Effect of Dietary Betaine on Muscle Protein Deposition, Nucleic Acid and Amino Acid Contents, and Proteomes of Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, Diets, and Management
2.2. Sample Collection
2.3. Serum Parameters Determination
2.4. Muscle Protein Deposition and Nucleic Acids Contents Measurement
2.5. Muscle Amino Acids Contents Measurement
2.6. iTRAQ Experiments
2.7. Protein Identification, Quantification, and Bioinformatics Analysis
2.8. Quantitative Real-Time PCR Validation
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Parameters
3.3. Muscle Protein Deposition and Nucleic Acids Contents
3.4. Muscle Amino Acids Contents
3.5. Muscle Protein Identification and Quantification
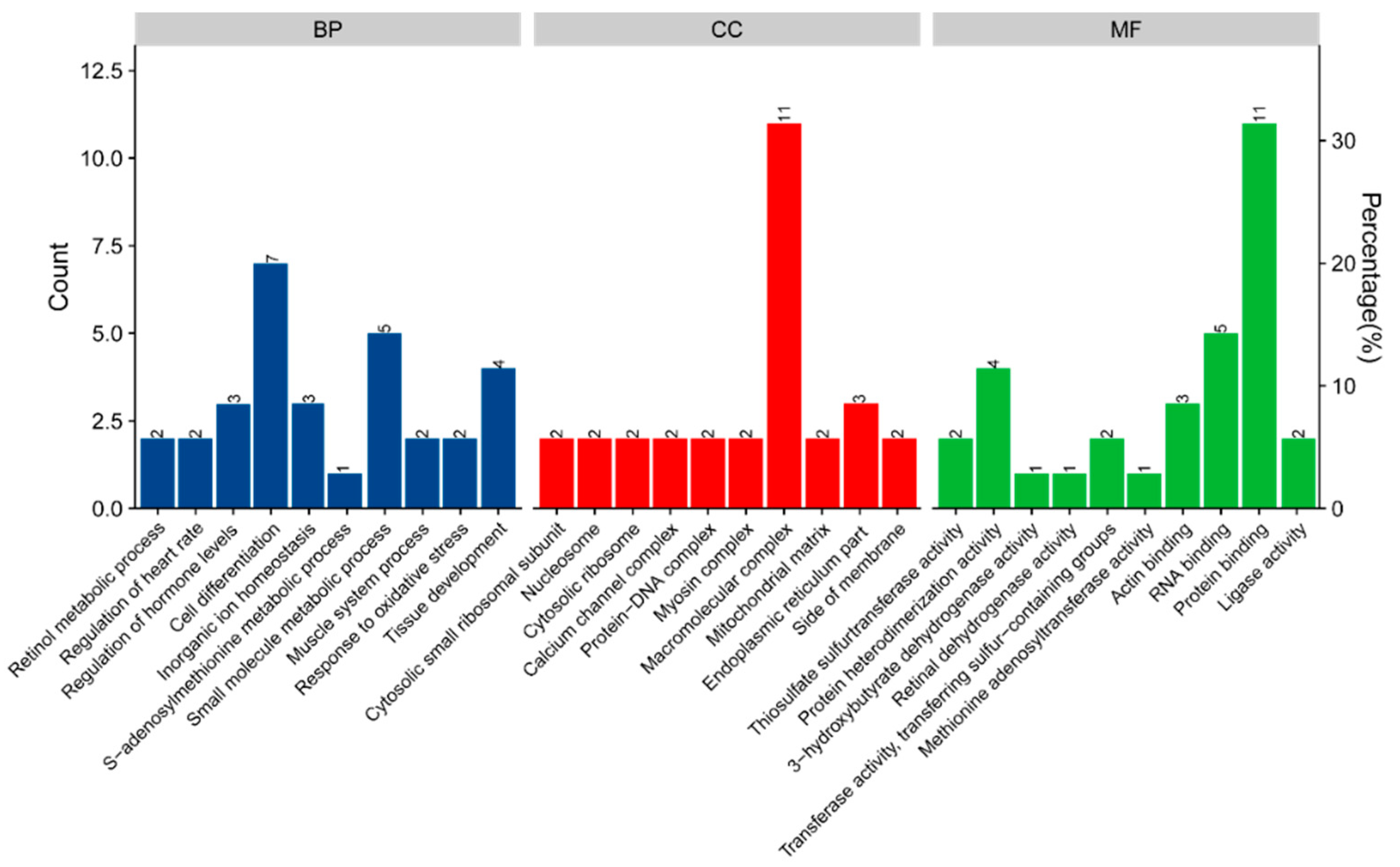
3.6. Bioinformatics Analysis
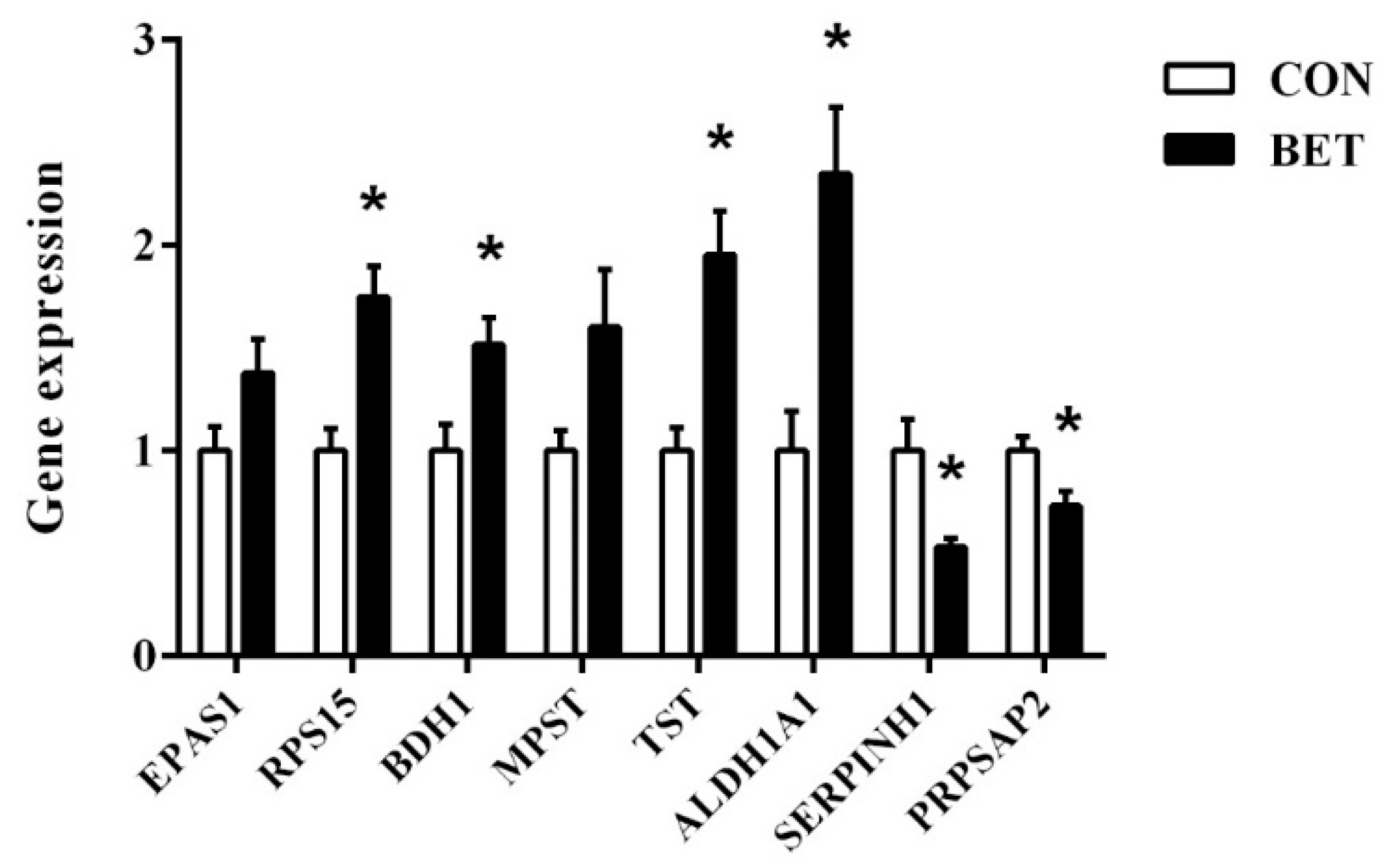
3.7. Validation of Differentially Abundant Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratriyanto, A.; Mosenthin, R.; Bauer, E.; Eklund, M. Metabolic, osmoregulatory and nutritional functions of betaine in monogastric animals. Asian. Australas. J. Anim. Sci. 2009, 22, 1461–1476. [Google Scholar] [CrossRef]
- Eklund, M.; Bauer, E.; Wamatu, J.; Mosenthin, R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005, 18, 31–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.C.; Xu, Z.R.; Han, X.Y.; Li, W.F. Changes in hormones, growth factor and lipid metabolism in finishing pigs fed betaine. Livest. Sci. 2006, 105, 78–85. [Google Scholar] [CrossRef]
- Nutautaite, M.; Alijoius, S.; Bliznikas, S.; Sasyte, V.; Viliene, V.; Pockevicius, A.; Raceviciute, A. Effect of betaine, a methyl group donor, on broiler chicken growth performance, breast muscle quality characteristics, oxidative status, and amino acid content. Ital. J. Anim. Sci. 2020, 19, 621–629. [Google Scholar] [CrossRef]
- Wang, H.C.; Li, S.S.; Xu, S.Y.; Feng, J. Betaine improves growth performance by increasing digestive enzymes activities, and enhancing intestinal structure of weaned piglets. Anim. Feed Sci. Technol. 2020, 267, 114545. [Google Scholar] [CrossRef]
