Influence of Genotype on Endometrial Angiogenesis during Early Pregnancy in Piau and Commercial Line Gilts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Local
2.2. Animals and Experimental Design
2.3. Artificial Insemination
2.4. Slaughter and Collection of Uterine Samples
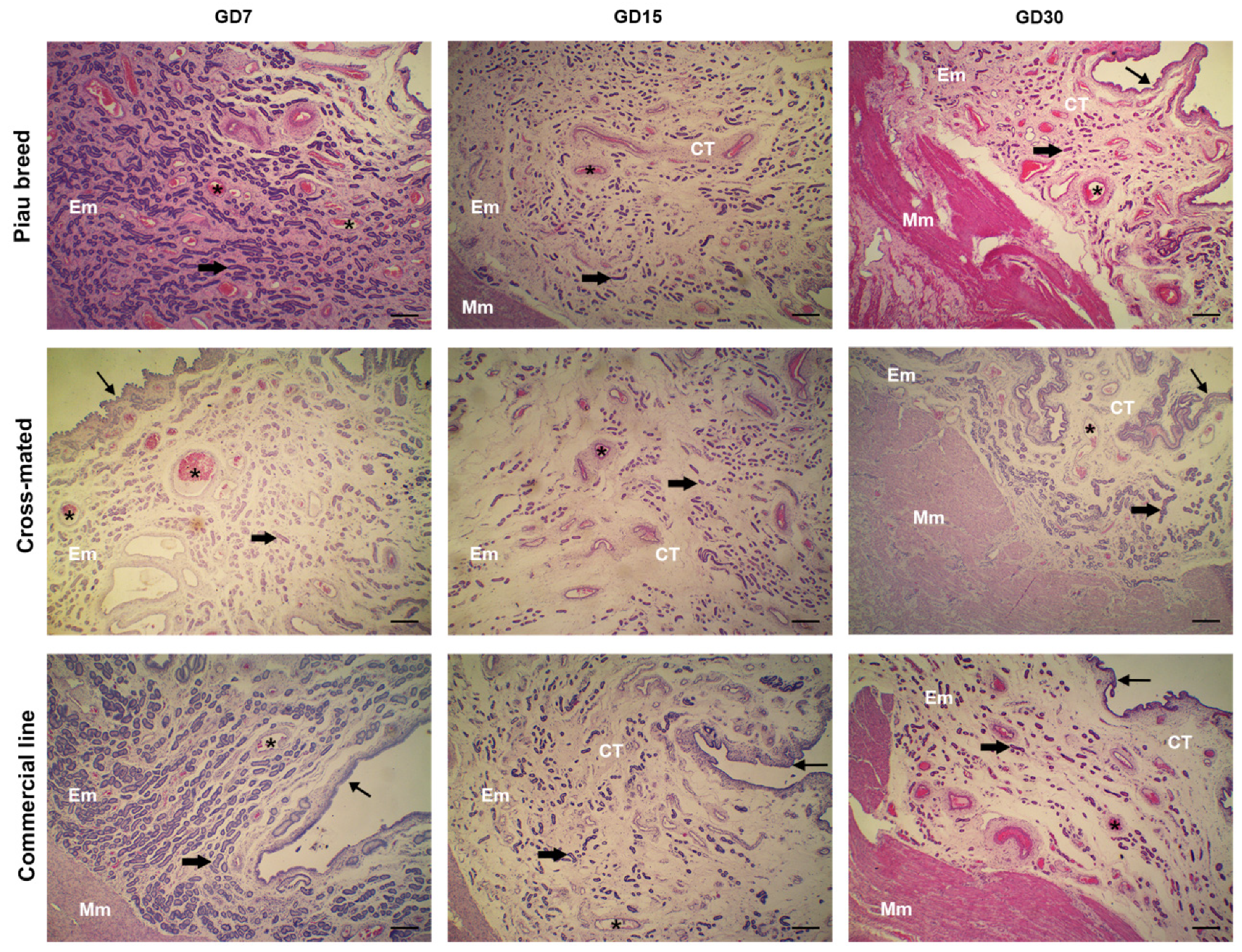
2.5. Histological Analysis
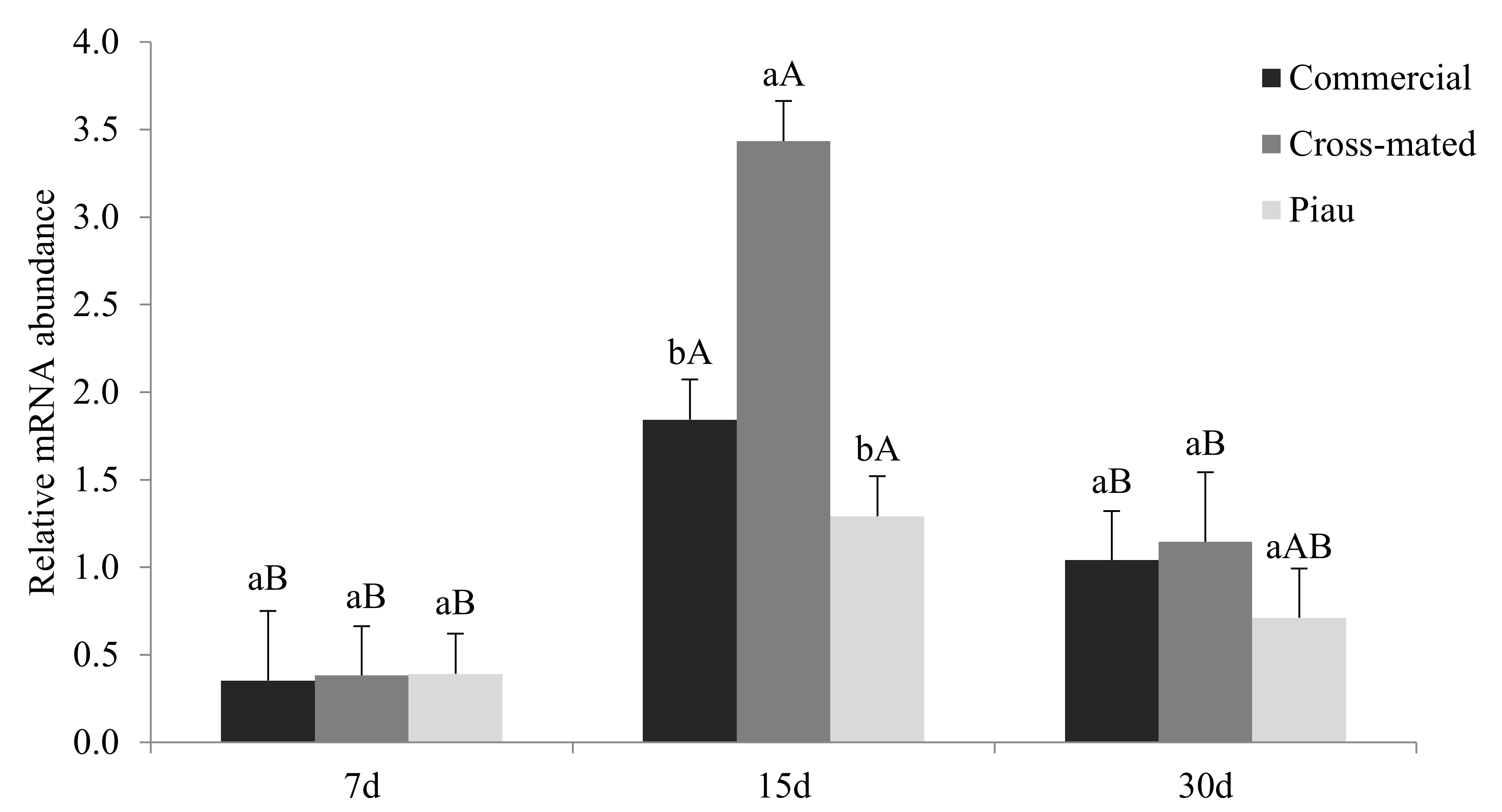
2.6. Gene Relative Transcript Abundance Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sollero, B.P.; Paiva, S.R.; Faria, D.A.; Guimarães, S.E.F.; Castro, S.T.R.; Egito, A.A.; Albuquerque, M.S.M.; Piovezan, U.; Bertani, G.R.; Mariante, A.D.S. Genetic diversity of Brazilian pig breeds evidenced by microsatellite markers. Livest. Sci 2009, 123, 8–15. [Google Scholar] [CrossRef]
- Silva, P.V.; Guimarães, S.E.F.; Guimarães, J.D.; Neto, J.B.; Lopes, P.S.; Nascimento, C.S.D.; De Campos, C.F.; A Weller, M.M.C.; E Botelho, M.; Faria, V.R. Gene expression in swine granulosa cells and ovarian tissue during the estrous cycle. Genet. Mol. Res. 2011, 10, 2258–2267. [Google Scholar] [PubMed]
- Silva, P.V.; Guimarães, S.E.F.; Guimarães, J.D.; Nascimento, C.S.; Lopes, P.S.; Siqueira, J.B.; Amorim, L.S.; E Silva, F.F.; Foxcroft, G.R. Follicular dynamics and gene expression in granulosa cells, corpora lutea and oocytes from gilts of breeds with low and high ovulation rates. Reprod. Fertil. Dev. 2014, 26, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.C.; Penitente-Filho, J.M.; Guimarães, S.E.F.; Lopes, P.S.; Camilo, B.S.; Shiomi, H.H.; Lima, D.A.; Pinho, R.O.; Pereira, J.V.T.D.N.; Okano, D.S.; et al. Aspects of sexual precocity and morphometry of uterus, placenta and embryos/fetuses in Piau breed and Commercial line gilts. Theriogenology 2018, 105, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Keys, J.L.; King, G.J.; Kennedy, T.G. Increased uterine vascular permeability at the time of embryonic attachment in the pig. Biol. Reprod. 1986, 34, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Keys, J.L.; King, G.J. Morphological evidence for increased uterine vascular permeability at the time of embryonic attachment in the pig. Biol. Reprod. 1988, 39, 473–487. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar]
