Upper Body Movement Symmetry in Reining Quarter Horses during Trot In-Hand, on the Lunge and during Ridden Exercise
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Gait Analysis Sensors
2.3. Assessment Conditions
2.4. Data Processing
2.5. Data Normalization
2.6. Statistical Testing
3. Results
3.1. Number of Strides and Stride Time
3.2. Baseline and Lunge Movement Symmetry Prior to Normalization
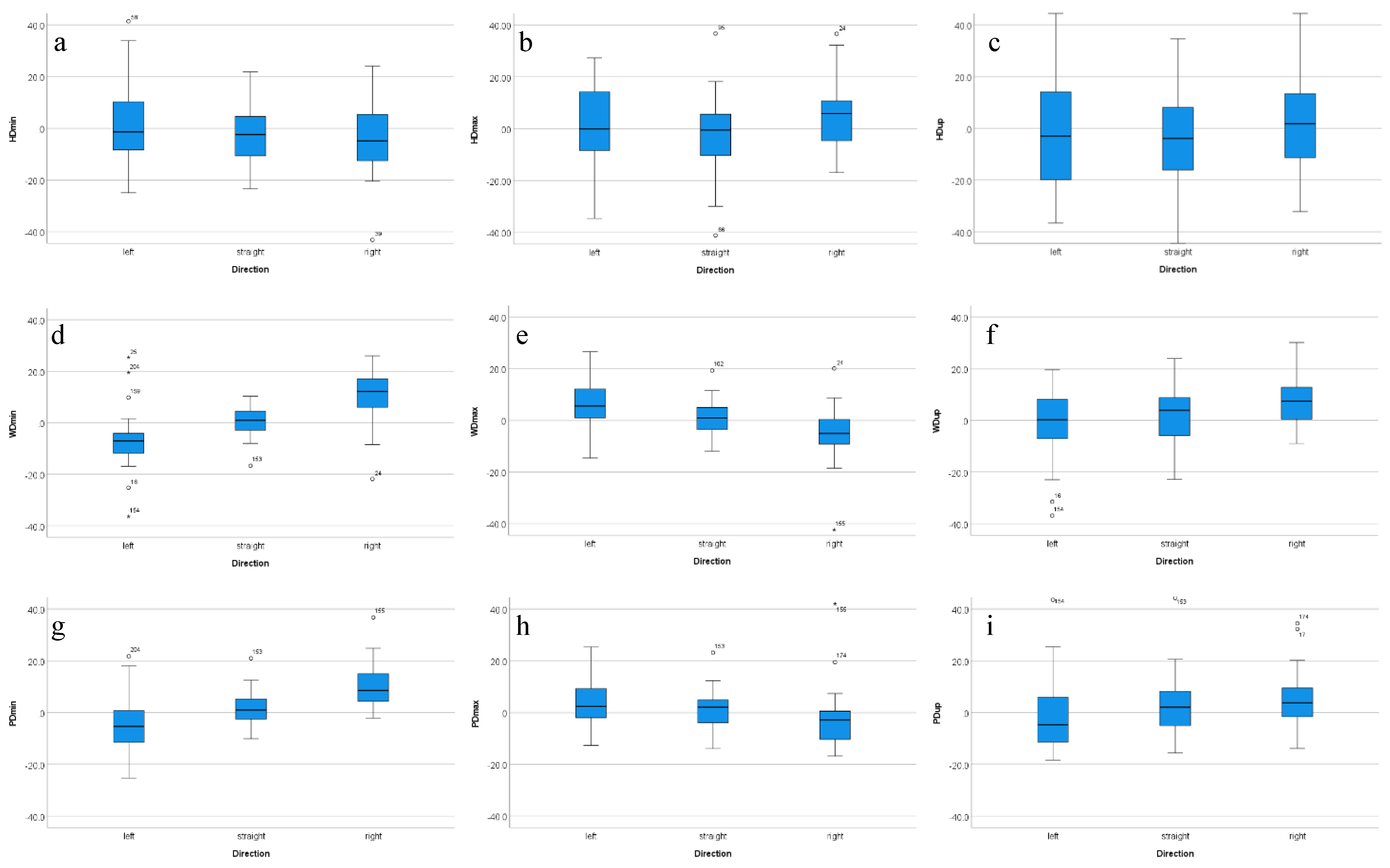
3.3. Effects of Movement Direction, Ridden Exercise and Stride Time
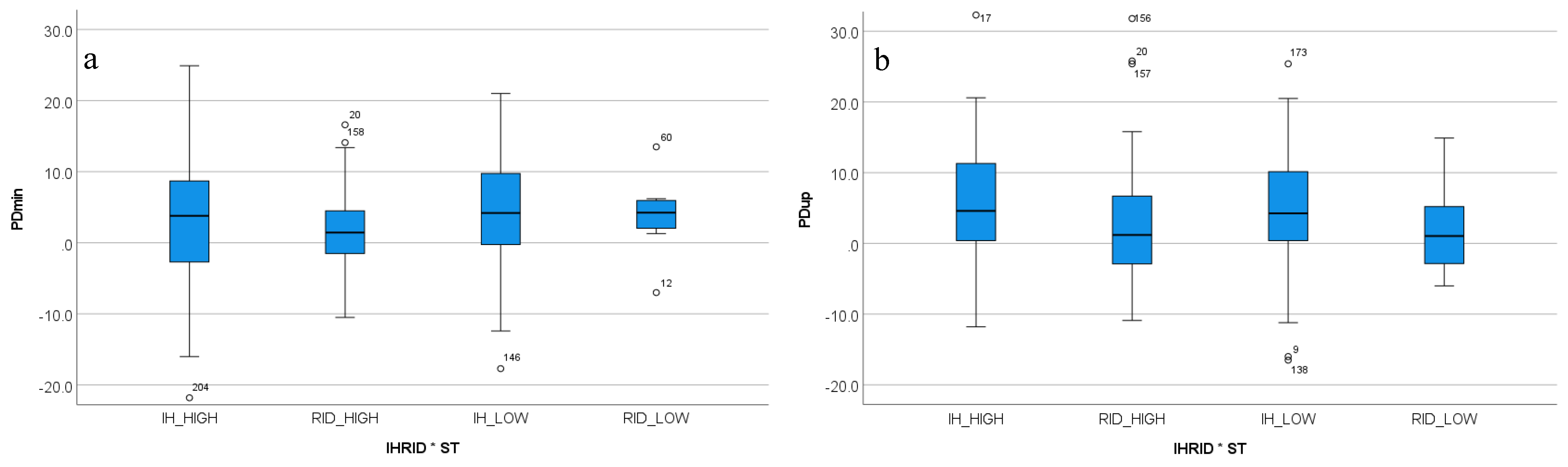
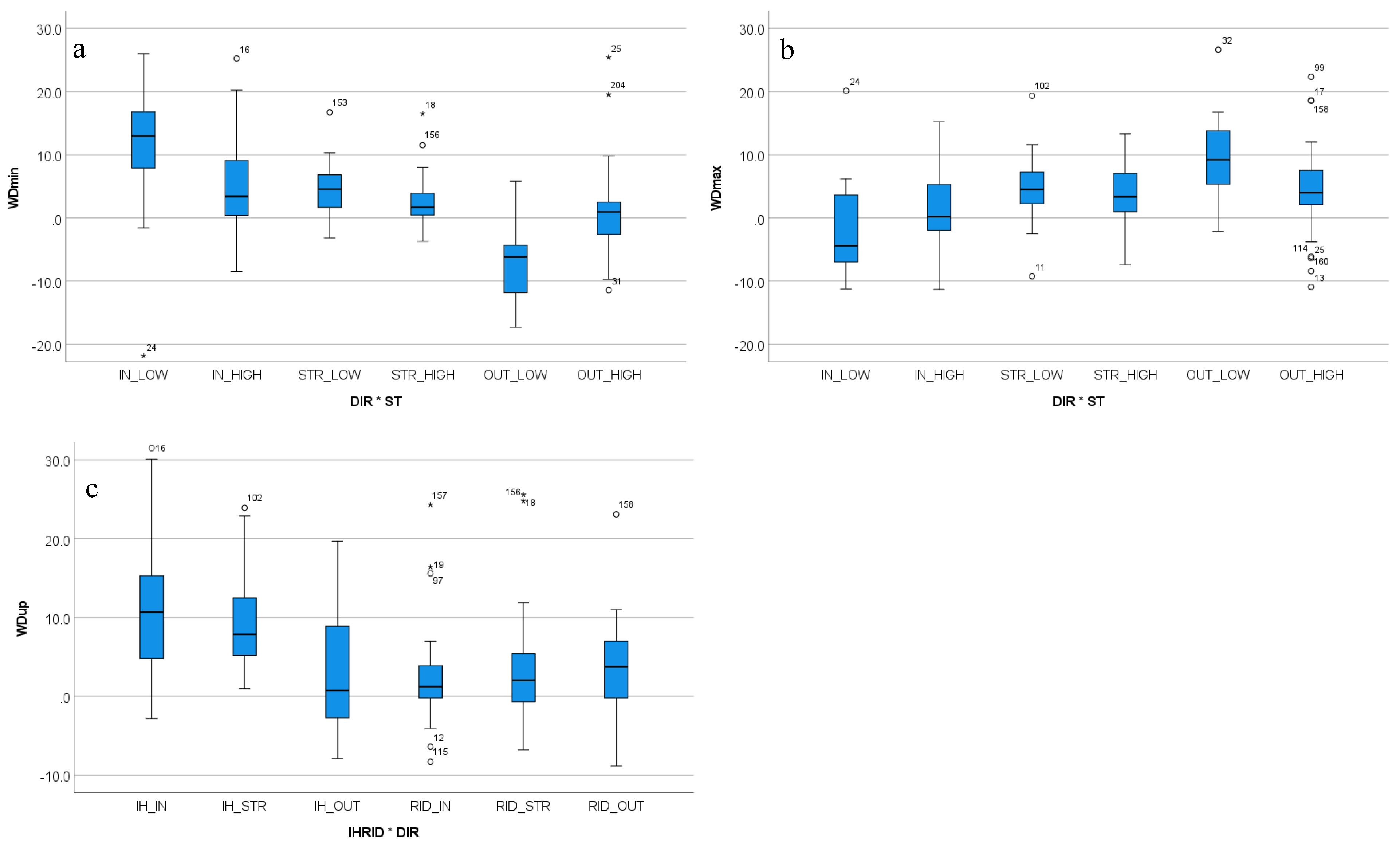
3.4. Two-Way Interactions between In-Hand Versus Ridden Exercise, Movement Direction and Stride Time
3.4.1. Sacral Movement Asymmetry
3.4.2. Withers Movement Asymmetry
4. Discussion
4.1. Ridden Exercise
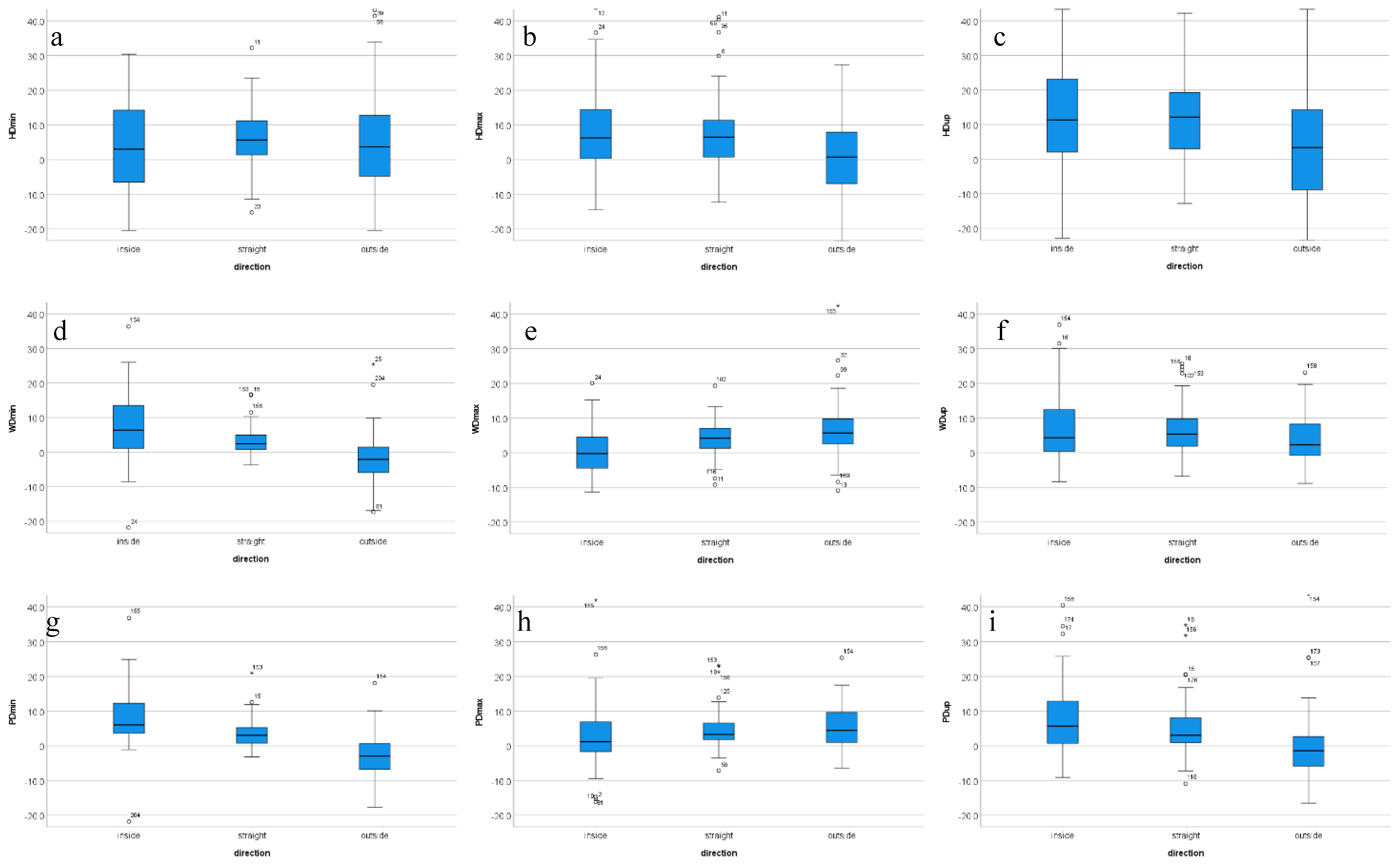
4.2. Movement Direction
4.3. Riding Style
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, M.W. Components of the Lameness exam and lameness strategy. In Diagnosis and Management of Lameness in the Horse; Dyson, S.J., Ross, M.W., Eds.; Saunders: Philadelphia, PA, USA, 2011; p. 7. ISBN 978-1-4160-6069-7. [Google Scholar]
- Ross, M.W. Movement. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 64–79. [Google Scholar]
- Bathe, A.; Judy, C.E.; Dyson, S.J. Letter to the editor: Do we have to redefine lameness in the era of quantitative gait analysis? Equine Vet. J. 2018, 50, 273. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.G.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Dent, E.V.; Kellerman, T.E.; Wilson, D.A.; Reed, S.K. Assessment of repeatability of a wireless inertial sensor-based lameness evaluation system for horses. Am. J. Vet. Res. 2011, 72, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; Serra Bragança, F.; Marin-Perianu, M.; Marin-Perianu, R.; van der Zwaag, J.B.; Voskamp, J.; Back, W.; van Weeren, R. EquiMoves: A wireless networked inertial measurement system for objective examination of horse gait. Sensors 2018, 18, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keegan, K.G.; Yonezawa, Y.; Pai, P.F.; Wilson, D.A.; Kramer, J. Evaluation of a sensor-based system of motion analysis for detection and quantification of forelimb and hind limb lameness in horses. Am. J. Vet. Res. 2004, 65, 665–670. [Google Scholar] [CrossRef]
- Pfau, T.; Weller, R. Comparison of a standalone consumer grade smartphone to a specialist inertial measurement unit for quantification of movement symmetry in the trotting horse. Equine Vet. J. 2017, 49, 124–129. [Google Scholar] [CrossRef]
