Assessment of Chilling Injury in Boar Spermatozoa by Kinematic Patterns and Competitive Sperm-Oviduct Binding In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Semen Collection and Processing
2.3. CASA Assessment of Sperm Kinematics
2.4. Assessment of Plasma and Acrosomal Membrane Integrity
2.5. Sperm Binding in the cOEA
- E1, E2 = explant from sow no. 1, explant from sow no. 2
- Al1, Al2, Al3 = Area of location no. 1, 2, and 3, respectively
- Nl1, Nl2, Nl3 = number of spermatozoa at location no. 1, 2, and 3, respectively
- %spermBTS = percentage of bound sperm from the extender BTS
- E1 = explant from sow no. 1
- NBTS + NAndrostar® Plus = number of spermatozoa from BTS or Androstar® Plus
- E1, E2 = explant from sow no. 1, explant from sow no. 2
2.6. Statistical Analysis
3. Results
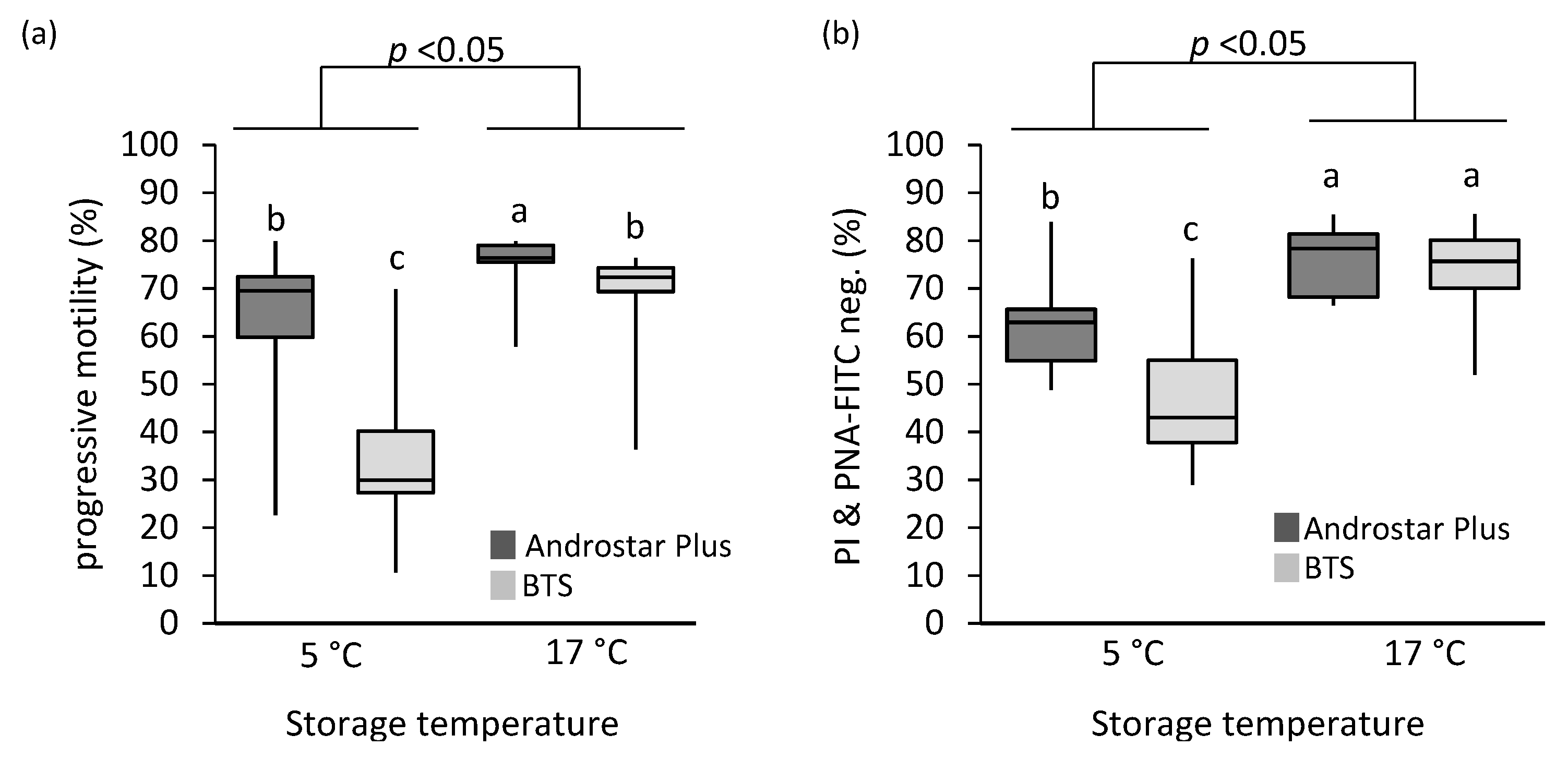
3.1. Sperm Kinematics
3.2. Plasma Membrane and Acrosome Integrity
3.3. Cluster Analysis
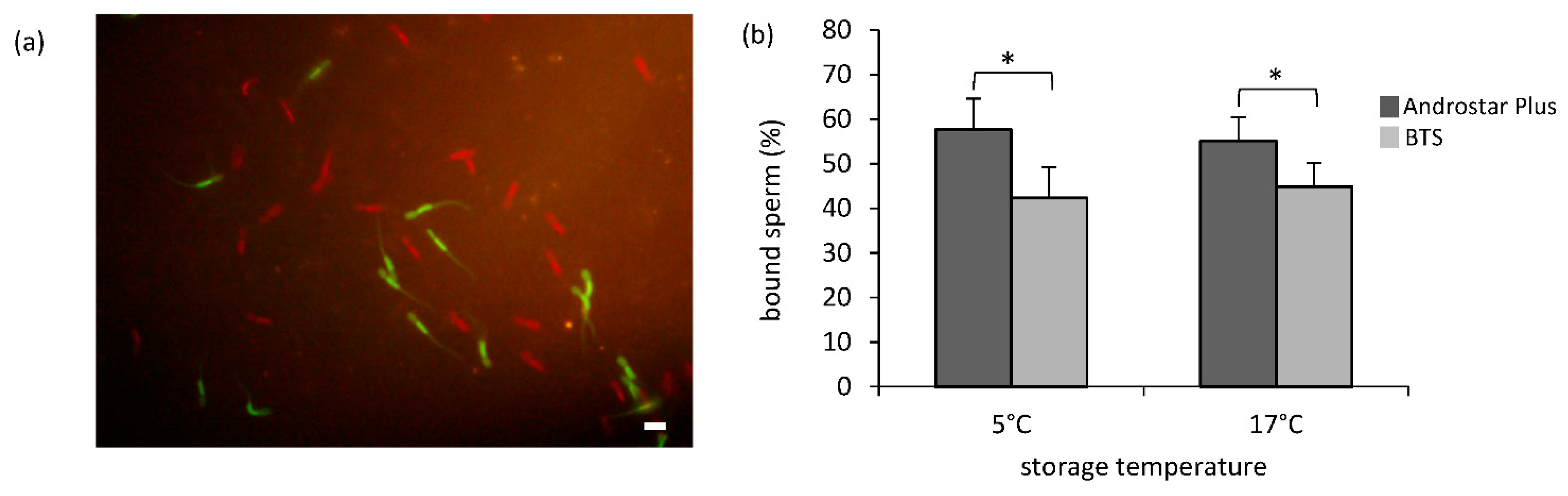
3.4. Sperm Binding to Oviduct Explants
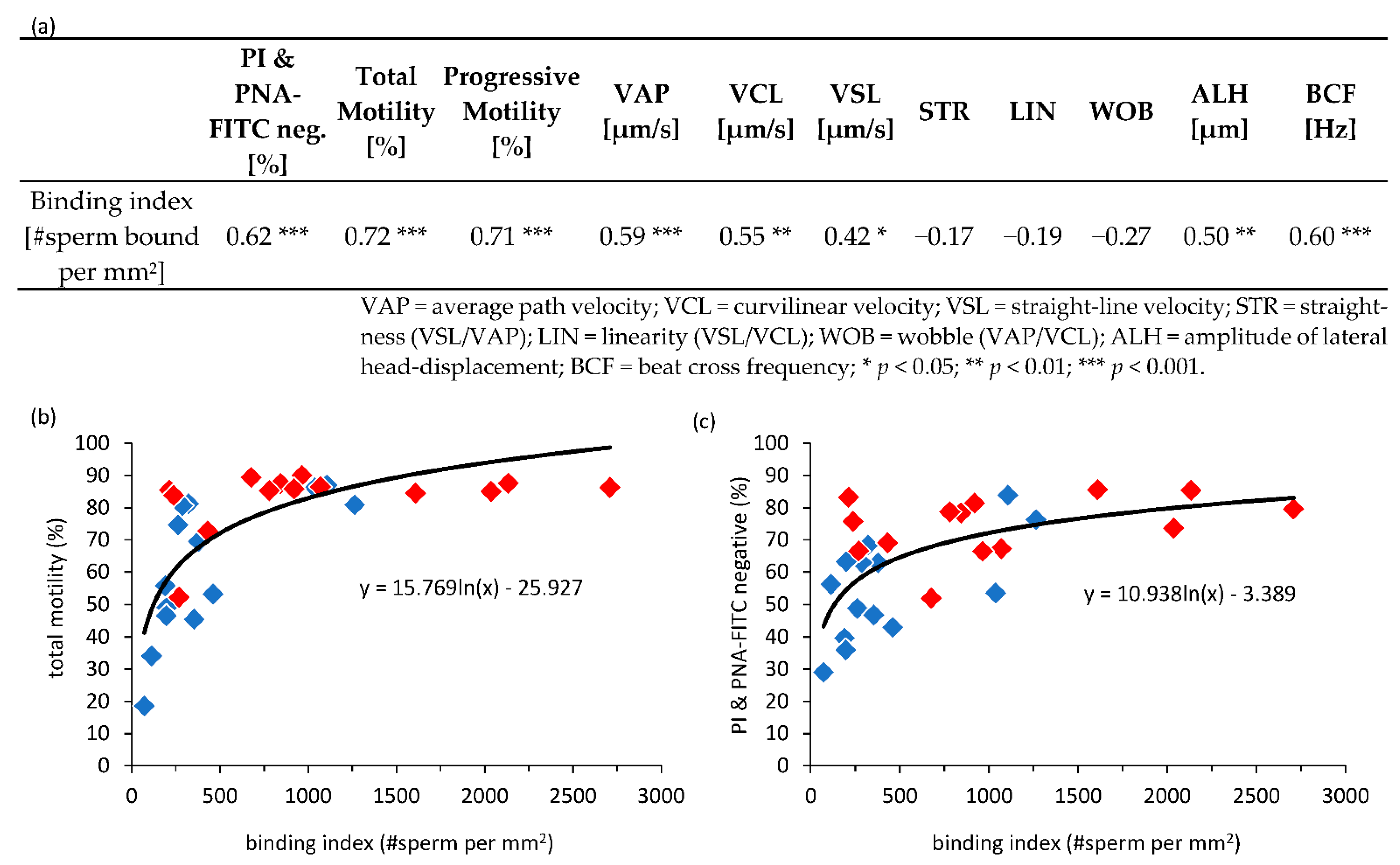
3.5. Correlation of Sperm Parameters and Number of Bound Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menezes, T.d.A.; Bustamante-Filho, I.C.; Paschoal, A.F.L.; Dalberto, P.F.; Bizarro, C.V.; Bernardi, M.L.; Da Ulguim, R.R.; Bortolozzo, F.P.; Mellagi, A.P.G. Differential seminal plasma proteome signatures of boars with high and low resistance to hypothermic semen preservation at 5 °C. Andrology 2020, 8, 1907–1922. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Luther, A.-M.; Grünther, B.; Jäkel, H.; Henning, H.; Vogel, C.; Peralta, W.; Weitze, K.F. Sperm function in vitro and fertility after antibiotic-free, hypothermic storage of liquid preserved boar semen. Sci. Rep. 2019, 9, 14748. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Watson, P.F. Comparison of the capacitation-like state of cooled boar spermatozoa with true capacitation. Reproduction 2001, 122, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Pursel, V.G.; Schulman, L.L.; Johnson, L.A. Effect of holding time on storage of boar spermatozoa at 5C. J. Anim. Sci. 1973, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Althouse, G.; Wilson, M.; Kuster, C.; Parsley, M. Characterization of lower temperature storage limitations of fresh-extended porcine semen. Theriogenology 1998, 50, 535–543. [Google Scholar] [CrossRef]
