Staphylococcus aureus Induces Goat Endometrial Epithelial Cells Apoptosis via the Autophagy and Endoplasmic Reticulum Stress Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Regents and Antibodies
2.2. Bacterial Strains and Growth Conditions
2.3. Cells and Infection
2.4. Cytotoxicity Assay
2.5. Transmission Electron Microscopy (TEM)
2.6. Western Blot
2.7. Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
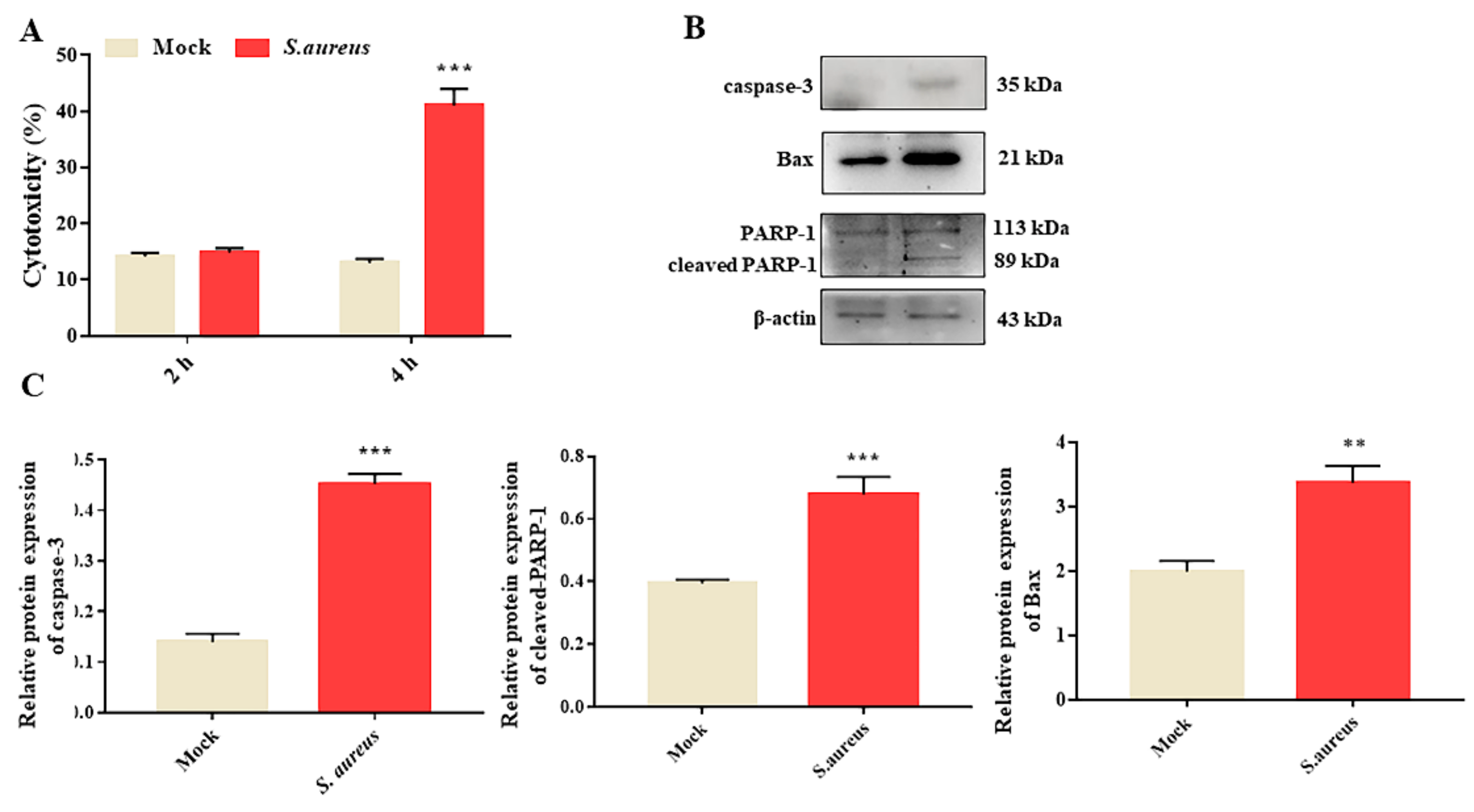
3.1. S. aureus Induces gEECs Apoptosis
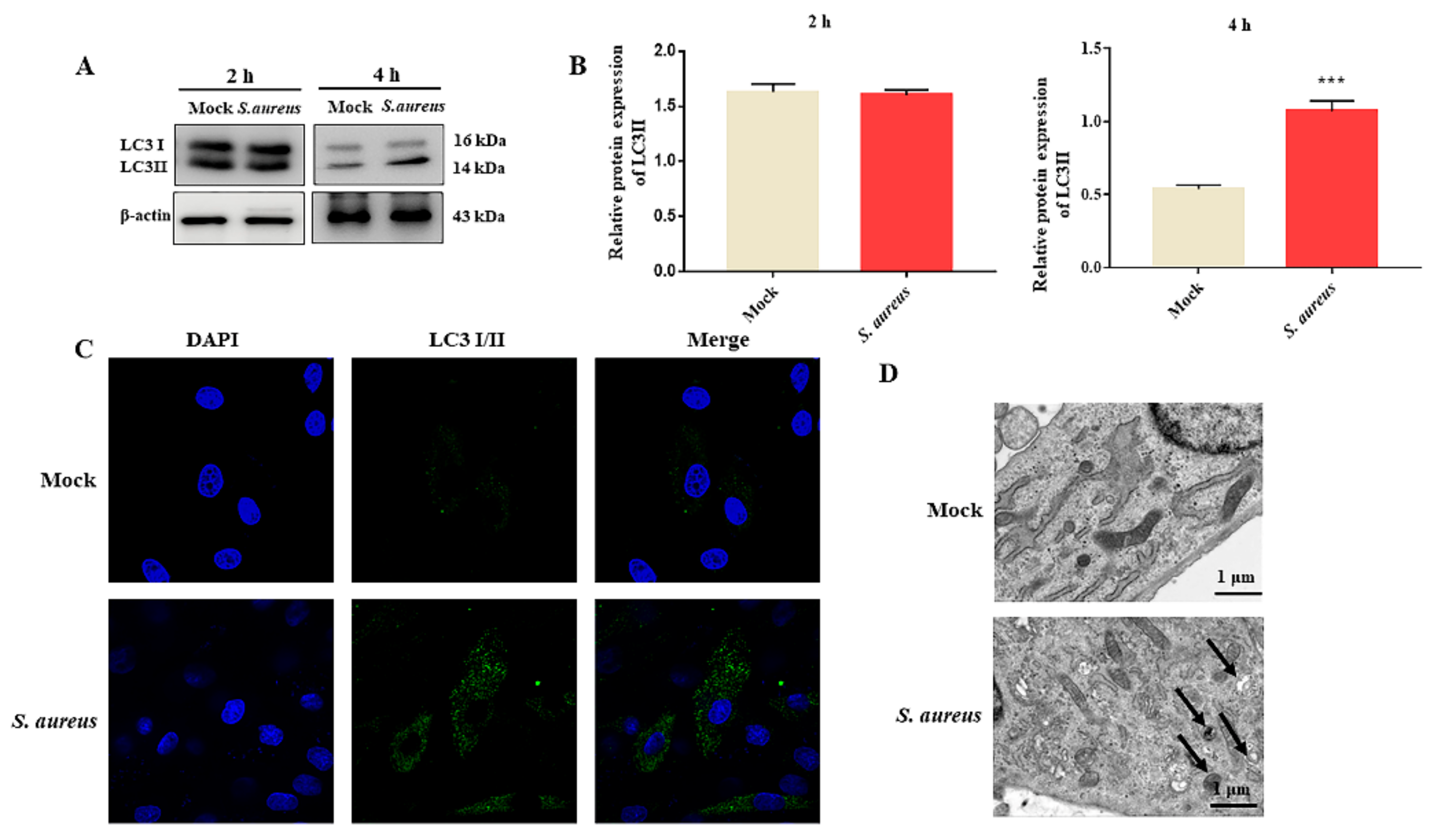
3.2. S. aureus Induces gEECs Autophagy
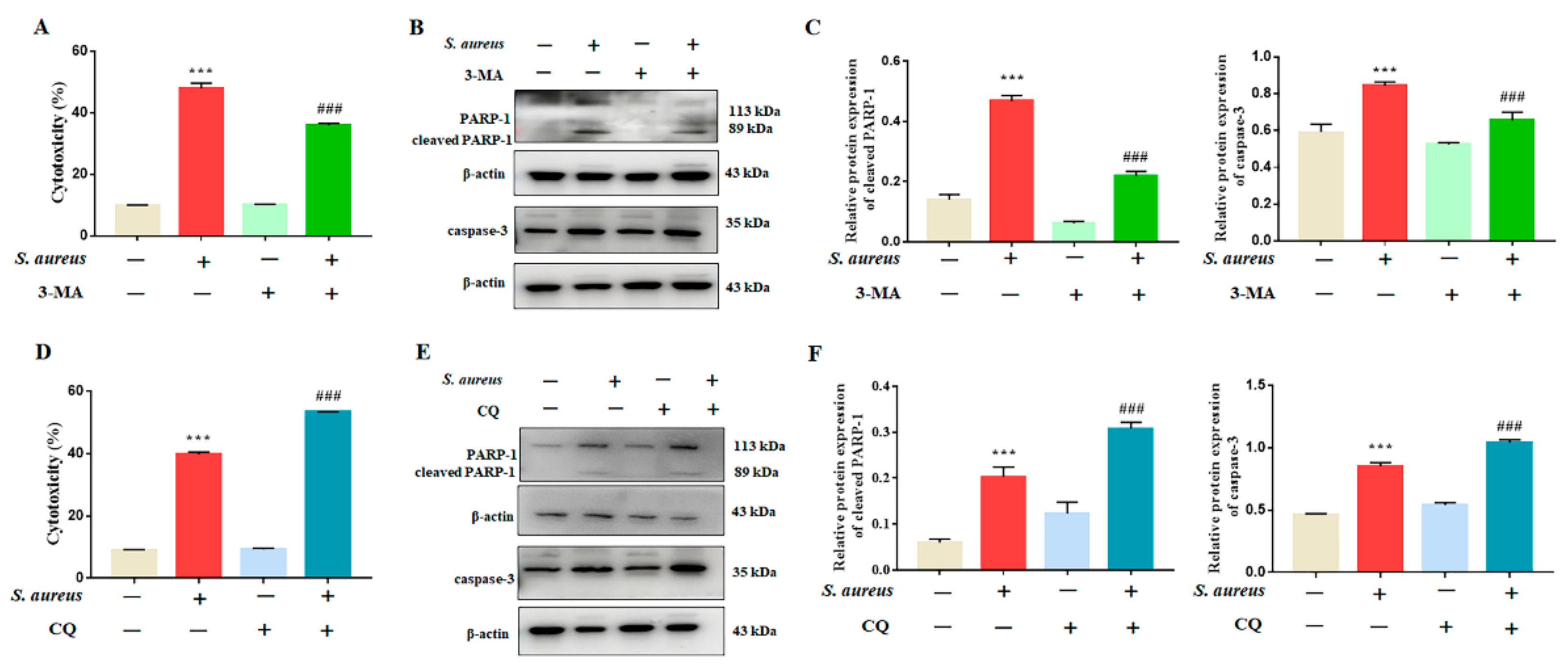
3.3. Inhibition of Autophagy Rescued S. aureus-Induced gEECs Apoptosis
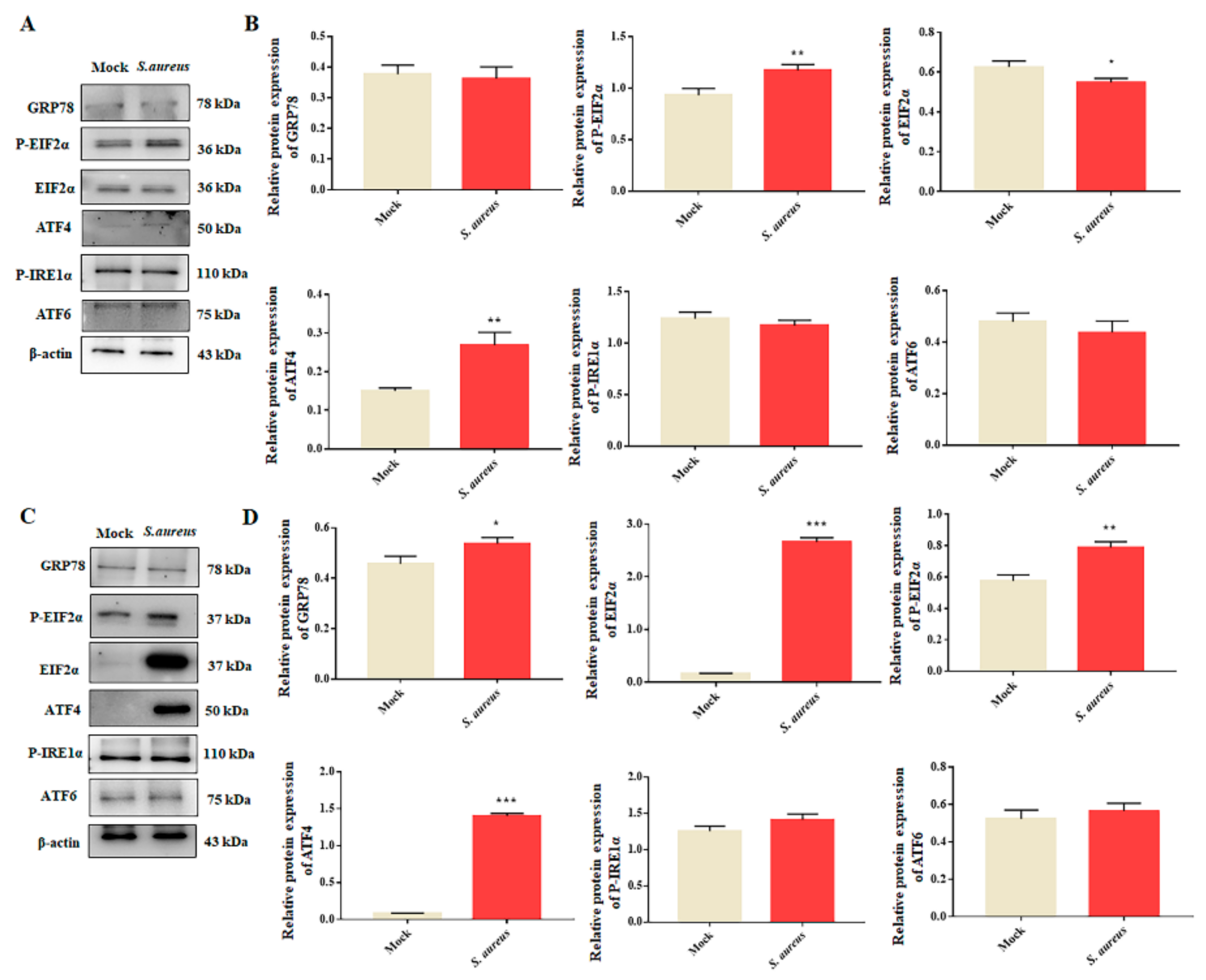
3.4. S. aureus Induces ER Stress in gEECs
3.5. Autophagy Rescues S. aureus-Induced gEECs Apoptosis via ER Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velázquez, M.M.L.; Peralta, M.B.; Angeli, E.; Stassi, A.F.; Gareis, N.C.; Durante, L.; Cainelli, S.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Immune status during postpartum, peri-implantation and early pregnancy in cattle: An updated view. Anim. Reprod. Sci. 2019, 206, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle. Annu. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Pei, Y.; Wang, X.; Feng, J.; Zhang, Y.; Gao, M.Q. LncRNA XIST mediates bovine mammary epithelial cell inflammatory response via NF-κB/NLRP3 inflammasome pathway. Cell Prolif. 