- Kaczmarek, M.M.; Kiewisz, J.; Schams, D.; Ziecik, A.J. Expression of VEGF-receptor system in conceptus during peri-implantation period and endometrial and luteal expression of soluble VEGFR-1 in the pig. Theriogenology 2009, 71, 1298–1306. [Google Scholar] [CrossRef]
- Ford, S.P.; Vonnahme, K.A.; Wilson, M.E. Uterine capacity in the pig reflects a combination of uterine environment and conceptus genotype effects. J. Anim Sci 2002, 80, E66–E73. [Google Scholar]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Vonnahme, K.A.; Luther, J.S.; Buchanan, D.S.; Hafez, S.A.; Grazul-Bilska, A.T.; Redmer, D.A. Uteroplacental vascular development and placental function: An update. Int. J. Dev. Biol. 2010, 54, 355–366. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Vonnahme, K.A.; Luther, J.S.; Hammer, C.J.; Maddock Carlin, K.R.; Grazulbilska, A.T.; Redmer, D.A. Development programming: The concept, large animal models, and the key role of uteroplacental vascular development. J. Anim. Sci. 2010, 88, E61–E72. [Google Scholar] [CrossRef]
- Tayade, C.; Fang, Y.; Croy, B.A. A review of gene expression in porcine endometrial lymphocytes, endothelium and trophoblast during pregnancy success and failure. J. Reprod. Dev. 2007, 53, 455–463. [Google Scholar] [CrossRef]
- Wessels, J.; Linton, N.F.; Croy, B.A.; Tayade, C. A review of molecular contrast between arresting and viable porcine attachment sites. Am. J. Reprod. Immunol. 2007, 58, 470–480. [Google Scholar] [CrossRef]
- Tammela, T.; Enholm, B.; Alitalo, K.; Paavonen, K. The biology of vascular endothelial growth factors. Cardiovasc. Res. 2005, 65, 550–563. [Google Scholar] [CrossRef]
- Oliver, G.; Novak, S.; Patterson, J.L.; Pasternak, J.A.; Paradis, F.; Norrby, M.; Oxtoby, K.; Dyck, M.K.; Dixon, W.T.; Foxcroft, G.R. Restricted feed intake in lactating primiparous sows: II. Effects on subsequente litter sex ratio and embryonic gene expression. Reprod. Fertil. Dev. 2011, 23, 899–911. [Google Scholar] [CrossRef]
- Østrup, E.; Bauersachs, S.; Blum, H.; Wolf, E.; Hyttel, P. Differential endometrial gene expression in pregnant and nonpregnant sows. Biol. Reprod. 2010, 83, 277–285. [Google Scholar] [CrossRef]
- Samborski, A.; Graf, A.; Krebs, S.; Kessler, B.; Bauersachs, S. Deep Sequencing of the Porcine Endometrial Transcriptome on Day 14 of Pregnancy. Biol. Reprod. 2013, 88, 1–13. [Google Scholar] [CrossRef]
