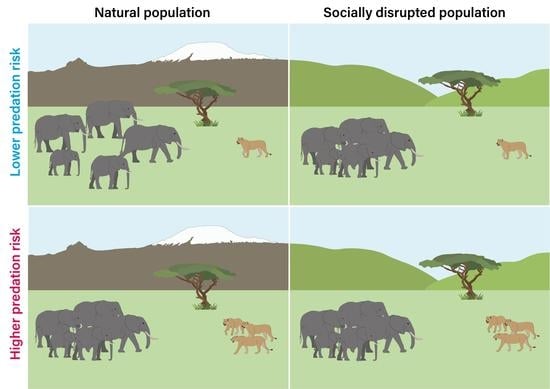
Social Disruption Impairs Predatory Threat Assessment in African Elephants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Playback Procedure
2.3. Data Analysis
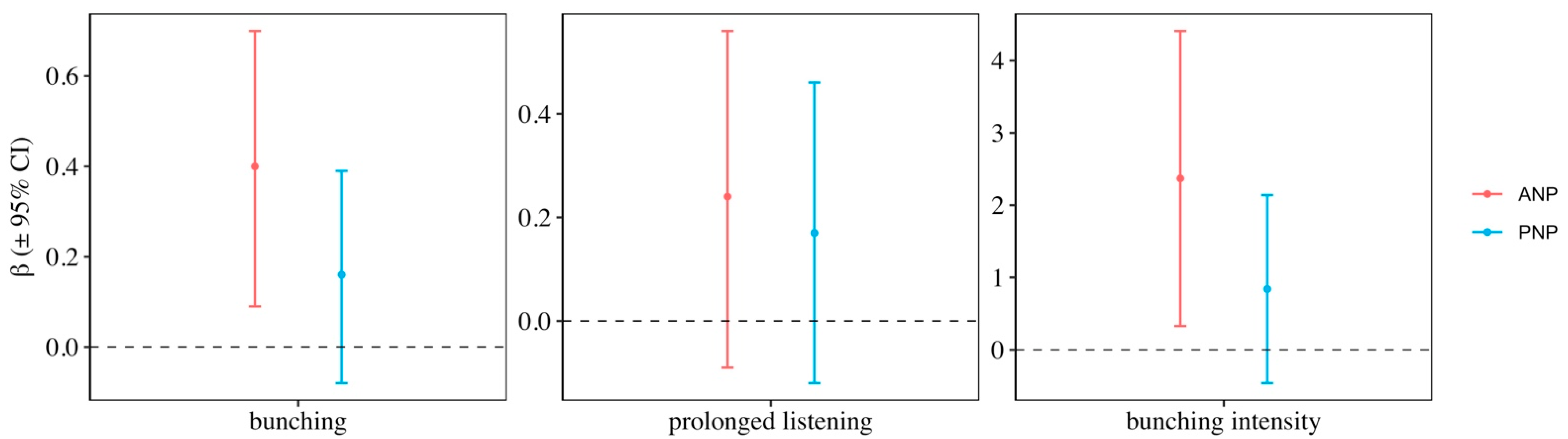
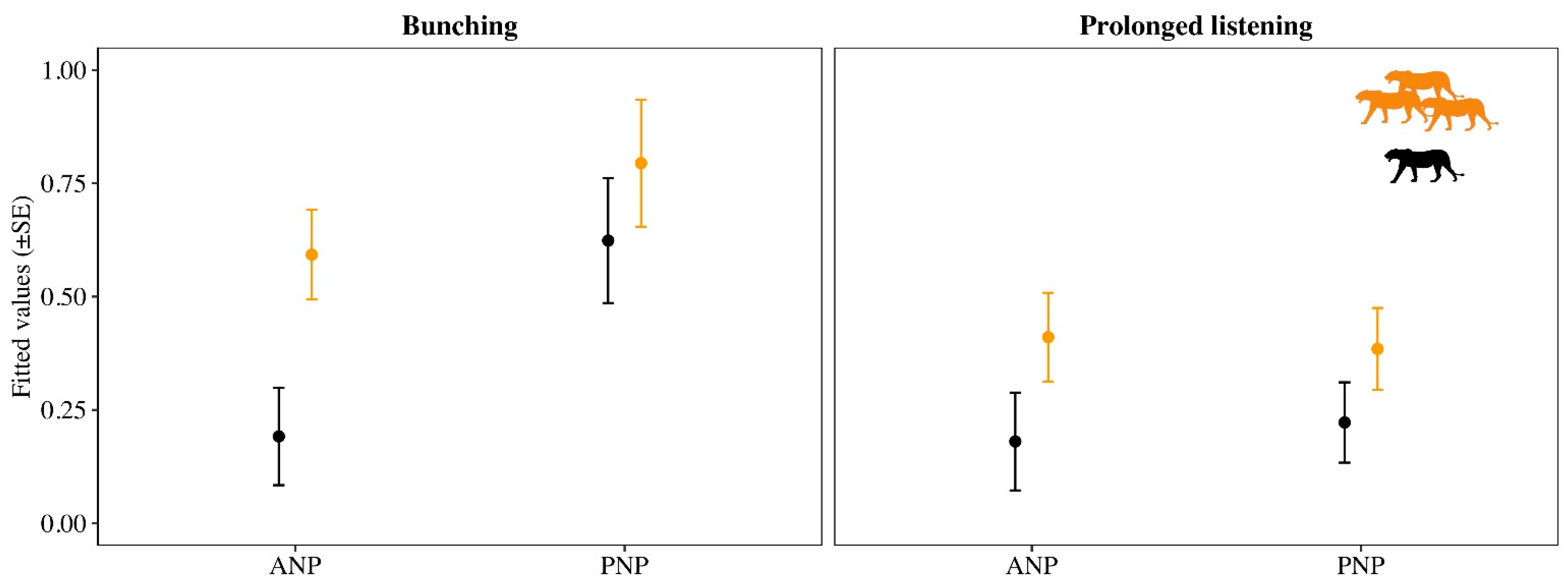
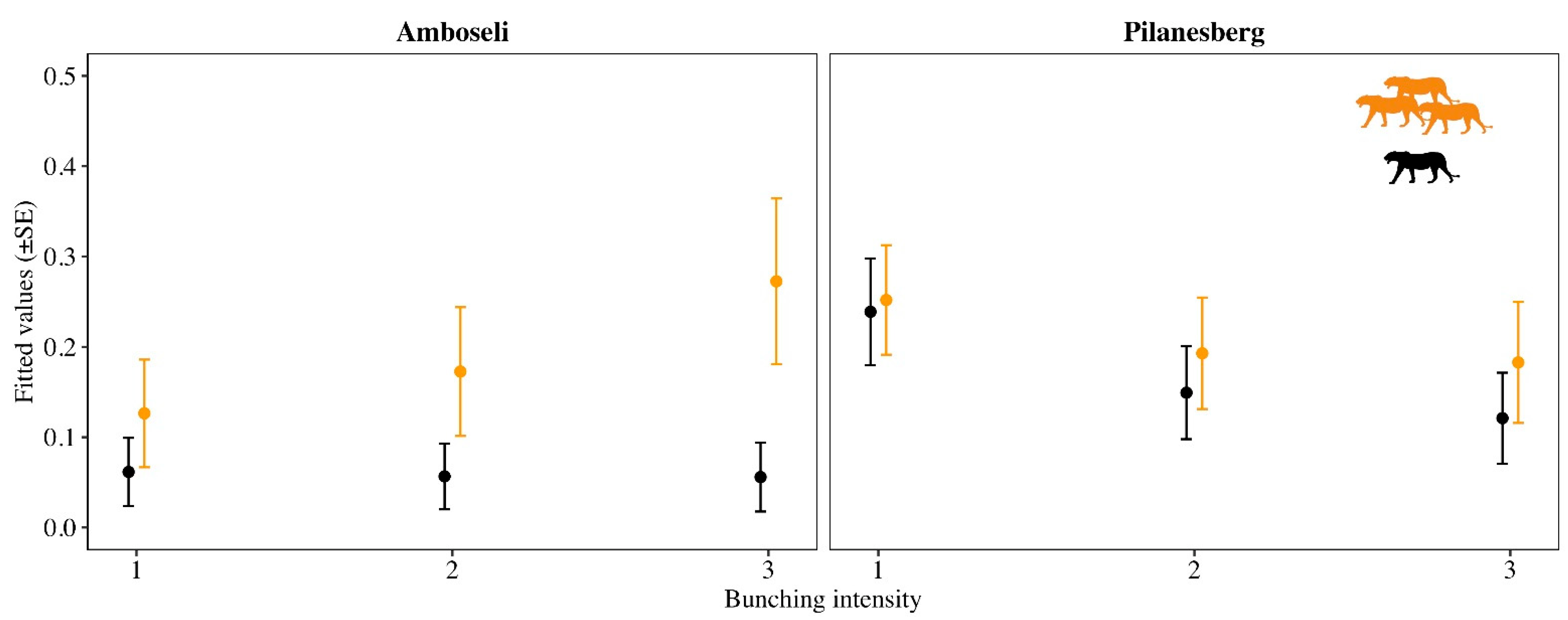
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couzin, I.D. Collective Cognition in Animal Groups. Trends Cogn. Sci. 2009, 13, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Biro, D. Cumulative Culture Can Emerge from Collective Intelligence in Animal Groups. Nat. Commun. 2017, 8, 15049. [Google Scholar] [CrossRef] [PubMed]
- McComb, K.; Moss, C.; Durant, S.M.; Baker, L.; Sayialel, S. Matriarchs as Repositories of Social Knowledge in African Elephants. Science 2001, 292, 491–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McComb, K.; Shannon, G.; Durant, S.M.; Sayialel, K.; Slotow, R.; Poole, J.; Moss, C. Leadership in Elephants: The Adaptive Value of Age. Proc. R. Soc. B Biol. Sci. 2011, 278, 3270–3276. [Google Scholar] [CrossRef] [Green Version]
- Brent, L.J.N.; Franks, D.W.; Foster, E.A.; Balcomb, K.C.; Cant, M.A.; Croft, D.P. Ecological Knowledge, Leadership, and the Evolution of Menopause in Killer Whales. Curr. Biol. 2015, 25, 746–750. [Google Scholar] [CrossRef] [Green Version]
- Van De Waal, E.; Borgeaud, C.; Whiten, A. Potent Social Learning and Conformity Shape a Wild Primate’s Foraging Decisions. Science 2013, 340, 483–485. [Google Scholar] [CrossRef]
- Kawamura, S.; Hinde, R.A.; Fisher, J.; Whiten, A.; Slagsvold, T.; Wiebe, K.L.; Thornton, A.; Rendell, L.; Whitehead, H.; Mesoudi, A.; et al. Lobtail Feeding in Humpback Whales. Science 2013, 340, 485–488. [Google Scholar]
- Alberts, S.C. Social Influences on Survival and Reproduction: Insights from a Long-Term Study of Wild Baboons. J. Anim. Ecol. 2019, 88, 47–66. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, H.; Smith, T.D.; Rendell, L. Adaptation of Sperm Whales to Open-Boat Whalers: Rapid Social Learning on a Large Scale? Biol. Lett. 2021, 17, 20210030. [Google Scholar] [CrossRef]