- Pfau, T.; Robilliard, J.J.; Weller, R.; Jespers, K.; Eliashar, E.; Wilson, A.M. Assessment of mild hindlimb lameness during over ground locomotion using linear discriminant analysis of inertial sensor data. Equine Vet. J. 2007, 39, 407–413. [Google Scholar] [CrossRef]
- Marunova, E.; Dod, L.; Witte, S.; Pfau, T. Smartphone-based pelvic movement asymmetry measures for clinical decision making in equine lameness assessment. Animals 2021, 11, 1665. [Google Scholar] [CrossRef]
- Martin, P.; Cheze, L.; Pourcelot, P.; Desquilbet, L.; Duray, L.; Chateau, H. Effects of the rider on the kinematics of the equine spine under the saddle during the trot using inertial measurement units: Methodological study and preliminary results. Vet. J. 2017, 221, 6–10. [Google Scholar] [CrossRef]
- MacKechnie-Guire, R.; MacKechnie-Guire, E.; Fairfax, V.; Fisher, M.; Hargreaves, S.; Pfau, T. The effect that induced rider asymmetry has on equine locomotion and the range of motion of the thoracolumbar spine when ridden in rising trot. J. Equine Vet. Sci. 2020, 88, 102946. [Google Scholar] [CrossRef]
- Warner, S.M.; Koch, T.O.; Pfau, T. Inertial Sensors for assessment of back movement in horses during locomotion over ground. Equine Vet. J. 2010, 42, 417–424. [Google Scholar] [CrossRef]
- Marshall, J.F.; Lund, D.G.; Voute, L.C. Use of a wireless, inertial sensor-based system to objectively evaluate flexion tests in the horse. Equine Vet. J. 2012, 44, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Starke, S.D.; Willems, E.; Head, M.; May, S.A.; Pfau, T. Proximal hindlimb flexion in the horse: Effect on movement symmetry and implications for defining soundness. Equine Vet. J. 2012, 44, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Spicer-Jenkins, C.; Smith, R.K.; Bolt, D.M.; Fiske-Jackson, A.; Witte, T.H. Identifying optimal parameters for quantification of changes in pelvic movement symmetry as a response to diagnostic analgesia in the hindlimbs of horses. Equine Vet. J. 2014, 46, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Greve, L.; Pfau, T.; Dyson, S. Alterations in body lean angle in lame horses before and after diagnostic analgesia in straight lines in hand and on the lunge. Vet. J. 2018, 239, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greve, L.; Dyson, S.; Pfau, T. Alterations in Thoracolumbosacral movement when pain causing lameness has been improved by diagnostic analgesia. Vet. J. 2017, 224, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Maliye, S.; Voute, L.; Lund, D.; Marshall, J.F. An inertial sensor-based system can objectively assess diagnostic anaesthesia of the equine foot. Equine Vet. J. 2013, 45, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Maliye, S.; Marshall, J.F. Objective assessment of the compensatory effect of clinical hind limb lameness in horses: 37 cases (2011–2014). Am. J. Vet. Res. 2016, 249, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Maliye, S.; Voute, L.C.; Marshall, J.F. Naturally-occurring forelimb lameness in the horse results in significant compensatory load redistribution during trotting. Vet. J. 2015, 204, 208–213. [Google Scholar] [CrossRef]
- Pfau, T.; Jennings, C.; Mitchell, H.; Olsen, E.; Walker, A.; Egenvall, A.; Tröster, S.; Weller, R.; Rhodin, M. Lungeing on hard and soft surfaces: Movement symmetry of trotting horses considered sound by their owners. Equine Vet. J. 2016, 48, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Pfau, T.; Persson-Sjodin, E.; Gardner, H.; Orssten, O.; Hernlund, E.; Rhodin, M. Effect of speed and surface type on individual rein and combined left–right circle movement asymmetry in horses on the lunge. Front. Vet. Sci. 2021, 8, 692031. [Google Scholar] [CrossRef]
- Rhodin, M.; Pfau, T.; Roepstorff, L.; Egenvall, A. Effect of lungeing on head and pelvic movement asymmetry in horses with induced lameness. Vet. J. 2013, 198, e39–e45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodin, M.; Roepstorff, L.; French, A.; Keegan, K.G.; Pfau, T.; Egenvall, A. Head and pelvic movement asymmetry during lungeing in horses with symmetrical movement on the straight. Equine Vet. J. 2015, 48, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Stubbs, N.C.; Kaiser, L.J.; Brown, L.E.A.; Clayton, H.M. Effect of trotting speed and circle radius on movement symmetry in horses during lunging on a soft surface. Am. J. Vet. Res. 2012, 73, 1890–1899. [Google Scholar] [CrossRef]
- Starke, S.D.; Willems, E.; May, S.A.; Pfau, T. Vertical head and trunk movement adaptations of sound horses trotting in a circle on a hard surface. Vet. J. 2012, 193, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Persson-sjodin, E.; Hernlund, E.; Pfau, T.; Andersen, P.H.; Rhodin, M. Influence of seating styles on head and pelvic vertical movement symmetry in horses ridden at trot. PLoS ONE 2018, 13, e0195341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robartes, H.; Fairhurst, H.; Pfau, T. Head and pelvic movement symmetry in horses during circular motion and in rising trot. Vet. J. 2013, 198, e52–e58. [Google Scholar] [CrossRef] [PubMed]
- Barstow, A.; Bailey, J.; Campbell, J.; Harris, C.; Weller, R.; Pfau, T. Does “Hacking” surface type affect equine forelimb foot placement, movement symmetry or hoof impact deceleration during ridden walk and trot exercise. Equine Vet. J. 2018, 51, 108–114. [Google Scholar] [CrossRef]