- Bazer, F.W.; Wu, G.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Bayless, K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol. Hum. Reprod. 2010, 16, 135–152. [Google Scholar] [CrossRef]
- Serret, C.G.; Alvarenga, M.V.F.D.; Coria, A.L.P.; Dias, C.P.; Corcini, C.D.; Corrêa, M.N.; Deschamps, J.C.; Bianchi, I.; Lucia, T., Jr. Intrauterine artificial insemination of swine with different sperm concentrations, parities, and methods for prediction of ovulation. Anim. Reprod. 2005, 2, 250–256. [Google Scholar]
- Panzardi, A.; Megalli, P.G.; Bernardi, M.; Wentz, I.; Bortolozzo, F.P. Eventos cronológicos da gestação: Da deposição dos espermatozoides no trato reprodutivo feminino ao desenvolvimento dos fetos. In Suinocultura em Ação: A Fêmea Suína Gestante, 4th ed.; Universidade Federal de Rio Grande do Sul: Porto Alegre, Brazil, 2007; pp. 43–71. [Google Scholar]
- Vonnahme, K.A.; Ford, S.P. Placental vascular endothelial growth factor receptor system mRNA expression in pigs selected for placental efficiency. J. Physiol. 2003, 54, 194–201. [Google Scholar] [CrossRef]
- Monteiro, C.M.R.; Carvalho, R.G. Caracterização histológica do útero, tubas uterinas e ovários de fêmeas recém-nascidas, pré-púberes e púberes de suínos mestiços (Sus scrofa domestica–L.1758). ARS Veterinaria 2006, 22, 223–228. [Google Scholar]
- Skowronski, M.T.; Kwon, T.H.; Nielsen, S. Immunolocalization of aquaporin 1, 5, and 9 in the female pig reproductive system. J. Histochem. Cytochem. 2009, 57, 61–67. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the −2ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.0 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Spencer, T.E.; Hayashi, K.; Hu, J.; Carpenter, K.D. Comparative development biology of mammalian uterus. Curr. Top. Dev. Biol. 2005, 68, 85–122. [Google Scholar]
- Spencer, T.E.; Bazer, F.W. Uterine and placental factors regulating conceptus growth in domestic animals. J. Anim. Sci. 2004, 82, E4–E13. [Google Scholar] [PubMed]
