752 |
| Atolla type B | 6 December 2017 | D991ss5 | 1783 | 2.363 | 34.592 | 1.271 | 36.548400 | −122.542593 |
| Nausithoe sp. | 23 March 2017 | KOK2017-BW22 | 30 | NA | NA | NA | 20.756111 | −157.255833 |
| Paraphyllina sp. | 27 May 2019 | D0026 ss8 | 2385 | 1.858 | 34.592 | 1.943 | 36.116665 | −122.75 |
| Paraphyllina sp. | 9 March 2015 | D0723 ss10 | 651 | 6.199 | 34.512 | 0.025 | 25.442516 | −109.852324 |
| Paraphyllina sp. | 18 November 2019 | D1221 D12 | 2088 | 2.006 | 34.815 | 1.851 | 36.545798 | −122.538197 |
| Periphyllopsis sp. | 27 February 2015 | D0715 ss1 | 1761 | 2.839 | 34.819 | 1.087 | 28.182585 | −119.599956 |
| Periphylla periphylla | 1 July 2015 | D0780 D11 | 534 | 5.231 | 34.169 | 0.433 | 36.15082 | −124.2852 |
| Periphylla periphylla | 15 November 2015 | D1218 ss12 | 384 | 7.542 | 34.158 | 0.996 | 36.695557 | −122.004649 |
| Periphylla periphylla | 15 November 2015 | D1218 ss6 | 392 | 6.940 | 34.184 | 0.763 | 36.698180 | −122.010072 |
| Periphylla periphylla | 16 November 2015 | D1219 D10 | 923 | 4.237 | 34.416 | 0.321 | 36.544387 | −122.537005 |

Table A2.
Potential diagnostic trait table for species identification of Atolla based on existing keys [8,9,10,11] as well as original descriptions and our observations. Potential valid diagnostic traits are highlighted in yellow and we have grouped A. tenella with A. aff. tennella and A. wyvillei with A. aff. wyvillei as the molecular analysis shows that they are similar (Appendix A Table A3). Septa shape (divergent or straight), degree of extension into coronal muscle, and gonad shape are not considered by us to be valid diagnostic traits.
Table A2.
Potential diagnostic trait table for species identification of Atolla based on existing keys [8,9,10,11] as well as original descriptions and our observations. Potential valid diagnostic traits are highlighted in yellow and we have grouped A. tenella with A. aff. tennella and A. wyvillei with A. aff. wyvillei as the molecular analysis shows that they are similar (Appendix A Table A3). Septa shape (divergent or straight), degree of extension into coronal muscle, and gonad shape are not considered by us to be valid diagnostic traits.
| Species | A. chuni * | A. parva | A. vanhoeffeni | A. tenella and A. aff. tenella | A. gigantea | A. wyvillei and A. aff. wyvillei | A. reynoldsi sp. nov. | Atolla species A—tall, rounded dome | Atolla species B—White, very flat |
|---|---|---|---|---|---|---|---|---|---|
| Pigment spots | No | No | Yes 2 per quadrant | Yes? 2 per tentacle a | No | No | No | No | no |
| Papillae | Yes | No | No | No | No | No | Yes | No | Yes, varies b |
| Ridges with spikes on rhopalia | No | No | No | No | No | No | Yes | No | No |
| Stomach | Clover shaped | Clover shaped | Cross shaped | Clover shaped | Clover shaped | Clover shaped | Greek-cross shaped | Greek-cross shaped—evaginated | Greek-cross shaped |
| Tentacles | 24 | 18–24 | 18–20 | 22–30 | 24, 28 | 22–30 | 26–39 coiled | 59–64 | 32–60 |
| Trailing tentacle | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Septa path c | ? | Straight or slightly divergent, club shaped | Straight | Divergent in description; straight in our specimens | Divergent (preserved look straight) | Divergent | Straight or slightly divergent | Straight or slightly divergent | Straight |
| Septa extend to muscle d | ? | Yes | No | No | Yes | Yes e | Yes | Yes | Yes |
| Gonad shape f | Oval/bean | oval | Horseshoe | Circular with irregular edges | Horseshoe | Oval to large auricular | Oval but horseshoe shaped when mature | Horseshoe | Immature, horseshoe |
Footnotes: * not observed or collected in this study; a. Pigment spots not observed in our specimen or photos of A. tenella; b. Papillae in Atolla sp. B vary from a few solitary papilla to two rows of papillae on the rhopalia, loss of papillae may be a result of fixation; c. Path of the septa is not a good trait, as it is somewhat arbitrary and changes with preservation; d. Septa extending into coronal muscle is also not a robust trait; e. Septa not extending into coronal muscle is supposed to be diagnostic for A. wyvillei, but they do extend into the muscle; f. Gonads change shape as they mature, going from a C-shaped outline to oval and then to fully folded horseshoe. Other species in the literature: A. russelli—might have a Greek-cross shaped gut (Lindsay et al. 2004 [30]) (16–22 tentacles divergent septa); 22 tentacles in original description; A. bairdii—Smithsonian holotype photo online, looks like A gigantea (22 tentacles, divergent septa); A. verrilli—28 tentacles, straight septa—doubtful species, sometimes considered a synonym of A. wyvillei; A. valdiviae—doubtful species, sometimes considered a synonym of A. wyvillei. ? refers to unknown status as we have not observed a specimen of A. chuni.