- Scott, M. Musculoskeletal injuries in nonracing quarter horses. Vet. Clin. North Am. Equine Pract. 2008, 24, 133–152. [Google Scholar] [CrossRef]
- Black, J.B.; Dabareiner, R.M. The Western performance horse. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 1165–1185. [Google Scholar]
- Dabareiner, R.M.; Cohen, N.D.; Carter, G.K.; Nunn, S.; Moyer, W. Lameness and poor performance in horses used for team roping: 118 cases (2000–2003). J. Am. Vet. Med. Assoc. 2005, 226, 1694–1699. [Google Scholar] [CrossRef]
- Dabareiner, R.M.; Cohen, N.D.; Carter, G.K.; Nunn, S.; Moyer, W. Musculoskeletal problems associated with lameness and poor performance among horses used for barrel racing: 118 cases (2000–2003). J. Am. Vet. Med. Assoc. 2005, 227, 1646–1650. [Google Scholar] [CrossRef]
- Galley, R.H. Injuries of the team roping horse. Proc. AAEP 2001, 47, 7. [Google Scholar]
- Lewis, R.D. Lameness in the rodeo horse. AAEP Proc. 2001, 47, 5. [Google Scholar]
- Rhodin, M.; Egenvall, A.; Andersen, P.H.; Pfau, T. Head and pelvic movement asymmetries at trot in riding horses in training and perceived as free from lameness by the owner. PLoS ONE 2017, 12, e0176253. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Martin, C.; Bondi, A.; Ellis, A.D. The influence of rider skill on ridden horse behaviour, assessed using the ridden horse pain ethogram, and gait quality. Equine Vet. Educ. 2020, eve.13434. [Google Scholar] [CrossRef]
- Pfau, T.; Witte, T.H.; Wilson, A.M. A Method for deriving displacement data during cyclical movement using an inertial sensor. J. Exp. Biol. 2005, 208, 2503–2514. [Google Scholar] [CrossRef] [Green Version]
- Starke, S.D.; Witte, T.H.; May, S.A.; Pfau, T. Accuracy and precision of hind limb foot contact timings of horses determined using a pelvis-mounted inertial measurement unit. J. Biomech. 2012, 45, 1522–1528. [Google Scholar] [CrossRef]
- Bell, R.P.; Reed, S.K.; Schoonover, M.J.; Whitfield, C.T.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Keegan, K.G. Associations of force plate and body-mounted inertial sensor measurements for identification of hind limb lameness in horses. Am. J. Vet. Res. 2016, 77, 337–345. [Google Scholar] [CrossRef]
- Keegan, K.G.; MacAllister, C.G.; Wilson, D.A.; Gedon, C.A.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F. Comparison of an inertial sensor system with a stationary force plate for evaluation of horses with bilateral forelimb lameness. Am. J. Vet. Res. 2012, 73, 368–374. [Google Scholar] [CrossRef]