- Strobant, H.W.; Taverne, N.; Lagenfeld, K.; Barends, P.M. The ultrastructure of the uterine epithelium of the pig during the estrous cycle and early pregnancy. Cell Tissue Res. 1986, 246, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Seagroves, T.N.; Ryan, H.E.; Lu, H.; Wouters, B.G.; Knapp, M.; Thibault, P.; Laderoute, K.; Johnson, R.S. Transcription Factor HIF-1 is a necessary mediator of the Pasteur Effect in mammalian cells. Mol. Cell Biol. 2001, 21, 3436–3444. [Google Scholar] [CrossRef]
- Masson, N.; Ratcliffe, P.J. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O2 levels. J. Cell Sci. 2003, 116, 3041–3049. [Google Scholar] [CrossRef]
- Tayade, C.; Fang, Y.; Hilchie, D.; Croy, B.A. Lymphocyte contributions to altered endometrial angiogenesis during early and midgestation fetal loss. J. Leukoc. Biol. 2007, 82, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Seagroves, T.N.; Hadsell, D.; McManaman, J.; Palmer, C.; Liao, D.; McNulty, W.; Welm, B.; Wagner, K.U.; Neville, M.; Johnson, R.S. HIF1alpha is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development 2003, 130, 1713–1724. [Google Scholar] [CrossRef][Green Version]
- Valdés, G.; Corthorn, J. Review: The angiogenic and vasodilatory utero-placental network. Placenta 2011, 32, S170–S175. [Google Scholar] [CrossRef]
- Shan, B.; Gerez, J.; Haedo, M.; Fuertes, M.; Theodoropoulou, M.; Buchfelder, M.; Losa, M.; Stalla, G.K.; Arzt, E.; Renner, U. RSUME is implicated in HIF-1-induced VEGF-A production in pituitary tumour cells. Endocr. Relat. Cancer 2012, 19, 13–27. [Google Scholar] [CrossRef]
- Song, K.H.; Song, J.; Jeong, G.B.; Kim, J.M.; Jung, S.H.; Song, J. Vascular endothelial growth factor- its relation to neovascularization and their significance as prognostic factors in renal cell carcinoma. Yonsei Med. J. 2001, 42, 539–546. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, H.J.; Ko, D.S.; Lee, H.C.; Park, W.I. The regulators of VEGF expression in mouse ovaries. Yonsei Med. J. 2005, 46, 679–686. [Google Scholar] [CrossRef][Green Version]
- Rossiter, H.; Barresi, C.; Ghannadan, M.; Gruber, F.; Mildner, M.; Födinger, D.; Tschachler, E. Inactivation of VEGF in mammary gland epithelium severely compromises mammary gland development and function. FASEB J. 2007, 21, 3994–4004. [Google Scholar] [CrossRef]
- Cheung, C.Y. Vascular endothelial growth factor: Possible role in fetal development and placental function. J. Soc. Gynecol. Investig. 1997, 4, 169–177. [Google Scholar] [CrossRef]
