Structuring Broiler Barns: How a Perforated Flooring System Affects Animal Behavior
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Housing
2.2. Behavioral Observations
2.2.1. Use of the Perforated Floor
2.2.2. Focal Animal Observations
2.3. Statistical Analyses
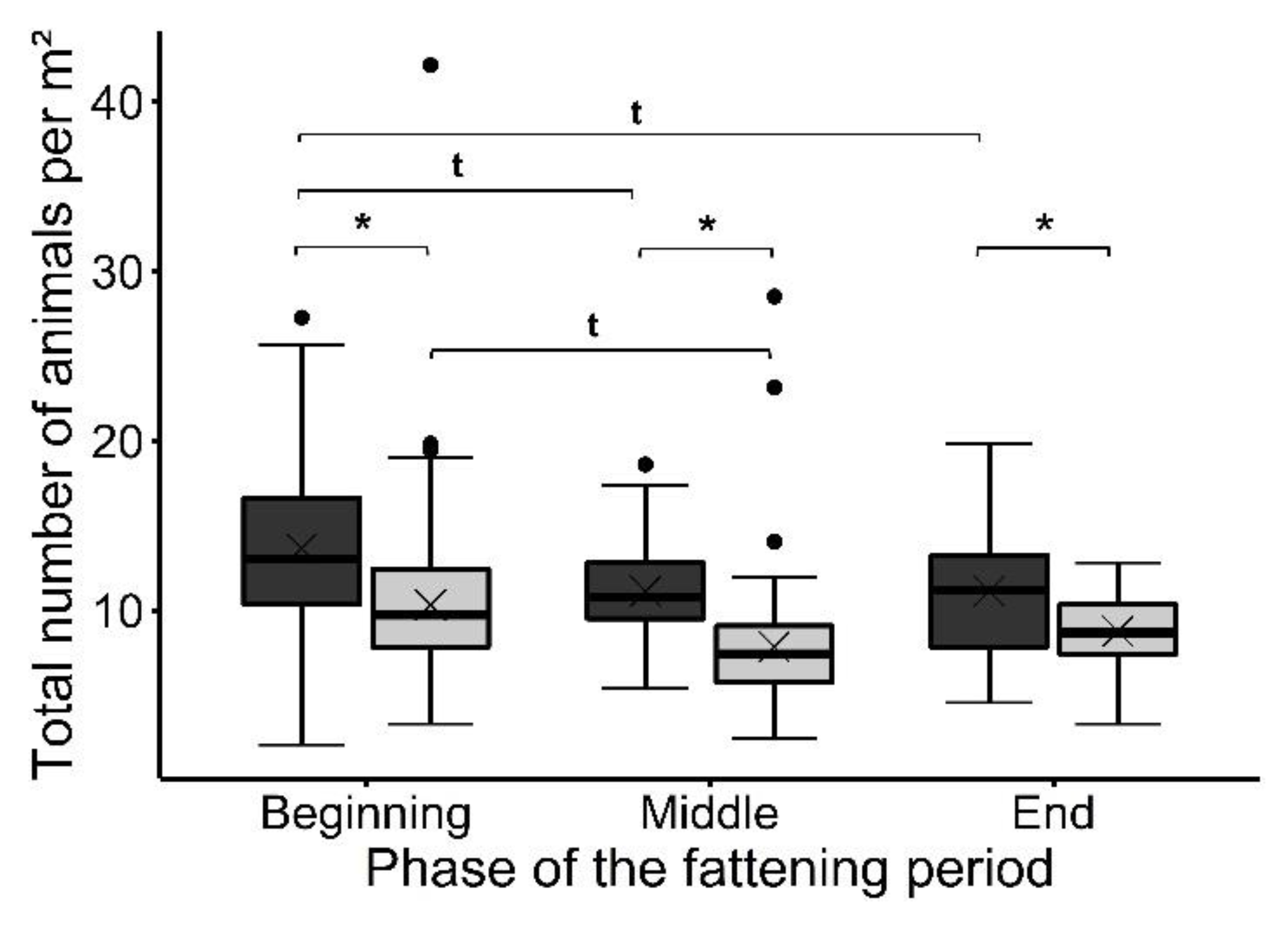
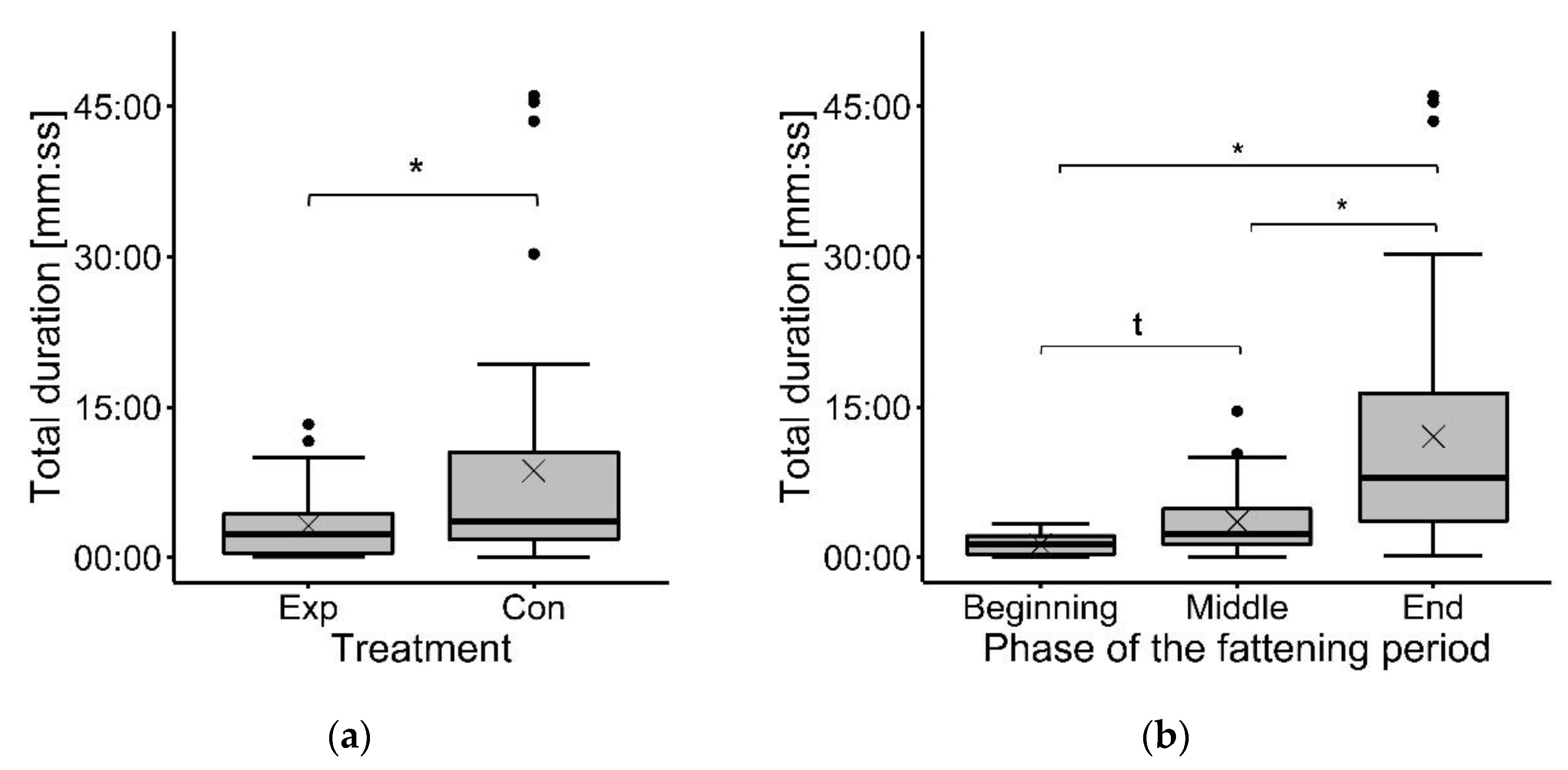
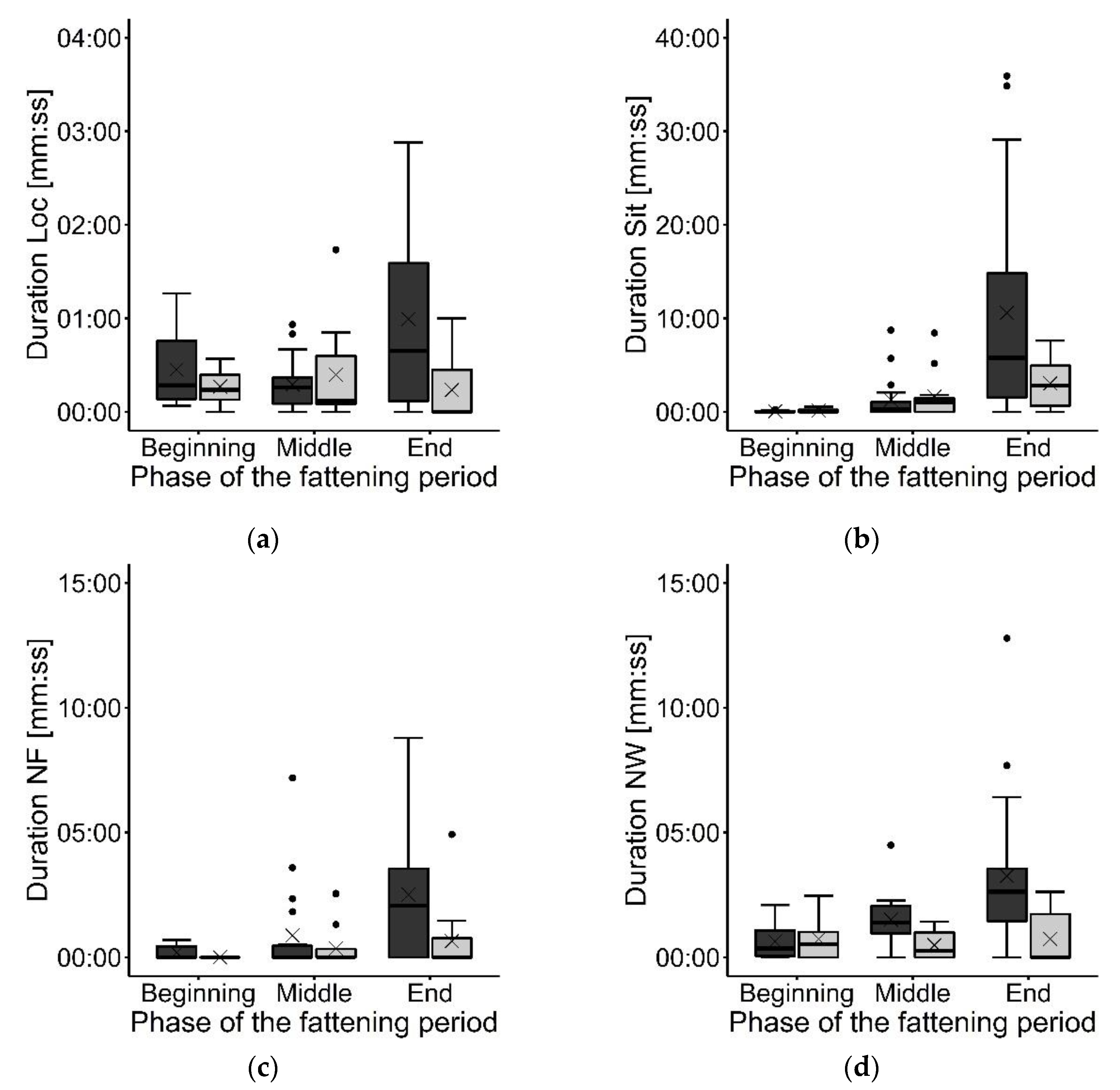
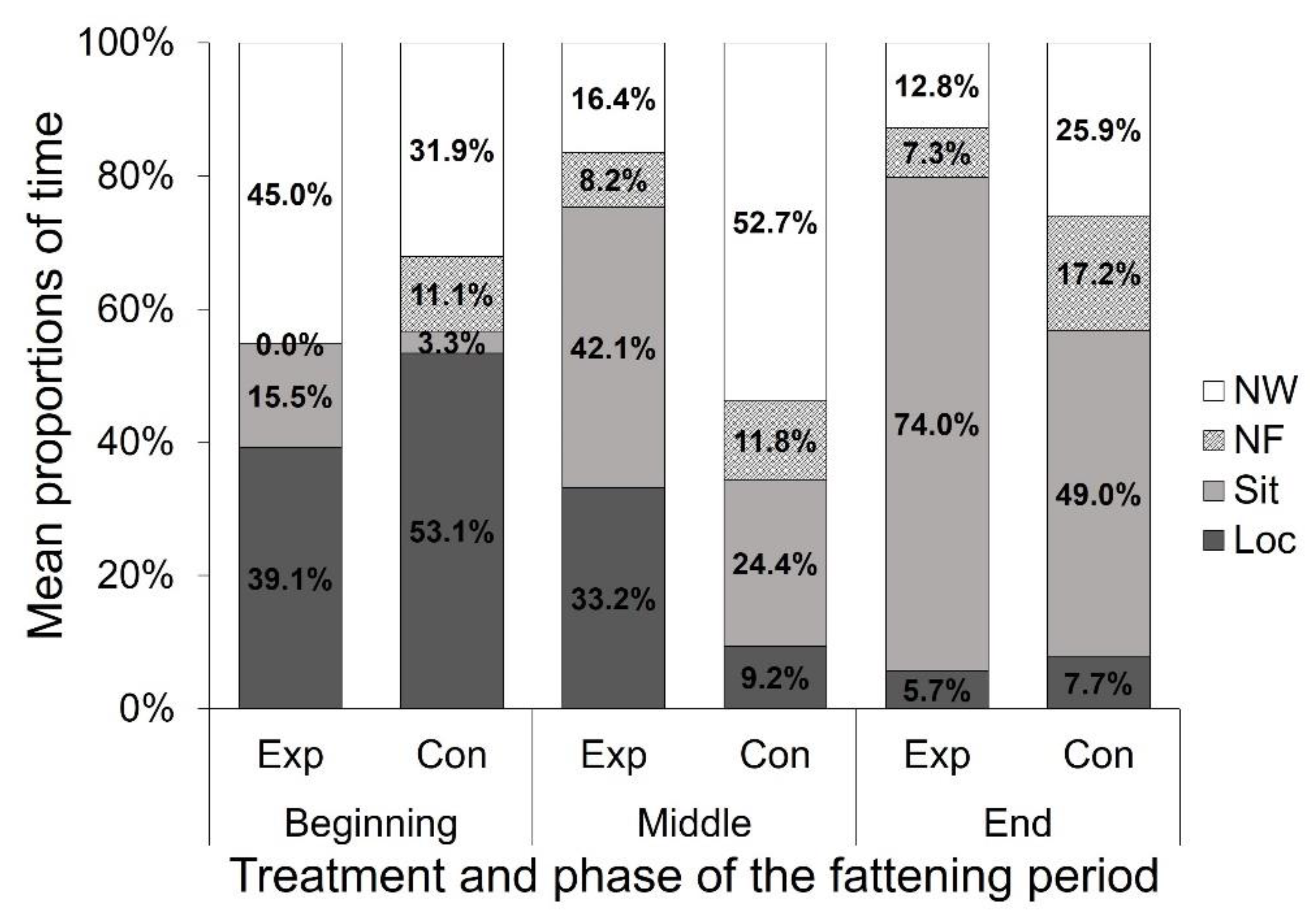
3. Results
3.1. Use of the Perforated Floor
3.2. Focal Animal Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission, Committee on Animal Health and Animal Welfare. The Welfare of Chickens Kept for Meat Production (Broilers). 2000. Available online: https://ec.europa.eu/food/horizontal-topics/expert-groups/scientific-committees/scientific-committee-animal-health-and-animal_en (accessed on 10 January 2022).