- Whiten, A. The Burgeoning Reach of Animal Culture. Science 2021, 372, eabe6514. [Google Scholar] [CrossRef]
- McComb, K.; Shannon, G.; Sayialel, K.N.; Moss, C. Elephants Can Determine Ethnicity, Gender, and Age from Acoustic Cues in Human Voices. Proc. Natl. Acad. Sci. USA 2014, 111, 5433–5438. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.A.; Sayialel, K.N.; Njiraini, N.W.; Moss, C.J.; Poole, J.H.; Byrne, R.W. Elephants Classify Human Ethnic Groups by Odor and Garment Color. Curr. Biol. 2007, 17, 1938–1942. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.; Stanton, M.A.; Patterson, E.M.; Bienenstock, E.J.; Singh, L.O. Social Networks Reveal Cultural Behaviour in Tool-Using Using Dolphins. Nat. Commun. 2012, 3, 980. [Google Scholar] [CrossRef] [Green Version]
- Wild, S.; Allen, S.J.; Krutzen, M.; King, S.L.; Gerber, L.; Hoppitt, W.J.E. Multi-Network-Based Diffusion Analysis Reveals Vertical Cultural Transmission of Sponge Tool Use within Dolphin Matrilines. Biol. Lett. 2019, 15, 20190227. [Google Scholar] [CrossRef]
- Brakes, P.; Dall, S.R.X.; Aplin, L.M.; Bearhop, S.; Carroll, E.L.; Ciucci, P.; Fishlock, V.; Ford, J.K.B.; Garland, E.C.; Keith, S.A.; et al. Animal Cultures Matter for Conservation. Science 2019, 363, 1032–1034. [Google Scholar] [CrossRef] [Green Version]
- Brakes, P.; Carroll, E.L.; Dall, S.R.X.; Keith, S.A.; McGregor, P.K.; Mesnick, S.L.; Noad, M.J.; Rendell, L.; Robbins, M.M.; Rutz, C.; et al. A Deepening Understanding of Animal Culture Suggests Lessons for Conservation. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202718. [Google Scholar] [CrossRef]
- Polansky, L.; Kilian, W.; Wittemyer, G. Elucidating the Significance of Spatial Memory on Movement Decisions by African Savannah Elephants Using State–Space Models. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143042. [Google Scholar] [CrossRef] [Green Version]
- Power, R.J.; Compion, R.X.S. Lion Predation on Elephants in the Savuti, Chobe National Park, Botswana. Afr. Zool. 2009, 44, 36–44. [Google Scholar] [CrossRef]