- Milkiewicz, M.; Ispanovic, E.; Doyle, J.L.; Haas, T.L. Regulators of angiogenesis and strategies for their respective manipulation. Int. J. Biochem. Cell Biol. 2006, 38, 333–357. [Google Scholar] [CrossRef]
- Suri, C.; McClain, J.; Thurston, G.; McDonald, D.M.; Zhou, H.; Oldmixon, E.H.; Sato, T.N.; Yancopoulos, G.D. Increased vascularization in mice overexpressing angiopoietin-1. Science 1998, 282, 468–471. [Google Scholar] [CrossRef]
- Thurston, G. Complementary actions of VEGF and Angiopoietin-1 on blood vessel growth and leakage. J. Anat. 2002, 200, 575–580. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef] [PubMed]
- De Spiegelaere, W.; Cornillie, P.; Simoens, P.; Van den Broeck, W. Immunohistochemical detection of the angiopoietins during porcine metanephric kidney development. Acta Histochem. 2011, 113, 585–590. [Google Scholar] [CrossRef]
- Tello-Montoliu, A.; Patel, J.V.; Lip, G.Y.H. Angiogenin: A review of the pathophysiology and potential clinical applications. J. Thromb. Haemost. 2006, 4, 1864–1874. [Google Scholar] [CrossRef]
- Perry, J.S.; Rowlands, I.W. Early pregnancy in the pig. J. Reprod. Fertil. 1962, 4, 175–188. [Google Scholar] [CrossRef]
- Geisert, R.D.; Renegar, R.H.; Thatcher, W.W.; Roberts, R.M.; Bazer, F.W. Establishment of pregnancy in pig: II. Cellular remodeling of the porcine blastocyst during elongation on day 12 of pregnancy. Biol. Reprod. 1982, 4, 941–955. [Google Scholar] [CrossRef]
- Rajashekhar, G.; Loganath, A.; Roy, A.C.; Chong, S.S.; Wong, Y.C. Hypoxia up-regulated angiogenin and down-regulated vascular cell adhesion molecule-1 expression and secretion in human placentaltrophoblasts. J. Soc. Gynecol. Investig. 2005, 12, 310–319. [Google Scholar] [CrossRef]


| Gene | Protein | Access Number | Primer Sequence |
|---|---|---|---|
| HIF1α | Hypoxia inducer factor-1α | NM_001123124.1 | F: GCCAGATCTCGACGAAGTAAAG R: AGCTGATGGTAAGCCTCATAAC |
| FGF9 | Fibroblast growth factor-9 | NM_213801.1 | F: CAGTCACGGACTTGGATCATT R: TTCCTGGTTCCCTGGATAGT |
| ANG1 | Angiogenin-1 | NM_001044573.2 | F: GAAGACAGGTACACACACTTCC R: CAGGCCTCGTTGCTTCATTA |
| TEK | Tyrosine endothelial kinase | XM_001926034.5 | F: CGGCACGAAGTACCTGATATT R: GGTGAAGAGGTTTCCTCCTATG |