- Zhan, X.A.; Li, J.X.; Xu, Z.R.; Zhao, R.Q. Effects of methionine and betaine supplementation on growth performance, carcase composition and metabolism of lipids in male broilers. Br. Poult. Sci. 2006, 47, 576–580. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Newmire, D.E.; Rossi, F.E.; Guimares-Ferreira, L.; Zanchi, N.E. An overview of betaine supplementation, sports performance, and body composition. In Nutrition and Enhanced Sports Performance, 2nd ed.; Bagchi, D., Nair, S., Sen, C.K., Eds.; Academic Press: Washington, DC, USA, 2019; pp. 691–706. [Google Scholar]
- Every, D.V.; Plotkin, D.L.; Delcastillo, K.; Cholewa, J.; Schoenfeld, B.J. Betaine supplementation: A critical review of its efficacy for improving muscle strength, power, and body composition. Strength. Cond. J. 2021, 43, 53–61. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Rashti, S.L.; Faigenbaum, A.D. Effect of betaine supplementation on power performance and fatigue. J. Int. Soc. Sports Nutr. 2009, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.J.; Shen, L.Y.; Zhang, P.W.; Tan, Z.D.; Cheng, X.; Luo, J.; Zhao, X.; Yang, Q.; Hao, G. The regulation of skeletal muscle fiber-type composition by betaine is associated with NFATc1/MyoD. J. Mol. Med. 2018, 96, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Senesi, P.; Luzi, L.; Montesano, A.; Mazzocchi, N.; Terruzzi, I. Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J. Transl. Med. 2013, 11, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Zhuang, S.; Chen, Y.P.; Cheng, Y.F.; Wen, C.; Zhou, Y.M. Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway. Poult. Sci. 2018, 97, 4297–4305. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.L. Proteins in the cell. Protein. Sci. 2019, 28, 1175–1176. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Satoh, K.; Yamada, H.; Takebe, T.; Nikaido, H.; Shiozawa, S. Assessment of the nutritional status of field-caught larval Pacific bluefin tuna by RNA/DNA ratio based on a starvation experiment of hatchery-reared fish. J. Exp. Mar. Biol. Ecol. 2008, 354, 56–64. [Google Scholar] [CrossRef]