- Joubert, D. Hunting Behaviour of Lions (Panethea Leo) on Elephants (Loxodonta Africana) in Chobe National Park, Botswana. Afr. J. Ecol. 2006, 44, 279–281. [Google Scholar] [CrossRef]
- Gara, T.W.; Wang, T.; Dube, T.; Ngene, S.M.; Mpakairi, K.S. African Elephant (Loxodonta Africana) Select Less Fragmented Landscapes to Connect Core Habitats in Human-Dominated Landscapes. Afr. J. Ecol. 2021, 59, 370–377. [Google Scholar] [CrossRef]
- De Boer, W.F.; van Langevelde, F.; Prins, H.H.T.; De Ruiter, P.C.; Blanc, J.; Vis, M.J.P.; Gaston, K.J.; Hamilton, I.D. Understanding Spatial Differences in African Elephant Densities and Occurrence, A Continent-Wide Analysis. Biol. Conserv. 2013, 159, 468–476. [Google Scholar] [CrossRef]
- Wittemyer, G.; Northrup, J.M.; Blanc, J.; Douglas-Hamilton, I.; Omondi, P.; Burnham, K.P. Illegal Killing for Ivory Drives Global Decline in African Elephants. Proc. Natl. Acad. Sci. USA 2014, 111, 13117–13121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauenstein, S.; Kshatriya, M.; Blanc, J.; Dormann, C.F.; Beale, C.M. African Elephant Poaching Rates Correlate with Local Poverty, National Corruption and Global Ivory Price. Nat. Commun. 2019, 10, 2242. [Google Scholar] [CrossRef] [PubMed]
- Mpakairi, K.S.; Ndaimani, H.; Tagwireyi, P.; Zvidzai, M.; Madiri, T.H. Futuristic Climate Change Scenario Predicts a Shrinking Habitat for the African Elephant (Loxodonta Africana): Evidence from Hwange National Park, Zimbabwe. Eur. J. Wildl. Res. 2020, 66, 1. [Google Scholar] [CrossRef]
- Di Minin, E.; Slotow, R.; Fink, C.; Bauer, H.; Packer, C. A Pan-African Spatial Assessment of Human Conflicts with Lions and Elephants. Nat. Commun. 2021, 12, 2978. [Google Scholar] [CrossRef]
- Bradshaw, G.A.; Schore, A.N.; Brown, J.L.; Poole, J.H.; Moss, C.J. Elephant Breakdown. Nature 2005, 433, 807. [Google Scholar] [CrossRef]
- Slotow, R.; van Dyk, G. Delinquent Young Male Elephants in Mortality of Rhino. Koedoe 2001, 44, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Slotow, R.; van Dyk, G.; Poole, J.; Page, B.; Klocke, A. Older Bull Elephants Control Young Males. Nature 2000, 408, 425–426. [Google Scholar] [CrossRef]