- Hoyt, D.F.; Wickler, S.J.; Dutto, D.J.; Catterfeld, G.E.; Johnsen, D. What are the relations between mechanics, gait parameters, and energetics in terrestrial locomotion? J. Exp. Zool. 2006, 305A, 912–922. [Google Scholar] [CrossRef]
- Licka, T.; Kapaun, M.; Peham, C. Influence of rider on lameness in trotting horses. Equine Vet. J 2004, 36, 734–736. [Google Scholar] [CrossRef]
- Byström, A.; Rhodin, M.; Von Peinen, K.; Weishaupt, M.A.; Roepstorff, L. Basic kinematics of the saddle and rider in high-level dressage horses trotting on a treadmill. Equine Vet. J. 2009, 41, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Byström, A.; Rhodin, M.; von Peinen, K.; Weishaupt, M.A.; Roepstorff, L. Kinematics of saddle and rider in high-level dressage horses performing collected walk on a treadmill. Equine Vet. J. 2010, 42, 340–345. [Google Scholar] [CrossRef]
- McCracken, M.J.; Kramer, J.; Keegan, K.G.; Lopes, M.; Wilson, D.A.; Reed, S.K.; LaCarrubba, A.; Rasch, M. Comparison of an inertial sensor system of lameness quantification with subjective lameness evaluation. Equine Vet. J. 2012, 44, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Rungsri, P.K.; Staecker, W.; Leelamankong, P.; Estrada, R.J.; Schulze, T.; Lischer, C.J. Use of body-mounted inertial sensors to objectively evaluate the response to perineural analgesia of the distal limb and intra-articular analgesia of the distal interphalangeal joint in horses with forelimb lameness. J. Equine Vet. Sci. 2014, 34, 972–977. [Google Scholar] [CrossRef]
- Pfau, T.; Boultbee, H.; Davis, H.; Walker, A.; Rhodin, M. Agreement between two inertial sensor gait analysis systems for lameness examinations in horses. Equine Vet. Educ. 2016, 28, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda Caviedes, M.F.; Forbes, B.S.; Pfau, T. Repeatability of gait analysis measurements in thoroughbreds in training. Equine Vet. J. 2018, 50, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeman, A.M.; Serra Bragança, F.M.; Swagemakers, J.H.; Weeren, P.R.; Roepstorff, L. Variation in Gait parameters used for objective lameness assessment in sound horses at the trot on the straight line and the lunge. Equine Vet. J. 2019, 51, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guire, R.; Mackechnie, E.; Bush, R.; Lawson, A.; Fairfax, V.; Fisher, D.; Fisher, M.; Weller, R.; Pfau, T. Effect of Tree width on kinematics of the thirteenth thoracic vertebra, thoracolumbar dimensions, saddle pressures and limb kinematics. Equine Vet. J. 2018, 50, 18. [Google Scholar]