- Hunter, R.H.; Fléchon, B.; Fléchon, J.E. Pre- and peri-ovulatory distribution of viable spermatozoa in the pig oviduct: A scanning electron microscope study. Tissue Cell 1987, 19, 423–436. [Google Scholar] [CrossRef]
- Druart, X.; Cognié, J.; Baril, G.; Clément, F.; Dacheux, J.-L.; Gatti, J.-L. In vivo imaging of in situ motility of fresh and liquid stored ram spermatozoa in the ewe genital tract. Reproduction 2009, 138, 45–53. [Google Scholar] [CrossRef]
- Holt, C.; Holt, W.V.; Moore, H.D.; Reed, H.C.; Curnock, R.M. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: Results of two fertility trials. J. Androl. 1997, 18, 312–323. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.W.J.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef]
- van der Horst, G.; Maree, L.; Du Plessis, S.S. Current perspectives of CASA applications in diverse mammalian spermatozoa. Reprod. Fertil. Dev. 2018, 30, 875–888. [Google Scholar] [CrossRef]
- Ramón, M.; Martínez-Pastor, F. Implementation of novel statistical procedures and other advanced approaches to improve analysis of CASA data. Reprod. Fertil. Dev. 2018, 30, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pastor, F.; Garcia-Macias, V.; Alvarez, M.; Herraez, P.; Anel, L.; de Paz, P. Sperm subpopulations in Iberian red deer epididymal sperm and their changes through the cryopreservation process. Biol. Reprod. 2005, 72, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Dorado, J.; Gálvez, M.J.; Murabito, M.R.; Muñoz-Serrano, A.; Hidalgo, M. Identification of sperm subpopulations in canine ejaculates: Effects of cold storage and egg yolk concentration. Anim. Reprod. Sci. 2011, 127, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ramió, L.; Rivera, M.M.; Ramírez, A.; Concha, I.I.; Peña, A.; Rigau, T.; Rodríguez-Gil, J.E. Dynamics of motile-sperm subpopulation structure in boar ejaculates subjected to “in vitro” capacitation and further “in vitro” acrosome reaction. Theriogenology 2008, 69, 501–512. [Google Scholar] [CrossRef]