Table A3.
Fixed differences for each Atolla species for COI barcode sequence (shaded in gray). Position refers to base pair position in alignment with A. wyvillei (GQ120088).
Table A3.
Fixed differences for each Atolla species for COI barcode sequence (shaded in gray). Position refers to base pair position in alignment with A. wyvillei (GQ120088).
| COI mtDNA | Position | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | 91 | 94 | 97 | 100 | 103 | 106 | 109 | 118 | 122 | 124 | 125 | 127 | 133 | 151 | 158 | 160 | 163 | 166 | 172 | 179 | 181 | 196 | 199 | 205 | 208 | 220 | 223 |
| Atolla sp. B | G | T | T | T | C | A | A | T | T | A | T | A | A | T | A | A | A | A | T | C | A | A | T | A | T | T | A |
| A. gigantea | A | T | T | T | A | A | T | T | T | A | T | A | A | T | A | A | T | T | T | T | A | T | C | T | T | C | A |
| A. reynoldsi | A | G | C | C | T | C | C | C | C | T | G | G | T | A | G | G | T | T | A | T | A | G | G | G | C | A | G |
| A. parva | A | T | T | T | T | A | T | T | T | A | T | A | A | T | A | A | T | T | A | T | A | T | T | T | T | T | A |
| A. aff. tenella | A | T | T | T | G | A | T | T | T | A | T | A | A | T | A | A | T | T | T | T | G | T | T | T | T | C | A |
| A. tenella | A | T | T | T | T | A | T | T | T | A | C | A | A | T | A | A | C | T | G | T | A | T | T | T | T | T | A |
| A. vanhoeffeni | A | T | T | T | G | T | C | T | T | A | T | A | A | T | A | A | T | T | T | T | A | T | T | A | T | C | A |
| A. aff. wyvillei | A | T | T | T | T | A | T | T | T | A | T | A | A | T | A | A | T | T | A | T | A | T | T | T | T | T | A |
| A. wyvillei | A | T | T | T | A | A | T | T | T | A | T | A | A | T | A | A | C | T | T | T | A | T | C | T | T | C | A |
| COI cont. | Position | ||||||||||||||||||||||||||
| Species | 224 | 232 | 233 | 245 | 250 | 253 | 256 | 265 | 266 | 268 | 283 | 284 | 286 | 298 | 300 | 301 | 304 | 316 | 328 | 331 | 334 | 335 | 337 | 340 | 341 | 346 | 352 |
| Atolla sp. B | T | T | A | T | A | A | C | A | T | A | A | T | A | A | C | C | T | T | C | A | T | G | T | A | T | T | T |
| A. gigantea | T | T | A | C | A | A | T | T | T | A | T | T | A | A | T | T | T | T | C | A | T | G | T | T | T | T | T |
| A. reynoldsi | T | C | G | T | G | T | T | C | C | T | G | A | T | G | T | T | A | A | T | G | T | G | T | T | T | T | A |
| A. parva | T | T | A | C | C | A | T | T | T | A | T | T | A | A | T | A | T | T | T | A | T | G | T | T | T | T | T |
| A. aff. tenella | T | T | A | C | C | A | T | T | T | A | T | T | A | A | T | A | T | T | C | A | T | G | T | C | C | T | T |
| A. tenella | T | T | A | C | T | A | T | T | T | A | T | T | A | A | T | A | G | T | T | A | C | G | C | T | T | A | T |
| A. vanhoeffeni | C | T | A | C | A | A | T | C | T | A | T | T | A | A | T | T | T | T | A | A | T | A | T | T | A | T | T |
| A. aff. wyvillei | T | T | A | C | C | A | T | T | T | A | T | T | A | A | T | G | T | T | T | A | T | G | T | T | T | T | T |