- Mackechnie-Guire, R.; Mackechnie-Guire, E.; Fisher, M.; Mathie, H.; Bush, R.; Pfau, T.; Weller, R. Relationship Between saddle and rider kinematics, horse locomotion, and thoracolumbar pressures in sound horses. J. Equine Vet. Sci. 2018, 69, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.; Cheze, L.; Pourcelot, P.; Desquilbet, L.; Duray, L.; Chateau, H. Effect of the rider position during rising trot on the horse’s biomechanics (back and trunk kinematics and pressure under the saddle). J. Biomech. 2016, 49, 1027–1033. [Google Scholar] [CrossRef]
- Peham, C.; Licka, T.; Schobesberger, H.; Meschan, E. Influence of the rider on the variability of the equine gait. Hum. Mov. Sci. 2004, 23, 663–671. [Google Scholar] [CrossRef]
- Gunst, S.; Dittmann, M.T.; Arpagaus, S.; Roepstorff, C.; Latif, S.N.; Klaassen, B.; Pauli, C.A.; Bauer, C.M.; Weishaupt, M.A. Influence of functional rider and horse asymmetries on saddle force distribution during stance and in sitting trot. J. Equine Vet. Sci. 2019, 78, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.M.; Whitton, R.C.; Kawcak, C.E.; Stover, S.M.; Pandy, M.G. Relationship between Muscle forces, joint loading and utilization of elastic strain energy in equine locomotion. J. Exp. Biol. 2010, 213, 3998–4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuigan, M.P.; Wilson, A.M. The effect of gait and digital flexor muscle activation on limb compliance in the forelimb of the horse (Equus caballus). J. Exp. Biol. 2003, 206, 1325–1336. [Google Scholar] [CrossRef] [Green Version]
- Rhodin, M.; Persson-Sjodin, E.; Egenvall, A.; Serra Braganca, F.; Pfau, T.; Roepstorff, L.; Weishaupt, M.; Thomsen, M.H.; van Weeren, P.R.; Hernlund, E.; et al. Vertical movement symmetry of the withers in horses with induced forelimb and hindlimb lameness at trot. Equine Vet. J. 2018, 50, 818–824. [Google Scholar] [CrossRef] [PubMed]
- MacKechnie-Guire, R.; MacKechnie-Guire, E.; Fairfax, V.; Fisher, D.; Fisher, M.; Pfau, T. The effect of tree width on thoracolumbar and limb kinematics, saddle pressure distribution, and thoracolumbar dimensions in sports horses in trot and canter. Animals 2019, 9, 842. [Google Scholar] [CrossRef] [Green Version]
- MacKechnie-Guire, R.; Fisher, M.; Pfau, T. Effect of a half pad on pressure distribution in sitting trot and canter beneath a saddle fitted to industry guidelines. J. Equine Vet. Sci. 2021, 96, 103307. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, M.A.; Wiestner, T.; Peinen, K.; Waldern, N.; Roepstorff, L.; Weeren, R.; Meyer, H.; Johnston, C. Effect of head and neck position on vertical ground reaction forces and interlimb coordination in the dressage horse ridden at walk and trot on a treadmill. Equine Vet. J. 2006, 38, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Starke, S.; Raistrick, K.; May, S.; Pfau, T. The effect of trotting speed on the evaluation of subtle lameness in horses. Vet. J. 2013, 197, 245–252. [Google Scholar] [CrossRef]