- Henning, H.; Petrunkina, A.M.; Harrison, R.A.P.; Waberski, D. Cluster analysis reveals a binary effect of storage on boar sperm motility function. Reprod. Fertil. Dev. 2014, 26, 623–632. [Google Scholar] [CrossRef]
- Silva, E.; Frost, D.; Li, L.; Bovin, N.; Miller, D.J. Lactadherin is a candidate oviduct Lewis X trisaccharide receptor on porcine spermatozoa. Andrology 2017, 5, 589–597. [Google Scholar] [CrossRef]
- Wagner, A.; Ekhlasi-Hundrieser, M.; Hettel, C.; Petrunkina, A.; Waberski, D.; Nimtz, M.; Töpfer-Petersen, E. Carbohydrate-based interactions of oviductal sperm reservoir formation-studies in the pig. Mol. Reprod. Dev. 2002, 61, 249–257. [Google Scholar] [CrossRef]
- Fazeli, A.; Duncan, A.E.; Watson, P.F.; Holt, W.V. Sperm-oviduct interaction: Induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol. Reprod. 1999, 60, 879–886. [Google Scholar] [CrossRef]
- Winters, R.A.; Hamilton, D.N.; Bhatnagar, A.S.; Fitzgerald, R.; Bovin, N.; Miller, D.J. Porcine sperm binding to oviduct cells and glycans as supplements to traditional laboratory semen analysis. J. Anim. Sci. 2018, 96, 5265–5275. [Google Scholar] [CrossRef]
- Waberski, D.; Magnus, F.; Mendonca Ferreira, F.; Petrunkina, A.M.; Weitze, K.F.; Töpfer-Petersen, E. Importance of sperm-binding assays for fertility prognosis of porcine spermatozoa. Theriogenology 2005, 63, 470–484. [Google Scholar] [CrossRef]
- Daigneault, B.W.; McNamara, K.A.; Purdy, P.H.; Krisher, R.L.; Knox, R.V.; Rodriguez-Zas, S.L.; Miller, D.J. Enhanced fertility prediction of cryopreserved boar spermatozoa using novel sperm function assessment. Andrology 2015, 3, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Henning, H.H.W.; Batz-Schott, J.; Grünther, B.; Le Thi, X.; Waberski, D. Fluorescent labelling of boar spermatozoa for quantitative studies on competitive sperm-oviduct binding. Reprod. Fertil. Dev. 2019, 31, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Vincent, R.; Nadeau, D. Adjustment of the osmolality of Percoll for the isopycnic separation of cells and cell organelles. Anal. Biochem. 