| A. wyvillei | T | T | A | C | A | A | T | T | T | A | T | T | A | A | T | A | T | T | C | A | T | G | C | T | T | T | T |
| COI cont. | Position | ||||||||||||||||||||||||||
| Species | 358 | 361 | 364 | 368 | 371 | 373 | 376 | 379 | 382 | 385 | 394 | 398 | 410 | 415 | 421 | 430 | 439 | 440 | 442 | 445 | 452 | 454 | 455 | 457 | 461 | 465 | 466 |
| Atolla sp. B | A | T | A | T | A | A | A | T | T | T | T | T | T | T | C | T | T | A | A | T | A | C | A | A | C | T | T |
| A. gigantea | T | A | A | C | G | T | A | T | T | A | A | G | T | T | A | A | A | A | T | T | T | A | A | T | A | C | A |
| A. reynoldsi | T | A | G | T | G | C | T | C | T | A | A | C | C | A | A | C | T | G | G | C | G | T | C | T | C | C | A |
| A. parva | T | A | T | T | G | T | A | T | T | A | A | C | T | T | A | A | T | A | T | T | T | T | A | T | G | C | A |
| A. aff. tenella | T | A | A | T | G | C | A | T | T | A | A | G | T | T | A | A | A | A | A | T | T | A | A | T | G | C | A |
| A. tenella | T | A | T | T | G | T | A | T | T | A | A | A | T | T | A | A | C | A | A | T | T | T | A | T | G | C | A |
| A. vanhoeffeni | T | A | A | T | G | T | A | T | T | A | A | G | A | T | A | A | A | A | A | T | T | A | A | T | G | C | A |
| A. aff. wyvillei | T | A | T | T | G | T | A | T | C | A | A | C | T | T | A | A | T | A | A | T | T | T | A | T | G | C | A |
| A. wyvillei | T | A | A | T | G | T | A | T | T | A | A | G | T | T | A | A | A | A | T | T | T | A | A | T | A | C | A |
| COI cont. | Position | ||||||||||||||||||||||||||
| Species | 481 | 482 | 484 | 490 | 496 | 500 | 502 | 503 | 511 | 518 | 520 | 529 | 535 | 538 | 541 | 547 | 548 | 549 | 556 | 565 | 583 | 586 | 589 | 592 | 610 | 616 | 619 |
| Atolla sp. B | A | T | A | T | T | T | A | G | T | T | A | T | T | G | A | A | G | C | T | A | T | T | A | T | G | T | A |
| A. gigantea | T | T | A | G | A | T | A | G | T | C | T | T | A | A | A | A | G | C | A | A | T | T | C | T | A | T | T |
| A. reynoldsi | T | C | T | G | C | C | G | G | A | C | T | A | G | A | G | A | T | G | A | G | C | C | G | C | C | T | T |
| A. parva | C | T | A | A | T | T | A | G | T | C | T | T | A | T | A | G | G | C | A | A | T | T | T | T | T | T | T |
| A. aff. tenella | T | T | A | A | A | T | A | G | T | C | T | T | G | A | A | A | G | C | A | A | T | T | T | T | A | C | T |
| A. tenella | T | T | A | A | T | T | A | G | T | C | T | T | A | T | A | A | G | C | A | A | T | T | T | T | T | T | T |
| A. vanhoeffeni | T | T | A | A | A | T | A | A | C | C | T | T | A | A | A | A | G | C | A | A | T | T | T | T | G | T | T |
| A. aff. wyvillei | C | T | A | A | T | T | A | G | T | C | T | T | A | T | A | A | G | C | A | A | T | T | T | T | T | T | T |
| A. wyvillei | T | T | A | A | T | T | A | G | T | C | T | T | A | A | A | A | G | C | A | A | T | T | C | T | A | T | T |
| COI cont. | Position | ||||||||||||||||||||||||||
| Species | 640 | 646 | 652 | 664 | 670 | 671 | 673 | 676 | 677 | 685 | 694 | 695 | 697 | 700 | 712 | 718 | 721 | 724 | 760 | 763 | 769 | 778 | 779 | 781 | |||