- Damodaran, S. Amino acids, peptides, and proteins. Electrophoresis 2013, 34, 321–430. [Google Scholar]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Karp, N.A.; Huber, W.; Sadowski, P.G.; Charles, P.D.; Hester, S.V.; Lilley, K.S. Addressing accuracy and precision issues in iTRAQ quantitation. Mol. Cell. Proteom. 2010, 9, 1885–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.H.; Wang, D.Y.; Xu, X.L.; Xu, W.M.; Zhou, G.H. iTRAQ-based proteomic analysis of duck muscle related to lipid oxidation. Poult. Sci. 2021, 100, 101029. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Wang, C.; Zhao, X.; Dai, C.; Zhou, G.; Xu, X. Proteome analysis using iTRAQ reveals the alterations in stress-induced dysfunctional chicken muscle. J. Agric. Food Chem. 2017, 65, 2913–2922. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Gao, Y.; Lu, Q.P.; Sa, R.N.; Zhang, H.F. iTRAQ-based quantitative proteomic analysis of longissimus muscle from growing pigs with dietary supplementation of non-starch polysaccharide enzymes. J. Zhejiang Univ. Sci. B 2015, 16, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Med. 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rao, S.V.R.; Raju, M.; Panda, A.K.; Saharia, P.; Sunder, G.S. Effect of supplementing betaine on performance, carcass traits and immune responses in broiler chicken fed diets containing different concentrations of methionine. Asian. Australas. J. Anim. Sci. 2011, 24, 662–669. [Google Scholar] [CrossRef]
- Song, Y.D.; Chen, R.; Yang, M.; Liu, Q.; Zhou, Y.M.; Zhuang, S. Dietary betaine supplementation improves growth performance, digestive function, intestinal integrity, immunity, and antioxidant capacity of yellow-feathered broilers. Ital. J. Anim. Sci. 2021, 20, 1575–1586. [Google Scholar] [CrossRef]
- Wen, C.; Chen, R.; Chen, Y.P.; Ding, L.R.; Wang, T.; Zhou, Y.M. Betaine improves growth performance, liver health, antioxidant status, breast meat yield, and quality in broilers fed a mold-contaminated corn-based diet. Anim. Nutr. 2021, 7, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wan, X.L.; Zhang, L.L.; Wang, C.; He, J.T.; Bai, K.W.; Zhang, X.H.; Zhao, L.G.; Wang, T. Effect of different doses of fermented Ginkgo biloba leaves on serum biochemistry, antioxidant capacity hepatic gene expression in broilers. Anim. Feed Sci. Technol. 2019, 248, 132–140. [Google Scholar] [CrossRef]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of growth hormone on insulin signaling. Mol. Cell. Endocrinol. 2020, 518, 111038. [Google Scholar] [CrossRef]