- Dyson, S.; Ellis, A.D.; Mackechnie-Guire, R.; Douglas, J.; Bondi, A.; Harris, P. The influence of rider:horse bodyweight ratio and rider-horse-saddle fit on equine gait and behaviour: A pilot study. Equine Vet. Educ. 2020, 32, 527–539. [Google Scholar] [CrossRef] [Green Version]
- Roth, I.T.; Schielke, B.; Rensing, M.; Bernau, M. Comparison of american quarter horses competing in western pleasure, hunter under saddle, and reining using linear traits. Animals 2021, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Kotschwar, A.B.; Baltacis, A.; Peham, C. The effects of different saddle pads on forces and pressure distribution beneath a fitting saddle. Equine Vet. J. 2010, 42, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Meschan, E.M.; Peham, C.; Schobesberger, H.; Licka, T.F. The influence of the width of the saddle tree on the forces and the pressure distribution under the saddle. Vet. J. 2007, 173, 578–584. [Google Scholar] [CrossRef] [PubMed]
| Percentiles | ||||||||
|---|---|---|---|---|---|---|---|---|
| Direction | 5 | 10 | 25 | 50 | 75 | 90 | 95 | |
| HDmin | left | −21.1 | −16.4 | −9.1 | −1.4 | 10.8 | 19.3 | 37.3 |
| straight | −21.4 | −15.3 | −10.7 | −2.4 | 4.9 | 18.0 | 20.5 | |
| right | −30.6 | −19.9 | −12.6 | −4.9 | 6.1 | 16.8 | 20.5 | |
| HDmax | left | −31.0 | −23.0 | −9.5 | −0.1 | 14.3 | 22.4 | 24.8 |
| straight | −34.9 | −18.7 | −10.5 | −0.5 | 5.6 | 15.5 | 26.5 | |
| right | −16.8 | −14.3 | −5.4 | 5.9 | 11.0 | 20.9 | 34.2 | |
| HDup | left | −36.4 | −28.3 | −20.0 | −3.0 | 16.1 | 39.3 | 50.5 |
| straight | −45.6 | −28.2 | −16.9 | −3.9 | 9.0 | 25.3 | 33.5 | |
| right | −44.6 | −25.0 | −12.0 | 1.8 | 13.6 | 27.0 | 38.8 | |
| WDmin | left | −30.2 | −16.9 | −12.1 | −7.1 | −3.9 | 9.0 | 22.2 |
| straight | −11.9 | −7.3 | −3.1 | 1.0 | 4.5 | 6.8 | 8.9 | |
| right | −14.5 | −5.2 | 6.0 | 12.1 | 17.2 | 20.1 | 22.9 | |
| WDmax | left | −10.1 | −6.4 | 1.0 | 5.6 | 12.5 | 16.5 | 24.2 |
| straight | −9.9 | −7.7 | −3.7 | 0.9 | 5.2 | 10.1 | 15.1 | |
| right | −29.3 | −13.1 | −9.3 | −5.0 | 1.2 | 8.0 | 13.8 | |
| WDup | left | −33.9 | −22.2 | −7.2 | 0.3 | 8.3 | 16.9 | 19.3 |
| straight | −20.9 | −14.6 | −6.2 | 3.9 | 8.9 | 12.9 | 18.0 | |
| right | −26.6 | −8.1 | 0.5 | 7.4 | 13.0 | 21.4 | 29.4 | |
| PDmin | left | −22.0 | −14.1 | −11.6 | −5.4 | 1.2 | 9.3 | 19.8 |
| straight | −9.3 | −7.8 | −2.7 | 1.0 | 5.5 | 11.0 | 16.4 | |
| right | −0.2 | 1.5 | 4.2 | 8.6 | 15.3 | 24.2 | 30.3 | |