| Atolla sp. B | T | T | G | A | T | T | A | A | C | T | A | A | A | A | T | T | T | T | T | G | G | T | T | A | |||
| A.gigantea | T | T | A | A | T | T | A | T | T | T | A | G | A | T | A | T | T | T | T | T | T | T | A | A | |||
| A. reynoldsi | C | C | T | G | T | C | T | T | T | C | G | G | T | T | A | C | T | T | C | T | A | C | C | T | |||
| A. parva | T | T | C | T | T | T | A | T | T | T | A | G | G | T | A | T | T | T | T | T | T | T | A | C | |||
| A. aff. tenella | T | T | C | T | T | T | A | T | T | T | A | G | A | T | A | T | C | C | T | T | T | T | A | A | |||
| A. tenella | T | T | C | C | C | T | G | T | T | T | A | G | A | T | A | T | T | T | T | T | T | T | A | A | |||
| A. vanhoeffeni | T | T | T | T | T | T | A | T | T | T | A | G | A | T | A | T | T | T | T | T | T | T | A | A | |||
| A. aff wyvillei | T | T | C | T | T | T | A | T | T | T | A | G | A | T | A | T | T | T | T | C | T | T | A | C | |||
| A. wyvillei | T | T | A | C | T | T | A | T | T | T | A | G | A | T | A | T | T | T | T | T | T | T | A | A | |||
References
- Götte, A. Entwickelungsgeschichte der Aurelia aurita und Cotylorhiza tuberculata. Abh. Entwickelungsgeschichte Tiere 1887, 4, 1–79. (In German) [Google Scholar]
- Calder, D.R. Cubozoan and scyphozoan jellyfishes of the Carolinian Biogeographic Province, southeastern USA. R. Ont. Mus. Contrib. Sci. 2009, 3, 1–58. [Google Scholar]
- Vanhöffen, E. Die Akalephen der Plankton-Expedition. Ergeb. Plankton-Exped. Humboldt-Stift. 1892, 2, 3–28. (In German) [Google Scholar]
- Hickson, S.J. Coelenterata and Ctenophora. In The Cambridge Natural History; Harmer, S.F., Shipley, A.E., Eds.; MacMillan and Company: London, UK, 1906; pp. 243–424. [Google Scholar]
- Haeckel, E. System der Acraspeden. Zweite Halfte des Systems der Me dusen. Denkschr. Medicinisch-Nat. Ges. Jena 1880, 2, 361–672. (In German) [Google Scholar]
- Collins, A.G.; Jarms, G.; Morandini, A.C. World List of Scyphozoa Atolla Haeckel, 1880. World Register of Marine Species. 2021. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=135248 (accessed on 30 November 2021).
- Pages, F.; Gill, J.; Bouillon, J. Medusae (Hydrozoa, Scyphozoa, Cubozoa) of the Benguela current (southeast Atlantic). Sci. Mar. 1992, 56, 1–64. [Google Scholar]
- Larson, R.J. Pelagic scyphomedusae (Scyphozoa: Coronatae and Semaestomeae) of the southern ocean. Biol. Antarct. Seas XVI Antarct. Res. Ser. 1986, 41, 59–165. [Google Scholar]
- Bigelow, H.B. The Medusae. In Reports on the Scientific Results of the Expedition to the Eastern Tropical Pacific, in Charge of Alexander Agassiz, by the U.S. Fish Commission Steamer “Albatross” from October 1904 to March 1905. XVI; Memoirs of the Museum of Comparative Zoölogy at Harvard College: Cambridge, MA, USA, 1909; Volume 37, pp. 1–243. Available online: http://www.biodiversitylibrary.org/item/30084 (accessed on 30 November 2021).