1984, 141, 322–328. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Gehlhaar, R.; Drommer, W.; Waberski, D.; Töpfer-Petersen, E. Selective sperm binding to pig oviductal epithelium in vitro. Reproduction 2001, 121, 889–896. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013; ISBN 9781134742707. [Google Scholar]
- Casas, I.; Althouse, G.C. The protective effect of a 17 °C holding time on boar sperm plasma membrane fluidity after exposure to 5 °C. Cryobiology 2013, 66, 69–75. [Google Scholar] [CrossRef]
- Orrego, M.T.; Melian, S.I.; Montenegro, J.; Cimato, A.N.; Cisale, H.; Piehl, L.L. Boar sperm protein tyrosine phosphorylation in the presence of egg yolk soluble and low density lipoprotein fractions during cooling. Theriogenology 2019, 123, 151–158. [Google Scholar] [CrossRef]
- Schmid, S.; Henning, H.; Petrunkina, A.M.; Weitze, K.F.; Waberski, D. Response to capacitating stimuli indicates extender-related differences in boar sperm function. J. Anim. Sci. 2013, 91, 5018–5025. [Google Scholar] [CrossRef][Green Version]
- Wasilewska, K.; Fraser, L. Boar variability in sperm cryo-tolerance after cooling of semen in different long-term extenders at various temperatures. Anim. Reprod. Sci. 2017, 185, 161–173. [Google Scholar] [CrossRef]
- Fair, S.; Romero-Aguirregomezcorta, J. Implications of boar sperm kinematics and rheotaxis for fertility after preservation. Theriogenology 2019, 137, 15–22. [Google Scholar] [CrossRef]
- Valverde, A.; Madrigal, M.; Caldeira, C.; Bompart, D.; de Murga, J.N.; Arnau, S.; Soler, C. Effect of frame rate capture frequency on sperm kinematic parameters and subpopulation structure definition in boars, analysed with a CASA-Mot system. Reprod. Domest. Anim. 2019, 54, 167–175. [Google Scholar] [CrossRef]
- Menegat, M.B.; Mellagi, A.P.G.; Bortolin, R.C.; Menezes, T.A.; Vargas, A.R.; Bernardi, M.L.; Wentz, I.; Gelain, D.P.; Moreira, J.C.F.; Bortolozzo, F.P. Sperm quality and oxidative status as affected by homogenization of liquid-stored boar semen diluted in short- and long-term extenders. Anim. Reprod. Sci. 2017, 179, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Magnus, F.; Ardón, F.; Petrunkina, A.M.; Weitze, K.F.; Töpfer-Petersen, E. Binding