- Singhal, V.; Goh, B.C.; Bouxsein, M.L.; Faugere, M.C.; DiGirolamo, D.J. Osteoblast-restricted disruption of the growth hormone receptor in mice results in sexually dimorphic skeletal phenotypes. Bone Res. 2013, 1, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berneis, K.; Keller, U.O. Metabolic actions of growth hormone: Direct and indirect. Baillieres Clin. Endocrinol. Metab. 1996, 10, 337–352. [Google Scholar] [CrossRef]
- Florini, J.R.; Ewton, D.Z.; Coolican, S.A. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 1996, 17, 481–517. [Google Scholar]
- Coleman, M.E.; DeMayo, F.; Yin, K.C.; Lee, H.M.; Geske, R.; Montgomery, C.; Schwartz, R.J. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 1995, 270, 12109–12116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, H.S.; Li, H.L.; Park, J.H.; Kang, C.W.; Ryu, K.S. Effects of dietary betaine on the secretion of insulin-like growth factor-I and insulin-like growth factor binding protein-1 and-3 in laying hens. Asian. Australas. J. Anim. Sci. 2010, 23, 379–384. [Google Scholar] [CrossRef]
- Yusuf, M.S.; El, N.; Adel, A.; Hassan, M.A.; Mandour, M.A. Supplementary outcomes of betaine on economic and productive performance, some biochemical parameters, and lipoprotein lipase gene expression in finishing male broilers. Int. J. Vet. Sci. Med. 2018, 6, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Ingale, S.L.; Lee, S.H.; Kim, K.H.; Kim, J.S.; Lee, J.H.; Chae, B.J. Effects of energy levels of diet and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites in growing pigs. Anim. Feed Sci. Technol. 2013, 186, 64–70. [Google Scholar] [CrossRef]
- Islam, M.S.; Tanaka, M. Nutritional condition, starvation status and growth of early juvenile Japanese sea bass (lateolabrax japonicus) related to prey distribution and feeding in the nursery ground. J. Exp. Mar. Biol. Ecol. 2005, 323, 172–183. [Google Scholar] [CrossRef]