2019, 52, e12525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Zhang, K.; Lu, K.; Shi, T.; Shen, S.; Chen, X.; Dong, J.; Gong, W.; Bao, Z.; Shi, Y.; et al. Inhibition of pyroptosis attenuates Staphylococcus aureus-induced bone injury in traumatic osteomyelitis. Ann. Transl. Med. 2019, 7, 170. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Rao, X. Apoptosis induced by Staphylococcus aureus toxins. Microbiol. Res. 2017, 205, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, Q.; Zhao, X.; Wang, H.; Li, N.; Fang, Y.; Wang, K.; Jia, Y.; Zhu, P.; Gu, J.; et al. Shiga toxins induce autophagic cell death in intestinal epithelial cells via the endoplasmic reticulum stress pathway. Autophagy 2015, 11, 344–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, P.; Xue, J.; Zhang, Z.; Jia, Y.; Tong, Y.; Han, D.; Li, Q.; Xiang, Y.; Mao, X.; Tang, B. Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell Death Dis. 2017, 8, 3207. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.A.; Yang, D.; Liu, S.; Lin, P.; Mohamad, O.A.A.; Jin, Y. Endoplasmic reticulum stress is involved in lipopolysaccharide-induced inflammatory response and apoptosis in goat endometrial stromal cells. Mol. Reprod. Dev. 2019, 86, 908–921. [Google Scholar] [CrossRef]
- Wang, X.; Lin, P.; Yin, Y.; Zhou, J.; Lei, L.; Zhou, X.; Jin, Y.; Wang, A. Brucella suis vaccine strain S2-infected immortalized caprine endometrial epithelial cell lines induce non-apoptotic ER-stress. Cell Stress Chaperones. 