- Olsson, I.A.S.; Keeling, L.J. Night-time roosting in laying hens and the effect of thwarting access to perches. Appl. Anim. Behav. Sci. 2000, 68, 243–256. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Bizeray, D.; Estevez, I.; Leterrier, C.; Faure, J.M. Effects of increasing environmental complexity on the physical activity of broiler chickens. Appl. Anim. Behav. Sci. 2002, 79, 27–41. [Google Scholar] [CrossRef]
- Malchow, J.; Puppe, B.; Berk, J.; Schrader, L. Effects of elevated grids on growing male chickens differing in growth performance. Front. Vet. Sci. 2019, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, E.; Norring, M.; Valros, A. Perches and elevated platforms in commercial broiler farms: Use and effect on walking ability, incidence of tibial dyschondroplasia and bone mineral content. Animal 2017, 11, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Cavusoglu, E.; Petek, M. Effects of different floor materials on the welfare and behaviour of slow- and fast-growing broilers. Arch. Anim. Breed. 2019, 62, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Adler, C.; Tiemann, I.; Hillemacher, S.; Schmithausen, A.J.; Muller, U.; Heitmann, S.; Spindler, B.; Kemper, N.; Buscher, W. Effects of a partially perforated flooring system on animal-based welfare indicators in broiler housing. Poult. Sci. 2020, 99, 3343–3354. [Google Scholar] [CrossRef]
- Topal, E.; Petek, M. Effects of fully or partially slatted flooring designs on the performance, welfare and carcass characteristics of broiler chickens. Br. Poult. Sci. 2021, 62, 804–809. [Google Scholar] [CrossRef]
- Gebhardt-Henrich, S.G.; Stratmann, A.; Dawkins, M.S. Groups and individuals: Optical flow patterns of broiler chicken flocks are correlated with the behavior of individual birds. Animals 2021, 11, 568. [Google Scholar] [CrossRef]
- The Better Life Label. Available online: https://beterleven.dierenbescherming.nl/zakelijk/en/participate-2/company-type/livestock-farms/broilers/ (accessed on 10 January 2022).