| PDmax | left | −11.9 | −11.0 | −2.8 | 2.4 | 9.4 | 16.7 | 20.9 |
| straight | −13.2 | −6.8 | −4.0 | 2.2 | 5.0 | 8.7 | 17.3 | |
| right | −16.7 | −16.0 | −10.7 | −2.8 | 0.7 | 6.9 | 29.6 | |
| PDup | left | −17.4 | −16.1 | −11.5 | −4.7 | 6.6 | 11.2 | 33.5 |
| straight | −15.1 | −9.8 | −5.0 | 2.1 | 8.7 | 20.1 | 31.2 | |
| right | −11.8 | −7.9 | −1.7 | 3.8 | 9.9 | 31.1 | 54.4 | |
| IHvsRID | ST | DIR | IHvsRID*ST | IHvsRID*DIR | DIR*ST | |
|---|---|---|---|---|---|---|
| HDmin | 0.438 | 0.590 | 0.166 | / | / | / |
| HDmax | 0.999 | 0.042 | 0.052 | / | 0.055 | 0.085 |
| HDup | 0.296 | 0.007 | 0.026 | / | / | / |
| WDmin | 0.022 | 0.430 | <0.001 | / | / | <0.001 |
| WDmax | 0.264 | 0.463 | <0.001 | / | / | <0.001 |
| WDup | <0.001 | 0.785 | 0.004 | / | <0.001 | / |
| PDmin | 0.044 | 0.126 | <0.001 | 0.042 | / | / |
| PDmax | 0.470 | 0.388 | <0.001 | / | / | / |
| PDup | 0.009 | 0.146 | <0.001 | 0.012 | / | / |
| IHvsRID | In-Hand | < / > | Ridden |
|---|---|---|---|
| (mm) | (mm) | ||
| WDmin | 4.48 | > | 1.25 |
| WDup | 8.54 | > | 3.38 |
| PDmin | 1.60 | < | 1.93 |
| PDup | 3.98 | > | 2.55 |
| DIR | Inside Rein | Straight-Line | Outside Rein | Pairwise Comparison |
|---|---|---|---|---|
| (Bonferroni) | ||||
| HDup | 12.2 1 | 11.9 | 5.6 1 | 1 p = 0.049 |
| WDmin | 7.5 | 3.4 | −2.3 | 1,2,3 p < 0.001 |
| WDmax | 0.2 1,2 | 4.1 1,3 | 6.8 2,3 | 1,2,3 p < 0.001 |
| WDup | 7.4 1 | 6.3 | 4.2 1 | 1 p = 0.003 |
| PDmin | 6.8 1,2 | 2.7 1,3 | −4.2 2,3 | 1,2,3 p < 0.001 |
| PDmax | 2.5 1,2 | 4.8 1 | 5.8 2 | 1 p = 0.03; 2 p < 0.001 |
| PDup | 7.0 1,2 | 4.2 1,3 | −1.3 2,3 | 1 p = 0.059; 2,3 p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfau, T.; Scott, W.M.; Sternberg Allen, T. Upper Body Movement Symmetry in Reining Quarter Horses during Trot In-Hand, on the Lunge and during Ridden Exercise. Animals 2022, 12, 596. https://doi.org/10.3390/ani12050596
Pfau T, Scott WM, Sternberg Allen T. Upper Body Movement Symmetry in Reining Quarter Horses during Trot In-Hand, on the Lunge and during Ridden Exercise. Animals. 2022; 12(5):596. https://doi.org/10.3390/ani12050596
Chicago/Turabian StylePfau, Thilo, W. Michael Scott, and Tabitha Sternberg Allen. 2022. "Upper Body Movement Symmetry in Reining Quarter Horses during Trot In-Hand, on the Lunge and during Ridden Exercise" Animals 12, no. 5: 596. https://doi.org/10.3390/ani12050596
APA StylePfau, T., Scott, W. M., & Sternberg Allen, T. (2022). Upper Body Movement Symmetry in Reining Quarter Horses during Trot In-Hand, on the Lunge and during Ridden Exercise. Animals, 12(5), 596. https://doi.org/10.3390/ani12050596