2015, 20, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.; Liu, J.; Jiang, Q.; Niu, H.; Wang, X.; Zhou, D.; Lin, P.; Chen, H.; Wang, A.; Jin, Y. Trueperella pyogenes pyolysin inhibits lipopolysaccharide-induced inflammatory response in endometrium stromal cells via autophagy- and ATF6-dependent mechanism. Braz. J. Microbiol. 2021, 52, 939–952. [Google Scholar] [CrossRef]
- Liang, S.; Wang, F.; Bao, C.; Han, J.; Guo, Y.; Liu, F.; Zhang, Y. BAG2 ameliorates endoplasmic reticulum stress-induced cell apoptosis in Mycobacterium tuberculosis-infected macrophages through selective autophagy. Autophagy 2020, 16, 1453–1467. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Leli, N.M.; Koumenis, C.; Amaravadi, R.K. Regulation of autophagy by canonical and non-canonical ER stress responses. Semin. Cancer Biol. 2020, 66, 116–128. [Google Scholar] [CrossRef]
- Rashid, H.O.; Yadav, R.K.; Kim, H.R.; Chae, H.J. ER stress: Autophagy induction, inhibition and selection. Autophagy 2015, 11, 1956–1977. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Guo, J.; Xu, M.; Jiang, P.; Yuan, X.; Zhao, C.; Maimai, T.; Cao, Y.; Zhang, N.; Fu, Y. Clostridium tyrobutyricum alleviates Staphylococcus aureus-induced endometritis in mice by inhibiting endometrial barrier disruption and inflammatory response. Food Funct. 2019, 10, 6699–6710. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, M.; Pan, Y.; Xie, Y.; Han, J.; Zhang, X.; Niayale, R.; He, H.; Li, Q.; Zhao, T.; et al. Anti-inflammatory effect of astragalin and chlorogenic acid on Escherichia coli-induced inflammation of sheep endometrial epithelium cells. Front. Vet. Sci. 2020, 7, 201. [Google Scholar] [CrossRef]