- Shannon, G.; Slotow, R.; Durant, S.M.; Sayialel, K.N.; Poole, J.; Moss, C.; McComb, K. Effects of Social Disruption in Elephants Persist Decades after Culling. Front. Zool. 2013, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Gobush, K.; Kerr, B.; Wasser, S. Genetic Relatedness and Disrupted Social Structure in a Poached Population of African Elephants. Mol. Ecol. 2009, 18, 722–734. [Google Scholar] [CrossRef]
- Gobush, K.S.; Mutayoba, B.M.; Wasser, S.K. Long-Term Impacts of Poaching on Relatedness, Stress Physiology, and Reproductive Output of Adult Female African Elephants. Conserv. Biol. 2008, 22, 1590–1599. [Google Scholar] [CrossRef]
- Goldenberg, S.Z.; Wittemyer, G. Orphaned Female Elephant Social Bonds Reflect Lack of Access to Mature Adults. Sci. Rep. 2017, 7, 14408. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.M.; Webb, C.T.; Daballen, D.; Goldenberg, S.Z.; Lepirei, J.; Letitiya, D.; Lolchuragi, D.; Leadismo, C.; Douglas-Hamilton, I.; Wittemyer, G. Poaching of African Elephants Indirectly Decreases Population Growth through Lowered Orphan Survival. Curr. Biol. 2021, 31, 4156–4162.e5. [Google Scholar] [CrossRef]
- Archie, E.A.; Moss, C.J.; Alberts, S.C. The Ties That Bind: Genetic Relatedness Predicts the Fission and Fusion of Social Groups in Wild African Elephants. Proc. Biol. Sci. R. Soc. 2006, 273, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Davidson, Z.; Valeix, M.; Van Kesteren, F.; Loveridge, A.J.; Hunt, J.E.; Murindagomo, F.; Macdonald, D.W. Seasonal Diet and Prey Preference of the African Lion in a Waterhole-Driven Semi-Arid Savanna. PLoS ONE 2013, 8, e055182. [Google Scholar] [CrossRef] [Green Version]
- Loveridge, A.J.; Hunt, J.E.; Murindagomo, F.; Macdonald, D.W. Influence of Drought on Predation of Elephant (Loxodonta Africana) Calves by Lions (Panthera Leo) in an African Wooded Savannah. J. Zool. 2006, 270, 523–530. [Google Scholar] [CrossRef]