- Craig, A.S.S. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Wen, C.; Cheng, Y.F.; Chen, Y.P.; Zhuang, S.; Zhou, Y.M. Effects of dietary supplementation with betaine on muscle growth, muscle amino acid contents and meat quality in Cherry Valley ducks. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Puchala, R.; Sahlu, T.; Herselman, M.J.; Davis, J.J. Influence of betaine on blood metabolites of alpine and angora kids. Small Ruminant Res. 1995, 18, 137–143. [Google Scholar] [CrossRef]
- Chiang, P.K.; Gordon, R.K.; Tal, J.; Zeng, G.C.; Doctor, B.P.; Pardhasaradhi, K.; Mccann, P. S-Adenosylmethionine and methylation. FASEB J. 1996, 10, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idriss, A.A.; Hu, Y.; Hou, Z.; Hu, Y.; Sun, Q.; Omer, N.A.; Abobaker, H.; Ni, Y.; Zhao, R. Dietary betaine supplementation in hens modulates hypothalamic expression of cholesterol metabolic genes in F1 cockerels through modification of DNA methylation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 217, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Razin, A.; Riggs, A.D. DNA methylation and gene function. Science 1980, 210, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Serganov, A. Ribosomal protein S15 represses its own translation via adaptation of an rRNA-like fold within its mRNA. EMBO J. 2014, 22, 1898–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Items | 1–21 Days | 22–42 Days | 43–52 Days |
|---|---|---|---|
| Ingredients (g/kg) | |||
| Corn | 565 | 612 | 659 |
| Soybean meal | 322 | 260 | 234 |
| Corn gluten meal | 37.0 | 42.0 | 20.0 |
| Soybean oil | 26.1 | 38.7 | 39.7 |
| Dicalcium phosphate | 20.0 | 16.0 | 16.0 |
| Limestone | 12.0 | 14.0 | 14.0 |
| Premix 1 | 10.0 | 10.0 | 10.0 |
| L-Lysine·HCl | 3.40 | 3.50 | 3.50 |
| Sodium chloride | 3.00 | 3.00 | 3.00 |
| DL-Methionine | 1.50 | 0.80 | 0.80 |
| Calculated nutrient contents | |||
| Metabolizable energy (MJ/kg) | 12.4 | 13.0 | 13.1 |
| Crude protein (g/kg) | 218 | 197 | 177 |
| Lysine (g/kg) | 12.3 | 11.0 | 10.3 |
| Calcium (g/kg) | 10.0 | 9.6 | 9.5 |
| Total sulfur amino acids (g/kg) | 8.70 | 7.50 | 6.80 |
| Methionine (g/kg) | 5.10 | 4.20 | 3.80 |
| Available phosphorus (g/kg) | 4.60 | 3.90 | 3.90 |
| Genes 1 | GeneBank ID | Primer Sequence, Sense/Antisense | Product Size (bp) |