- Missiakas, D.; Winstel, V. Selective host cell death by Staphylococcus aureus: A strategy for bacterial persistence. Front. Immunol. 2021, 11, 621733. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Q.; Zhang, Z.; Lin, P.; Lei, L.; Wang, A.; Jin, Y. Endoplasmic reticulum stress cooperates in zearalenone-induced cell death of RAW 264.7 macrophages. Int. J. Mol. Sci. 2015, 16, 19780–19795. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Tan, J.; Miao, Y.; Li, M.; Zhang, Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell. Physiol. 2017, 232, 2977–2984. [Google Scholar] [CrossRef]
- Geng, N.; Wang, X.; Yu, X.; Wang, R.; Zhu, Y.; Zhang, M.; Liu, J.; Liu, Y. Staphylococcus aureus avoids autophagy clearance of bovine mammary epithelial cells by impairing lysosomal function. Front. Immunol. 2020, 11, 746. [Google Scholar] [CrossRef]
- Cai, J.; Li., J.; Zhou., Y.; Wang., J.; Li., J.; Cui., L.; Meng., X.; Zhu., G.; Wang., H. Staphylococcus aureus facilitates its survival in bovine macrophages by blocking autophagic flux. J. Cell. Mol. Med. 2020, 24, 3460–3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Guan, G.; Lei, L.; Lv, Q.; Liu, S.; Zhan, X.; Jiang, Z.; Gu, X. Palmitic acid induces human osteoblast-like Saos-2 cell apoptosis via endoplasmic reticulum stress and autophagy. Cell Stress Chaperones. 