- Wood-Gush, D.; Duncan, I. Some behavioural observations on domestic fowl in the wild. Appl. Anim. Ethol. 1976, 2, 255–260. [Google Scholar] [CrossRef]
- Norring, M.; Kaukonen, E.; Valros, A. The use of perches and platforms by broiler chickens. Appl. Anim. Behav. Sci. 2016, 184, 91–96. [Google Scholar] [CrossRef]
- Bailie, C.L.; Baxter, M.; O’Connell, N.E. Exploring perch provision options for commercial broiler chickens. Appl. Anim. Behav. Sci. 2018, 200, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Malchow, J.; Berk, J.; Puppe, B.; Schrader, L. Perches or grids? What do rearing chickens differing in growth performance prefer for roosting? Poult. Sci. 2019, 98, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.C.; Estevez, I.; Keeling, L.J. Group size and perching behaviour in young domestic fowl. Appl. Anim. Behav. Sci. 2001, 73, 117–129. [Google Scholar] [CrossRef]
- Tahamtani, F.M.; Pedersen, I.J.; Riber, A.B. Effects of environmental complexity on welfare indicators of fast-growing broiler chickens. Poult. Sci. 2020, 99, 21–29. [Google Scholar] [CrossRef]
- Heitmann, S.; Stracke, J.; Adler, C.; Ahmed, M.F.E.; Schulz, J.; Buscher, W.; Kemper, N.; Spindler, B. Effects of a slatted floor on bacteria and physical parameters in litter in broiler houses. Vet. Anim. Sci 2020, 9, 100115. [Google Scholar] [CrossRef]
- Chuppava, B.; Visscher, C.; Kamphues, J. Effect of different flooring designs on the performance and foot pad health in broilers and turkeys. Animals 2018, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Adler, C.; Schmithausen, A.J.; Trimborn, M.; Heitmann, S.; Spindler, B.; Tiemann, I.; Kemper, N.; Buscher, W. Effects of a partially perforated flooring system on ammonia amissions in broiler housing-conflict of objectives between animal welfare and environment? Animals 2021, 11, 707. [Google Scholar] [CrossRef]
- Chuppava, B.; Keller, B.; Abd El-Wahab, A.; Surie, C.; Visscher, C. Resistance reservoirs and multi-drug resistance of commensal escherichia coli from excreta and manure isolated in broiler houses with different flooring designs. Front. Microbiol. 2019, 10, 2633. [Google Scholar] [CrossRef] [Green Version]
- Aviagen. Ross 308 Broiler. Management Handbook . 2018. Available online: http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 24 October 2019).
- Baxter, M.; Bailie, C.L.; O’Connell, N.E. An evaluation of potential dustbathing substrates for commercial broiler chickens. Animal 2018, 12, 1933–1941. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.F.; Krippendorff, K. Answering the call for a standard reliability measure for coding data. Communication Methods and Measures 2007, 1, 77–89. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forslind, S.; Blokhuis, H.J.; Riber, A.B. Disturbance of resting behaviour of broilers under different environmental conditions. Appl. Anim. Behav. Sci. 2021, 242, 105425. [Google Scholar] [CrossRef]
- Yngvesson, J.; Wedin, M.; Gunnarsson, S.; Jönsson, L.; Blokhuis, H.; Wallenbeck, A. Let me sleep! Welfare of broilers (Gallus gallus domesticus) with disrupted resting behaviour. Acta Agric. Scand. Sect. A—Anim. Sci. 2018, 67, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Schwean-Lardner, K.; Fancher, B.I.; Laarveld, B.; Classen, H.L. Effect of day length on flock behavioural patterns and melatonin rhythms in broilers. Br. Poult. Sci. 2014, 55, 21–30. [Google Scholar] [CrossRef]
- Bessei, W. Das Verhalten von Broilern unter intensiven Haltungsbedingungen. Arch. Geflügelk. 1992, 56, 1–7. [Google Scholar]
- Bizeray, D.; Leterrier, C.; Constantin, P.; Picard, M.; Faure, J.M. Early locomotor behaviour in genetic stocks of chickens with different growth rates. Appl. Anim. Behav. Sci. 2000, 68, 231–242. [Google Scholar] [CrossRef]
- Ventura, B.A.; Siewerdt, F.; Estevez, I. Access to barrier perches improves behavior repertoire in broilers. PLoS ONE 2012, 7, e29826. [Google Scholar] [CrossRef] [Green Version]