|---|---|---|---|
| EPAS1 | NM_204807.2 | TTGACGATGAGCAGTGCCTTTGAG | 116 |
| CCAGGTGTTGGAGCCAGTTGTG | |||
| RPS15 | NM_205462.1 | ACAACGGCAAGACCTTCAACCAG | 86 |
| CGGCTTGTAGGTGATGGAGAACTC | |||
| BDH1 | NM_001006547.2 | GGGTCGTGTAGTGAACATCAGTAGC | 111 |
| TACCGCAGGCAGTCAGAGAAGG | |||
| MPST | NM_001277377.1 | CTGAAGAACTGGCTGCGAGAAGG | 150 |
| CACGACTTGGAAGCGATGGGAATC | |||
| TST | NM_001167731.1 | GATGGCTCCTGGTCTGAATGGTTC | 110 |
| GGCTACAGATACGCTAAGGGACAAC | |||
| ALDH1A1 | NM_204577.4 | TGGATTGACATGGAGGTGAGAGAGG | 100 |
| AGCCATTGCACGTACCACTCATTC | |||
| SERPINH1 | NM_205291.1 | GCCGAGAGGAGATGAGGAACCC | 106 |
| ACGAGCCTGCCAATGAAGAGAATG | |||
| PRPSAP2 | NM_001006165.1 | CATGGTCTGCTATCTTCGGATGCTC | 103 |
| ACTGGAGTTTCTGGATTTCGTGTGG | |||
| β-actin | NM_205518 | TGCTGTGTTCCCATCTATCG | 150 |
| TTGGTGACAATACCGTGTTCA | |||
| GAPDH | NM_204305 | AGAACATCATCCCAGCGTCC | 133 |
| CGGCAGGTCAGGTCAACAAC |
| Items 1 | Control | Betaine | p-Value |
|---|---|---|---|
| ADG (g) | 37.15 ± 0.38 | 38.51 ± 0.39 * | 0.025 |
| ADFI (g) | 81.58 ± 0.76 | 82.35 ± 0.87 | 0.509 |
| FCR (g/g) | 2.20 ± 0.02 | 2.14 ± 0.02 * | 0.040 |
| Items 1 | Control | Betaine | p-Value |
|---|---|---|---|
| GH (ng/mL) | 8.77 ± 0.52 | 9.70 ± 0.91 * | 0.024 |
| T3 (ng/mL) | 8.42 ± 0.26 | 9.15 ± 0.23 | 0.053 |
| T4 (ng/mL) | 91.04 ± 3.08 | 93.71 ± 5.91 | 0.277 |
| INS (U/mL) | 43.00 ± 1.64 | 44.61 ± 2.06 | 0.550 |
| IGF-1 (ng/mL) | 345.53 ± 6.50 | 368.94 ± 5.54 * | 0.016 |
| TP (g/L) | 41.46 ± 1.12 | 44.62 ± 0.71 * | 0.032 |
| ALB (g/L) | 14.05 ± 0.95 | 14.86 ± 0.62 | 0.061 |
| GLB (g/L) | 27.41 ± 1.22 | 29.75 ± 0.56 | 0.111 |
| GLU (mmol/L) | 13.44 ± 0.20 | 13.60 ± 0.29 | 0.649 |
| BUN (mmol/L) | 0.97 ± 0.05 | 0.75 ± 0.05 * | 0.013 |
| NH3 (μmol/L) | 173.03 ± 4.55 | 166.48 ± 4.90 | 0.344 |
| Items | Control | Betaine | p-Value |
|---|---|---|---|
| Absolute protein deposition (g) | 60.38 ± 1.96 | 68.07 ± 2.03 * | 0.016 |
| Relative protein deposition (g/kg BW) | 27.47 ± 0.47 | 29.49 ± 0.83 | 0.053 |
| RNA (ng/mg) | 1084.78 ± 24.43 | 1198.22 ± 34.87 * | 0.019 |
| DNA (ng/mg) | 722.54 ± 12.56 | 744.33 ± 13.73 | 0.261 |
| RNA/DNA | 1.50 ± 0.03 | 1.61 ± 0.04 * | 0.036 |