2018, 23, 1283–1294. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, X.; Sho, T.; Zhang, J.; Xu, W.; Yao, J.; Xu, J. ROS-induced autophagy regulates porcine trophectoderm cell apoptosis, proliferation, and differentiation. Am. J. Physiol. Cell Physiol. 2019, 316, C198–C209. [Google Scholar] [CrossRef]
- Chen, F.; Wen, X.; Lin, P.; Chen, H.; Wang, A.; Jin, Y. HERP depletion inhibits zearalenone-induced apoptosis through autophagy activation in mouse ovarian granulosa cells. Toxicol. Lett. 2019, 301, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Conza, G.; Ho, P.C. ER stress responses: An emerging modulator for innate immunity. Cells 2020, 9, 695. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Singh, P.K.; Zhang, K.; Kumar, A. Toll-like receptor 2 (TLR2) engages endoplasmic reticulum stress sensor IRE1α to regulate retinal innate responses in Staphylococcus aureus endophthalmitis. FASEB J. 2020, 34, 13826–13838. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jin, J.; Choi, J.A.; Cho, S.N.; Lim, Y.J.; Lee, J.H.; Seo, J.Y.; Chen, H.Y.; Rha, K.S.; Song, C.H. Staphylococcus aureus enterotoxin B-induced endoplasmic reticulum stress response is associated with chronic rhinosinusitis with nasal polyposis. Clin. Biochem. 2014, 47, 96–103. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y.; Zhao, K.; Chen, K.; Cao, Y.; Zhang, J.; Han, M.; Hu, L.; He, R.; Wang, D.; et al. Endoplasmic reticulum stress exacerbates inflammation in chronic rhinosinusitis with nasal polyps via the transcription factor XBP1. Clin. Immunol. 