- Gibbons, M.J.; Morandini, A.C.; Straehler-Pohl, I.; Bezio, N. Identification Guide to Macro Jellyfishes of West Africa; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Russell, F.S. The Medusae of the British Isles II. Pelagic Scyphozoa with a Supplement to the First Volume on Hydromedusae; Cambridge University Press: London, UK, 1970; 296p. [Google Scholar]
- Hartlaub, C. Méduses. In Croisière océanographique: Accomplie à bord de la Belgica dans la Mer du Grönland, 1905; C. Bulens: Bruxelles, Belgium, 1909; pp. 463–478. Available online: https://www.biodiversitylibrary.org/page/41467593 (accessed on 30 November 2021). (In French)
- Raskoff, K.A.; Purcell, J.E.; Hopcroft, R.R. Gelatinous zooplankton of the Arctic Ocean: In situ observations under the ice. Polar Biol. 2005, 28, 207–217. [Google Scholar] [CrossRef]
- Russell, F.S. Some observations on the scyphomedusa Atolla. J. Mar. Biol. Assoc. UK 1959, 3833, 40. [Google Scholar]
- Hunt, J.C.; Lindsay, D.J. Observations on the behavior of Atolla (Scyphozoa: Coronatae) and Nanomia (Hydrozoa: Physonectae): Use of the hypertrophied tentacle in prey capture. Plankton Biol. Ecol. 1998, 45, 239–242. [Google Scholar]
- Walker, A. The Form and Function of the Hypertrophied Tentacle of the Deep-Sea Jelly Atolla spp. Unpublished Intern Report. 2011, pp. 1–19. Available online: https://www.mbari.org/wp-content/uploads/2015/10/Walker.pdf (accessed on 30 November 2021). Unpublished Intern Report.
- Larson, R.J.; Mills, C.E.; Harbison, G.R. Western Atlantic midwater hydrozoan and scyphozoan medusae: In situ studies using manned submersibles. Hydrobiologia 1991, 216, 311–317. [Google Scholar] [CrossRef]
- Moore, P.G.; Rainbow, P.S.; Larson, R.J. The mesopelagic shrimp Notostomus robustus Smith (Decapoda: Oplophoridae) observed in situ feeding on the medusan Atolla wyvillei Haeckel in the Northwest Atlantic, with notes on gut contents and mouthpart morphology. J. Crustacean Biol. 1993, 13, 690–696. [Google Scholar] [CrossRef]
- Robison, B.H.; Reisenbichler, K.R.; Sherlock, R.E. The coevolution of midwater research and ROV technology at MBARI. Oceanography 2017, 30, 26–37. [Google Scholar] [CrossRef]
- Youngbluth, M.J. Manned Submersibles and Sophisticated Instrumentations: Tools for Oceanographic Research. In SUBTECH ’83 Symposium: The Design and Operation of Underwater Vehicles. Soc. Underw. Technol. 1984, 22, 351–355. [Google Scholar]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Folmer, O.; Black, M.B.; Hoeh, W.R.; Lutz, R.A.; Vrijenhoek, R.C. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Schmidt, H.A.; Chernoor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. Q-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Vanhöffen, E. Die Acraspeden Medusen der deutschen Tiefsee-Expedition 1898–1899. Wiss. Ergeb. Dtsch. Tiefsee Exped. Dampfer Valdivia 1898 1899, 1902, 3–52. (In German) [Google Scholar] [CrossRef] [Green Version]
- Russell, F.S. On a new species of scyphomedusa, Atolla vanhöffeni n. sp. J. Mar. Biol. Assoc. UK 1957, 36, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, D.; Furushima, Y.; Miyake, H.; Kitamura, M.; Hunt, J.C. The Scyphomedusan Fauna of the Japan Trench: Preliminary Results from a Remotely-Operated Vehicle. In Coelenterate Biology; Springer: Dordrecht, The Netherlands, 2004; pp. 537–547. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).