| Items | Control | Betaine | p-Value |
|---|---|---|---|
| Asp | 22.79 ± 0.29 | 23.72 ± 0.46 | 0.111 |
| Thr | 11.20 ± 0.18 | 11.59 ± 0.17 | 0.135 |
| Ser | 9.43 ± 0.14 | 10.06 ± 0.14 * | 0.008 |
| Glu | 36.08 ± 0.34 | 38.31 ± 0.67 * | 0.010 |
| Gly | 10.78 ± 0.12 | 11.25 ± 0.19 | 0.053 |
| Ala | 14.63 ± 0.21 | 15.19 ± 0.22 | 0.087 |
| Cys | 2.17 ± 0.14 | 2.12 ± 0.13 | 0.796 |
| Val | 12.72 ± 0.19 | 13.27 ± 0.30 | 0.140 |
| Met | 6.31 ± 0.19 | 6.91 ± 0.17 * | 0.036 |
| Ile | 11.54 ± 0.15 | 12.11 ± 0.27 | 0.082 |
| Leu | 20.31 ± 0.14 | 20.91 ± 0.24 | 0.053 |
| Tyr | 8.74 ± 0.17 | 9.10 ± 0.16 | 0.138 |
| Phe | 10.34 ± 0.11 | 10.83 ± 0.21 * | 0.047 |
| Lys | 22.01 ± 0.30 | 22.79 ± 0.23 | 0.057 |
| His | 9.43 ± 0.21 | 9.85 ± 0.21 | 0.179 |
| Arg | 15.56 ± 0.16 | 15.93 ± 0.18 | 0.154 |
| Pro | 6.85 ± 0.10 | 7.08 ± 0.13 | 0.177 |
| Accession | Protein Name | Gene Name | Fold Change | p-Value |
|---|---|---|---|---|
| Q5ZL59 | UBC core domain-containing protein | UBE2D3 | 1.247 | 0.017 |
| Q5ZKN8 | Transaldolase | RCJMB04_9n21 | 1.368 | 0.035 |
| A0A3Q2UHT5 | S-AdoMet_synt_C domain-containing protein | N/A | 1.384 | 0.012 |
| A0A1D5NVY0 | Uncharacterized protein | USMG5 | 1.458 | 0.007 |
| A0A1D5NVW6 | Myosin heavy chain 1G, skeletal muscle | MYH1G | 1.499 | 0.027 |
| P09540 | Myosin light chain, embryonic | N/A | 2.074 | 0.036 |
| Q5ZJZ5 | D-beta-hydroxybutyrate dehydrogenase, mitochondrial | BDH1 | 1.228 | 0.030 |
| A0A1D5PY54 | Uncharacterized protein | LANCL2 | 1.239 | 0.009 |
| Q5ZHQ4 | Thiolase_N domain-containing protein | RCJMB04_34i5 | 1.308 | 0.001 |
| Q800K9 | Surfeit locus protein 4 | SURF4 | 1.275 | 0.036 |
| A0A1D5PKN8 | Uncharacterized protein | MPST | 1.315 | 0.047 |
| Q5ZL61 | OBG-type G domain-containing protein | RCJMB04_7i14 | 1.385 | 0.012 |
| A0A218NER6 | Endothelial PAS domain protein 1 | EPAS1 | 1.327 | 0.040 |
| A0A3Q2TYL5 | Uncharacterized protein | N/A | 1.206 | 0.044 |
| A0A1L1RNY8 | Histone H2A | H2AFX | 1.337 | 0.046 |
| A0A3Q2U578 | Histone H3 | N/A | 1.205 | 0.019 |
| A0A1D5NY17 | Transmembrane protein 182 | TMEM182 | 1.223 | 0.033 |
| P27463 | Retinal dehydrogenase 1 | ALDH1A1 | 1.502 | 0.050 |