- Ruggiero, R.G. Opportunistic Predation on Elephant Calves. Afr. J. Ecol. 1991, 29, 86–89. [Google Scholar] [CrossRef]
- Funston, P.J.; Mills, M.G.L.; Biggs, H.C. Factors Affecting the Hunting Success of Male and Female Lions in the Kruger National Park. J. Zool. 2001, 253, 419–431. [Google Scholar] [CrossRef]
- Funston, P.J.; Mills, M.G.L.; Biggs, H.C.; Richardson, P.R.K. Hunting by Male Lions: Ecological Influences and Socioecological Implications. Anim. Behav. 1998, 56, 1333–1345. [Google Scholar] [CrossRef] [Green Version]
- Moss, C.J.; Croze, H.; Lee, P.C. The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; University of Chicago Press: Chicago, IL, USA, 2011. [Google Scholar]
- Moss, C.J. The Demography of an African Elephant (Loxodonta Africana) Population in Amboseli, Kenya. J. Zool. 2001, 255, 145–156. [Google Scholar] [CrossRef]
- Slotow, R.; van Dyk, G. Ranging of Older Male Elephants Introduced to an Existing Small Popula-Tion without Older Males: Pilanesberg National Park. Koedoe 2004, 47, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.M.; Funston, P.J. Rapid Growth Rates of Lion (Panthera Leo) Populations in Small, Fenced Reserves in South Africa: A Management Dilemma. South Afr. J. Wildl. Res. 2014, 44, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Huqa, T.J. The Impact of Climate Variability on the Ecology of a Lion (Panthera Leo Linnaeus 1758) Population and Lion Livestock Conflicts in the Amboseli Ecosystem-Kenya; Leiden University: Leiden, The Netherlands, 2015. [Google Scholar]
- Grinnell, J.; Packer, C.; Pusey, A.E. Cooperation in Male Lions: Kinship, Reciprocity or Mutualism? Anim. Behav. 1995, 49, 95–105. [Google Scholar] [CrossRef]
- McComb, K.; Packer, C.; Pusey, A. Roaring and Numerical Assessment in Contests between Groups of Female Lions, Panthera Leo. Anim. Behav. 1994, 47, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Christensen, R.H.B. Regression Models for Ordinal Data, R Package Version 2019.12-10; 2019. Available online: https://cran.r-project.org/package=ordinal (accessed on 12 October 2021).