of boar spermatozoa to oviductal epithelium in vitro in relation to sperm morphology and storage time. Reproduction 2006, 131, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Petrunkina, A.M.; Friedrich, J.; Drommer, W.; Bicker, G.; Waberski, D.; Töpfer-Petersen, E. Kinetic characterization of the changes in protein tyrosine phosphorylation of membranes, cytosolic Ca2+ concentration and viability in boar sperm populations selected by binding to oviductal epithelial cells. Reproduction 2001, 122, 469–480. [Google Scholar] [CrossRef]
- Luño, V.; López-Úbeda, R.; García-Vázquez, F.A.; Gil, L.; Matás, C. Boar sperm tyrosine phosphorylation patterns in the presence of oviductal epithelial cells: In vitro, ex vivo, and in vivo models. Reproduction 2013, 146, 315–324. [Google Scholar] [CrossRef]
- López-Úbeda, R.; García-Vázquez, F.A.; Gadea, J.; Matás, C. Oviductal epithelial cells selected boar sperm according to their functional characteristics. Asian J. Androl. 2017, 19, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Holt, W.V.; Fazeli, A. Sperm selection in the female mammalian reproductive tract. Focus on the oviduct: Hypotheses, mechanisms, and new opportunities. Theriogenology 2016, 85, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, K.E.; Hofmo, P.O.; Tverdal, A.; Miller, R.R. Within and between breed differences in freezing tolerance and plasma membrane fatty acid composition of boar sperm. Reproduction 2006, 131, 887–894. [Google Scholar] [CrossRef]
- Paschoal, A.F.L.; Luther, A.-M.; Jäkel, H.; Scheinpflug, K.; Mühldorfer, K.; Bortolozzo, P.F.; Waberski, D. Determination of a cooling-rate frame for antibiotic-free preservation of boar semen at 5 °C. PLoS ONE 2020, 15, e0234339. [Google Scholar] [CrossRef]
- Jäkel, H.; Scheinpflug, K.; Mühldorfer, K.; Gianluppi, R.; Lucca, M.S.; Mellagi, A.P.G.; Bortolozzo, F.P.; Waberski, D. In vitro performance and in vivo fertility of antibiotic-free preserved boar semen stored at 5 °C. J. Anim. Sci. Biotechnol. 2021, 12, 9. [Google Scholar] [CrossRef]