2021, 223, 108659. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Santano, N.; Ellis, J.K.; Mateos, L.M.; Calle, Y.; Keun, H.C.; Behrends, V.; Letek, M. Intracellular Staphylococcus aureus modulates host central carbon metabolism to activate autophagy. mSphere 2018, 3, e00374-18. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Gao, K.; Zhang, R.; Lin, P.; Wang, A.; Jin, Y. Staphylococcus aureus Induces Goat Endometrial Epithelial Cells Apoptosis via the Autophagy and Endoplasmic Reticulum Stress Pathway. Animals 2022, 12, 711. https://doi.org/10.3390/ani12060711
Yi Y, Gao K, Zhang R, Lin P, Wang A, Jin Y. Staphylococcus aureus Induces Goat Endometrial Epithelial Cells Apoptosis via the Autophagy and Endoplasmic Reticulum Stress Pathway. Animals. 2022; 12(6):711. https://doi.org/10.3390/ani12060711
Chicago/Turabian StyleYi, Yanyan, Kangkang Gao, Ruixue Zhang, Pengfei Lin, Aihua Wang, and Yaping Jin. 2022. "Staphylococcus aureus Induces Goat Endometrial Epithelial Cells Apoptosis via the Autophagy and Endoplasmic Reticulum Stress Pathway" Animals 12, no. 6: 711. https://doi.org/10.3390/ani12060711
APA StyleYi, Y., Gao, K., Zhang, R., Lin, P., Wang, A., & Jin, Y. (2022). Staphylococcus aureus Induces Goat Endometrial Epithelial Cells Apoptosis via the Autophagy and Endoplasmic Reticulum Stress Pathway. Animals, 12(6), 711. https://doi.org/10.3390/ani12060711