- Weeks, C.A.; Danbury, T.D.; Davies, H.C.; Hunt, P.; Kestin, S.C. The behaviour of broiler chickens and its modification by lameness. Appl. Anim. Behav. Sci. 2000, 67, 111–125. [Google Scholar] [CrossRef]
- Caplen, G.; Colborne, G.R.; Hothersall, B.; Nicol, C.J.; Waterman-Pearson, A.E.; Weeks, C.A.; Murrell, J.C. Lame broiler chickens respond to non-steroidal anti-inflammatory drugs with objective changes in gait function: A controlled clinical trial. Vet. J. 2013, 196, 477–482. [Google Scholar] [CrossRef]
- Giersberg, M.F.; Hartung, J.; Kemper, N.; Spindler, B. Floor space covered by broiler chickens kept at stocking densities according to Council Directive 2007/43/EC. Vet. Rec. 2016, 179, 124. [Google Scholar] [CrossRef] [PubMed]


| Method | Behavior/Location | Description |
|---|---|---|
| Scan sampling and focal animals | Located near the feeding trough (NF) | The bird is located with more than a half of its body within a radius of one animal’s length around the feeding trough and is holding its head in the direction of the feeding trough (regardless of whether upright position or sitting) |
| Scan sampling and focal animals | Located near the waterline (NW) | The bird is located with more than a half of its body within a radius of one animal’s length around the water line and is holding its head in the direction of a nipple (regardless of whether upright position or sitting) |
| Scan sampling | Other (Oth) | All animals not ‘located near the feeding trough’ or ‘located near the waterline’ |
| Focal animals | Locomotion 1 (Loc) | The bird is standing or walking (upright position) and is not ‘located near the feeding trough’ or ‘located near the waterline’ |
| Focal animals | Sitting inactive1 (Sit) | The bird is resting (sitting with head under the wing or resting on the ground) or lying (the bird is lying on one side with a leg and/or wing stretched out) and is not ‘located near the feeding trough’ or ‘located near the waterline’ |
| Parameter | Krippendorff’s Alpha Intra-Observer | Krippendorff’s Alpha Inter-Observer | |
|---|---|---|---|
| Scan sampling: | Oth | 0.84 | 0.42 |
| NF | 0.83 | 0.75 | |
| NW | 0.90 | 0.85 | |
| Focal animal observation: | 0.99 | 0.75 | |
| Location | Treatment | Phase of the Fattening Period | Treatment p | Phase p | Treatment × Phase | ||
|---|---|---|---|---|---|---|---|
| Beginning | Middle | End | p | ||||
| Oth | Exp | 47.29 (16.66) | 46.52 (12.24) | 36.86 (11.77) | <0.001 | <0.05 | <0.001 |
| Con | 38.51 (14.16) | 23.06 (10.89) | 21.01 (13.69) | ||||
| NF | Exp | 23.62 (12.71) | 26.12 (7.06) | 30.57 (10.46) | <0.001 | <0.1 | n.s. |
| Con | 28.37 (11.27) | 33.29 (10.20) | 37.56 (12.83) | ||||
| NW | Exp | 29.08 (11.63) | 27.36 (10.09) | 32.57 (9.47) | <0.001 | <0.05 | <0.001 |
| Con | 33.11 (11.59) | 43.65 (11.70) | 41.42 (12.62) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, F.; Stracke, J.; Heitmann, S.; Adler, C.; Krasny, A.; Kemper, N.; Spindler, B. Structuring Broiler Barns: How a Perforated Flooring System Affects Animal Behavior. Animals 2022, 12, 735. https://doi.org/10.3390/ani12060735
May F, Stracke J, Heitmann S, Adler C, Krasny A, Kemper N, Spindler B. Structuring Broiler Barns: How a Perforated Flooring System Affects Animal Behavior. Animals. 2022; 12(6):735. https://doi.org/10.3390/ani12060735
Chicago/Turabian StyleMay, Franziska, Jenny Stracke, Sophia Heitmann, Carolin Adler, Alica Krasny, Nicole Kemper, and Birgit Spindler. 2022. "Structuring Broiler Barns: How a Perforated Flooring System Affects Animal Behavior" Animals 12, no. 6: 735. https://doi.org/10.3390/ani12060735
APA StyleMay, F., Stracke, J., Heitmann, S., Adler, C., Krasny, A., Kemper, N., & Spindler, B. (2022). Structuring Broiler Barns: How a Perforated Flooring System Affects Animal Behavior. Animals, 12(6), 735. https://doi.org/10.3390/ani12060735