| P25324 | Thiosulfate sulfurtransferase | TST | 1.259 | 0.017 |
| Q90579 | Anion exchange protein | N/A | 1.649 | 0.016 |
| Q5F3G6 | PHD finger protein 20-like protein 1 | PHF20L1 | 1.538 | 0.007 |
| Q8QGU2 | Peptidylprolyl isomerase | FKBP12.6 | 1.256 | 0.048 |
| A0A3Q2U8Y0 | Uncharacterized protein | LOC107050760 | 1.313 | 0.030 |
| A0A1D5PUQ7 | Uncharacterized protein | PFKM | 1.516 | 0.030 |
| P62846 | 40S ribosomal protein S15 | RPS15 | 1.577 | 0.044 |
| Q5ZJ61 | Phenylalanyl-tRNA synthetase beta subunit | FARSB | 1.253 | 0.010 |
| Q5ZK08 | Asparagine-tRNA ligase | RCJMB04_13p14 | 1.224 | 0.041 |
| A0A3Q2U3Y3 | Calponin | CNN1 | 0.653 | 0.019 |
| P19966 | Transgelin | TAGLN | 0.810 | 0.042 |
| P27731 | Transthyretin | TTR | 0.820 | 0.019 |
| Q5ZL26 | Phosphoribosyl pyrophosphate synthase-associated protein 2 | PRPSAP2 | 0.815 | 0.026 |
| E1C4M0 | 40S ribosomal protein S2 | RPS2 | 0.809 | 0.004 |
| P13731 | Serpin H1 | SERPINH1 | 0.790 | 0.011 |
| A0A1D5PUM7 | Uncharacterized protein | IGFN1 | 0.813 | 0.016 |
| Q5ZL90 | Phosducin-domain-containing protein | RCJMB04_7d1 | 0.809 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Song, Y.; Yang, M.; Wen, C.; Liu, Q.; Zhuang, S.; Zhou, Y. Effect of Dietary Betaine on Muscle Protein Deposition, Nucleic Acid and Amino Acid Contents, and Proteomes of Broilers. Animals 2022, 12, 736. https://doi.org/10.3390/ani12060736
Chen R, Song Y, Yang M, Wen C, Liu Q, Zhuang S, Zhou Y. Effect of Dietary Betaine on Muscle Protein Deposition, Nucleic Acid and Amino Acid Contents, and Proteomes of Broilers. Animals. 2022; 12(6):736. https://doi.org/10.3390/ani12060736
Chicago/Turabian StyleChen, Rui, Yuduo Song, Mi Yang, Chao Wen, Qiang Liu, Su Zhuang, and Yanmin Zhou. 2022. "Effect of Dietary Betaine on Muscle Protein Deposition, Nucleic Acid and Amino Acid Contents, and Proteomes of Broilers" Animals 12, no. 6: 736. https://doi.org/10.3390/ani12060736
APA StyleChen, R., Song, Y., Yang, M., Wen, C., Liu, Q., Zhuang, S., & Zhou, Y. (2022). Effect of Dietary Betaine on Muscle Protein Deposition, Nucleic Acid and Amino Acid Contents, and Proteomes of Broilers. Animals, 12(6), 736. https://doi.org/10.3390/ani12060736