| VEGFA | Vascular endothelial growth factor A | NM_214084.1 | F: GCACATAGGAGAGATGAGCTTC R: CAAGGCCCACAGGGATTT |
| ANGPT1 | Angiopoietin-1 | NM_213959.1 | F: ACAGAGCCACCACCAATAAC R: GTGCAAAGGTTGACGAGATTATG |
| ANGPT2 | Angiopoietin-2 | NM_213808.1 | F: CTGAGCTGTGATCTCGTCTTG R: CTGAACCTGATACTGCCTCTTC |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | NM_001206359.1 | F: CAAAGTGGACATTGTCGCCATCA R: AGCTTCCCATTCTCAGCCTTGACT |
| Days of Pregnancy | Commercial | Cross-Mated | Piau |
|---|---|---|---|
| 7 | 78.5 ± 11.8 bA | 72.8 ± 6.8 bA | 136.6 ± 6.8 aA |
| 15 | 51.5 ± 6.8 bA | 69.3 ± 6.8 bA | 112.2 ± 6.8 aA |
| 30 | 66.1 ± 6.8 aA | 73.4 ± 6.8 aA | 77.2 ± 6.8 aB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Vergara, J.C.; Penitente-Filho, J.M.; Machado-Neves, M.; Machado, L.C.M.; Castaño-Villadiego, F.A.; Costa, K.A.; da Costa, E.P.; de Campos, C.F.; Ramírez-López, C.J.; Guimarães, S.E.F.; et al. Influence of Genotype on Endometrial Angiogenesis during Early Pregnancy in Piau and Commercial Line Gilts. Animals 2022, 12, 553. https://doi.org/10.3390/ani12050553
Montes-Vergara JC, Penitente-Filho JM, Machado-Neves M, Machado LCM, Castaño-Villadiego FA, Costa KA, da Costa EP, de Campos CF, Ramírez-López CJ, Guimarães SEF, et al. Influence of Genotype on Endometrial Angiogenesis during Early Pregnancy in Piau and Commercial Line Gilts. Animals. 2022; 12(5):553. https://doi.org/10.3390/ani12050553
Chicago/Turabian StyleMontes-Vergara, José Carlos, Jurandy Mauro Penitente-Filho, Mariana Machado-Neves, Lucas Corrêa Martins Machado, Faider Alberto Castaño-Villadiego, Karine Assis Costa, Eduardo Paulino da Costa, Carolina Filardi de Campos, Camilo José Ramírez-López, Simone Eliza Facioni Guimarães, and et al. 2022. "Influence of Genotype on Endometrial Angiogenesis during Early Pregnancy in Piau and Commercial Line Gilts" Animals 12, no. 5: 553. https://doi.org/10.3390/ani12050553
APA StyleMontes-Vergara, J. C., Penitente-Filho, J. M., Machado-Neves, M., Machado, L. C. M., Castaño-Villadiego, F. A., Costa, K. A., da Costa, E. P., de Campos, C. F., Ramírez-López, C. J., Guimarães, S. E. F., Lopes, P. S., & Guimarães, J. D. (2022). Influence of Genotype on Endometrial Angiogenesis during Early Pregnancy in Piau and Commercial Line Gilts. Animals, 12(5), 553. https://doi.org/10.3390/ani12050553