- Jäkel, H.; Henning, H.; Luther, A.-M.; Rohn, K.; Waberski, D. Assessment of chilling injury in hypothermic stored boar spermatozoa by multicolor flow cytometry. Cytom. A 2021, 99, 1033–1041. [Google Scholar] [CrossRef]

| BTS | Androstar® Plus | |||
|---|---|---|---|---|
| 5 °C | 17 °C | 5 °C | 17 °C | |
| total motility [%] * | 49.9 ± 18.4 a | 80.9 ± 12.7 b | 73.4 ± 18.4 c | 85.2 ± 5.6 d |
| progressive motility [%] * | 35.1 ± 18.5 a | 67.5 ± 14.0 b | 62.4 ± 19.0 b | 74.7 ± 7.7 c |
| VAP [µm/s] | 44.6 ± 12.6 a | 54.6 ± 7.1 b | 58.8 ± 7.7 b,c | 62.2 ± 5.6 c |
| VCL [µm/s] | 73.7 ± 28.4 a | 91.7 ± 15.6 b | 99.1 ± 20.1 b,c | 100.9 ± 15.9 c |
| VSL [µm/s] | 33.1 ± 7.6 a | 41.6 ± 5.6 b | 43.0 ± 5.6 b | 48.3 ± 3.8 c |
| STR | 0.75 ± 0.09 a, b | 0.76 ± 0.06 a | 0.73 ± 0.07 b | 0.77 ± 0.05 a,b |
| LIN | 0.49 ± 0.12 | 0.46 ± 0.07 | 0.44 ± 0.10 | 0.48 ± 0.08 |
| WOB | 0.62 ± 0.09 a | 0.60 ± 0.05 a,b | 0.60 ± 0.08 b | 0.62 ± 0.06 a,b |
| ALH [µm] | 1.78 ± 0.69 a | 2.11 ± 0.47 a,b | 2.22 ± 0.53 b | 2.21 ± 0.45 b |
| BCF [Hz] | 25.8 ± 6.3 a | 33.6 ± 3.0 b | 33.9 ± 3.8 b | 37.1 ± 2.2 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henning, H.; Franz, J.; Batz-Schott, J.; Le Thi, X.; Waberski, D. Assessment of Chilling Injury in Boar Spermatozoa by Kinematic Patterns and Competitive Sperm-Oviduct Binding In Vitro. Animals 2022, 12, 712. https://doi.org/10.3390/ani12060712
Henning H, Franz J, Batz-Schott J, Le Thi X, Waberski D. Assessment of Chilling Injury in Boar Spermatozoa by Kinematic Patterns and Competitive Sperm-Oviduct Binding In Vitro. Animals. 2022; 12(6):712. https://doi.org/10.3390/ani12060712
Chicago/Turabian StyleHenning, Heiko, Jennifer Franz, Julia Batz-Schott, Xuyen Le Thi, and Dagmar Waberski. 2022. "Assessment of Chilling Injury in Boar Spermatozoa by Kinematic Patterns and Competitive Sperm-Oviduct Binding In Vitro" Animals 12, no. 6: 712. https://doi.org/10.3390/ani12060712
APA StyleHenning, H., Franz, J., Batz-Schott, J., Le Thi, X., & Waberski, D. (2022). Assessment of Chilling Injury in Boar Spermatozoa by Kinematic Patterns and Competitive Sperm-Oviduct Binding In Vitro. Animals, 12(6), 712. https://doi.org/10.3390/ani12060712