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q) AIC (c), R Package Version; 2016. Available online: https://rdrr.io/cran/AICcmodavg/ (accessed on 12 October 2021).
- Thornton, A.; Clutton-Brock, T. Social Learning and the Development of Individual and Group Behaviour in Mammal Societies. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 978–987. [Google Scholar] [CrossRef]
- Keen, S.C.; Cole, E.F.; Sheehan, M.J.; Sheldon, B.C. Social Learning of Acoustic Anti-Predator Cues Occurs between Wild Bird Species. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192513. [Google Scholar] [CrossRef] [Green Version]
- Shier, D.M.; Owings, D.H. Effects of Social Learning on Predator Training and Postrelease Survival in Juvenile Black-Tailed Prairie Dogs, Cynomys Ludovicianus. Anim. Behav. 2007, 73, 567–577. [Google Scholar] [CrossRef]
- Manassa, R.P.; McCormick, M.I. Social Learning Improves Survivorship at a Life-History Transition. Oecologia 2013, 171, 845–852. [Google Scholar] [CrossRef]
- Lee, P.C.; Moss, C.J. The Social Context for Learning and Behavioural Development among Wild African Elephants. In Mammalian Social Learning: Comparative and Ecological Perspectives; Cambridge University Press: Cambridge, UK, 1999; pp. 102–125. [Google Scholar]
- Bates, L.A.; Handford, R.; Lee, P.C.; Njiraini, N.; Poole, J.H.; Sayialel, K.; Sayialel, S.; Moss, C.J.; Byrne, R.W. Why Do African Elephants (Loxodonta Africana) Simulate Oestrus? An Analysis of Longitudinal Data. PLoS ONE 2010, 5, e010052. [Google Scholar] [CrossRef] [Green Version]
- Chiyo, P.I.; Moss, C.J.; Alberts, S.C. The Influence of Life History Milestones and Association Networks on Crop-Raiding Behavior in Male African Elephants. PLoS ONE 2012, 7, e031382. [Google Scholar] [CrossRef]
- Joyce, H.; Poole, P.L.; Tyack, A.S.; Stoeger-Horwath, S.W. Elephants Are Capable of Vocal Learning. Nature 2005, 434, 455–456. [Google Scholar] [CrossRef] [Green Version]
- Stoeger, A.S.; Manger, P. Vocal Learning in Elephants: Neural Bases and Adaptive Context. Curr. Opin. Neurobiol. 2014, 28, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Stoeger, A.S.; Mietchen, D.; Oh, S.; de Silva, S.; Herbst, C.T.; Kwon, S.; Fitch, W.T. An Asian Elephant Imitates Human Speech. Curr. Biol. 2012, 22, 2144–2148. [Google Scholar] [CrossRef] [Green Version]
- King, L.E.; Douglas-Hamilton, I.; Vollrath, F. African Elephants Run from the Sound of Disturbed Bees. Curr. Biol. 2007, 17, 832–833. [Google Scholar] [CrossRef] [Green Version]
- Foley, C.; Pettorelli, N.; Foley, L. Severe Drought and Calf Survival in Elephants. Biol. Lett. 2008, 4, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Zanette, L.Y.; Clinchy, M. Ecology and Neurobiology of Fear in Free-Living Wildlife. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 297–318. [Google Scholar] [CrossRef]
- Maldonado-Chaparro, A.A.; Chaverri, G. Why Do Animal Groups Matter for Conservation and Management? Conserv. Sci. Pract. 2021, 3, e550. [Google Scholar] [CrossRef]
- Snijders, L.; Blumstein, D.T.; Stanley, C.R.; Franks, D.W. Animal Social Network Theory Can Help Wildlife Conservation. Trends Ecol. Evol. 2017, 32, 567–577. [Google Scholar] [CrossRef]
- Hayward, M.W.; Slotow, R. Management of reintroduced wildlife populations. In Reintroduction of Fish and Wildlife Populations; Jachowski, D.S., Millspaugh, J.J., Angermeier, P.L., Slotow, R., Eds.; University of California Press: Oakland, CA, USA, 2016; pp. 319–340. [Google Scholar]
- Goldenberg, S.Z.; Owen, M.A.; Brown, J.L.; Wittemyer, G.; Oo, Z.M.; Leimgruber, P. Increasing conservation translocation success by building social functionality in released populations. Glob. Ecol. Conserv. 2019, 18, e00604. [Google Scholar] [CrossRef]
| Response | Pop | Explanatory | K | AICc | ΔAICc | AICc Weight |
|---|---|---|---|---|---|---|
| Bunching | ANP | number of lions | 4 | 60.2 | 0.00 | 0.85 |
| null | 3 | 64.5 | 4.30 | 0.10 | ||
| PNP | null | 3 | 81.4 | 0.00 | 0.65 | |
| number of lions + number of adults | 5 | 83.4 | 2.00 | 0.24 | ||
| number of lions | 4 | 85.0 | 3.60 | 0.11 | ||
| Prolonged listening | ANP | null | 3 | 60.7 | 0.00 | 0.67 |
| number of lions | 4 | 62.7 | 2.01 | 0.25 | ||
| number of lions + number of adults | 5 | 65.2 | 4.44 | 0.07 | ||
| PNP | null | 3 | 78.1 | 0.00 | 0.81 | |
| number of lions | 4 | 81.1 | 3.00 | 0.18 | ||
| Bunching intensity | ANP | number of lions + number of adults | 6 | 90.4 | 0.00 | 0.40 |
| number of lions | 5 | 91.1 | 0.73 | 0.28 | ||
| number of lions + age of matriarch | 6 | 91.9 | 1.50 | 0.19 | ||
| number of lions+ age of matriarch + number of adults | 7 | 92.8 | 2.42 | 0.12 | ||
| PNP | number of lions + number of adults | 6 | 141.7 | 0.00 | 0.61 | |
| number of lions+ age of matriarch + number of adults | 7 | 144.3 | 2.65 | 0.16 | ||
| null | 4 | 144.9 | 3.24 | 0.12 | ||
| number of lions | 5 | 145.8 | 4.16 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shannon, G.; Cordes, L.S.; Slotow, R.; Moss, C.; McComb, K. Social Disruption Impairs Predatory Threat Assessment in African Elephants. Animals 2022, 12, 495. https://doi.org/10.3390/ani12040495
Shannon G, Cordes LS, Slotow R, Moss C, McComb K. Social Disruption Impairs Predatory Threat Assessment in African Elephants. Animals. 2022; 12(4):495. https://doi.org/10.3390/ani12040495
Chicago/Turabian StyleShannon, Graeme, Line S. Cordes, Rob Slotow, Cynthia Moss, and Karen McComb. 2022. "Social Disruption Impairs Predatory Threat Assessment in African Elephants" Animals 12, no. 4: 495. https://doi.org/10.3390/ani12040495
APA StyleShannon, G., Cordes, L. S., Slotow, R., Moss, C., & McComb, K. (2022). Social Disruption Impairs Predatory Threat Assessment in African Elephants. Animals, 12(4), 495. https://doi.org/10.3390/ani12040495