Assessment of Selected Morphological, Physical and Chemical Parameters of the Teeth of the Offspring of Female Rats Exposed to 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), Taking into Account the Protective Role of Selected Antioxidants—Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Procedure
- I.
- A control group of females (C), not treated with any chemicals;
- II.
- A group of females (TCDD) who received a solution of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in a single dose of 5 μg/kg BW them;
- III.
- A group of females (TCDD + E) administered a solution of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in a single dose of 5 μg/kg BW them. and a solution of α-tocopherol acetate at the dose of 30 mg/kg BW/day s.c. was administered for a period of 3 weeks;
- IV.
- A group of females (TCDD + ASA) administered a solution of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in a single dose of 5 μg/kg BW them and administration of acetylsalicylic acid suspension in starch solution at a dose of 50 mg/kg BW/day per os (p.o.) for a period of 3 weeks.
- Newborns (NC)—10 individuals (from C females);
- Newborns (NTCDD)—4 individuals (from TCDD females);
- Newborns (NTCDD + E)—7 individuals (from TCDD + E females);
- Newborns (NTCDD + ASA)—6 individuals (from TCDD + ASA females).
- α-tocopherol acetate (an oil solution of the drug prepared on an individual order by Hasco-Lek S.A. in Wroclaw) (vitamin E);
- Acetylsalicylic acid—Aspirin (Bayer) (drug suspension in starch solution was prepared at the Department of Medical Biochemistry, Wroclaw Medical University);
- Thiopental (Biochemie GmbH, Kundl, Austria);
- Standard solution of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Greyhound Chromatography and Allied Chemicals, cat. at the Department of Organic Technology, Wroclaw University of Technology);
2.2. Methods
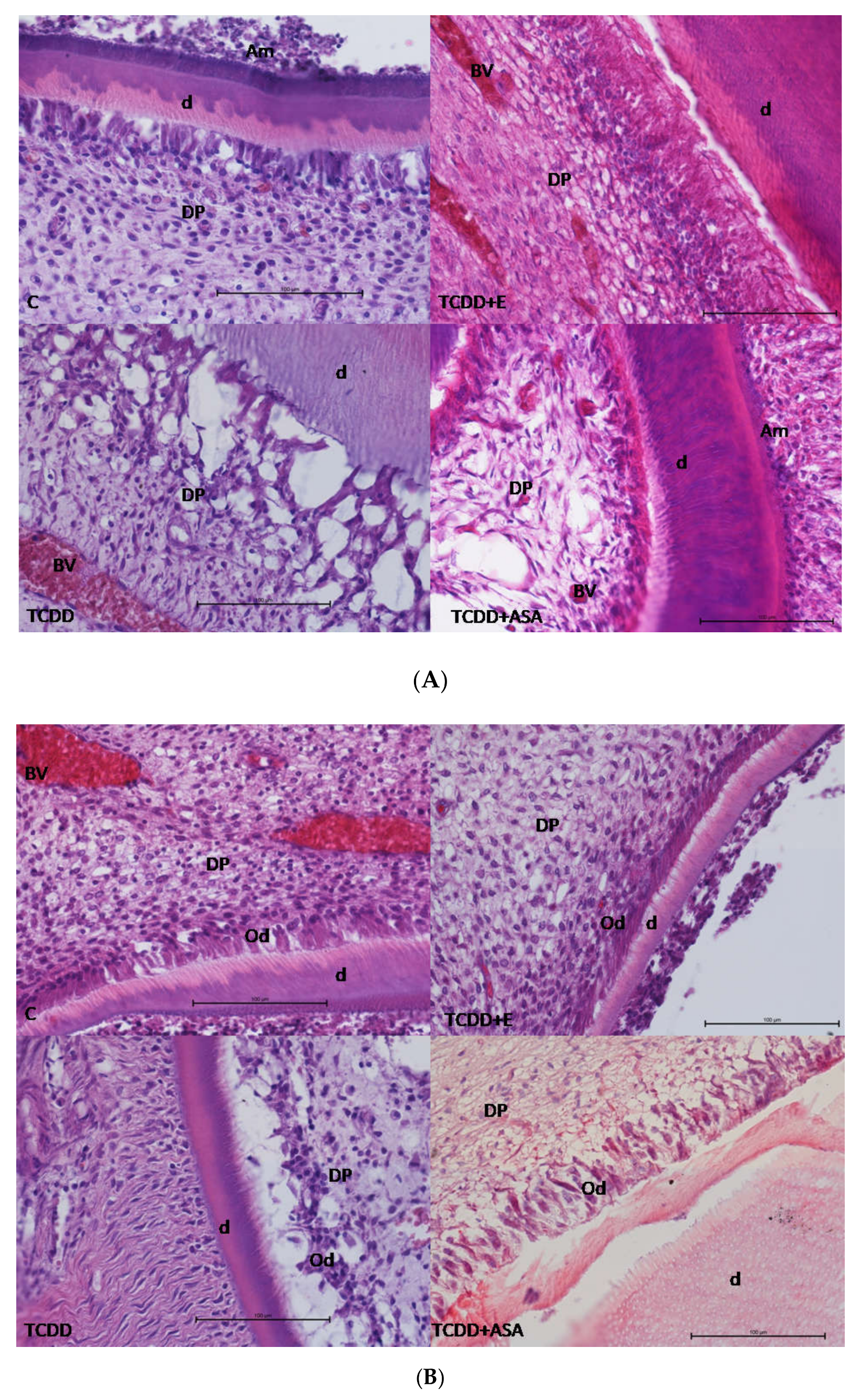
2.2.1. Histological Research
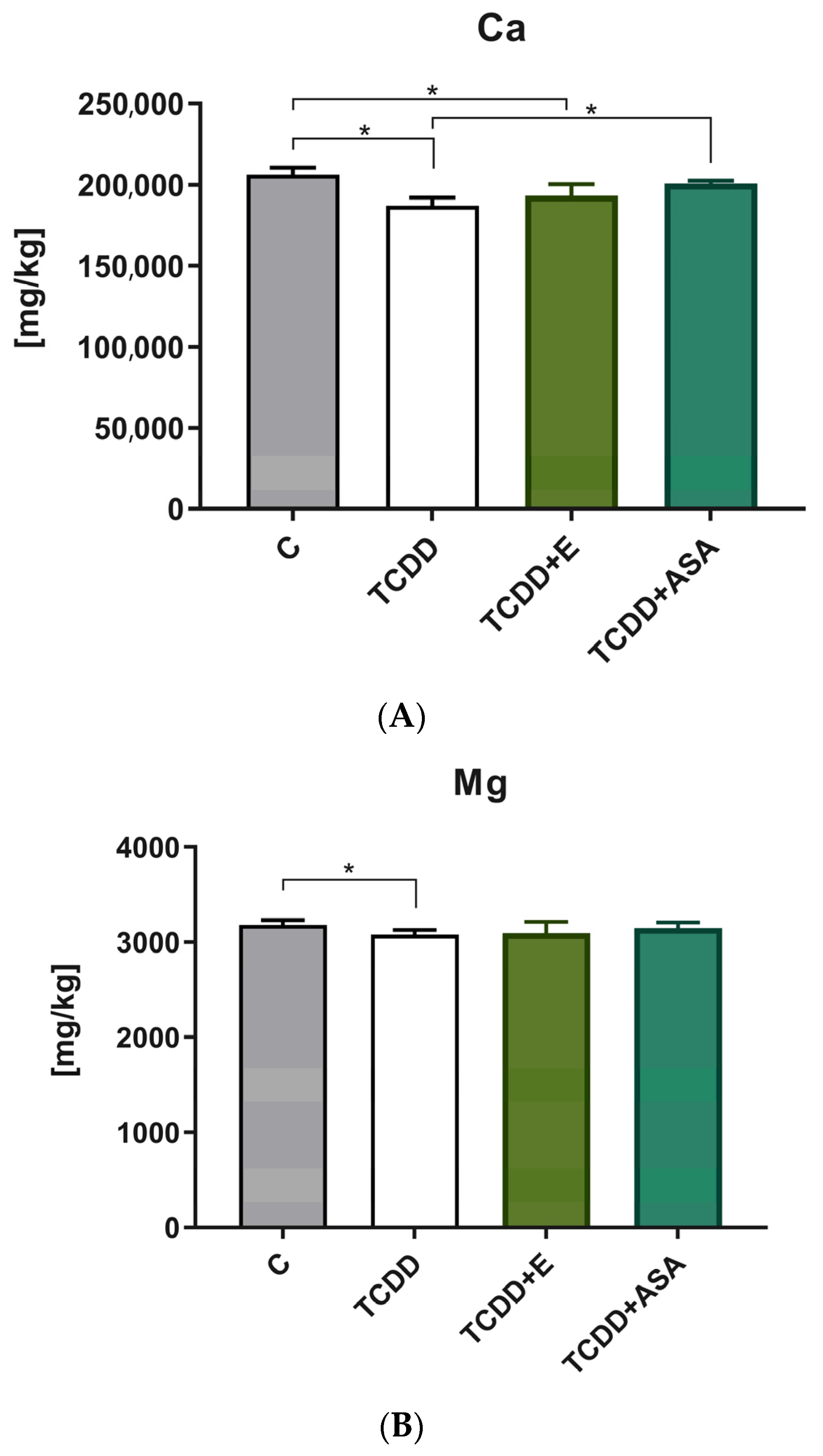
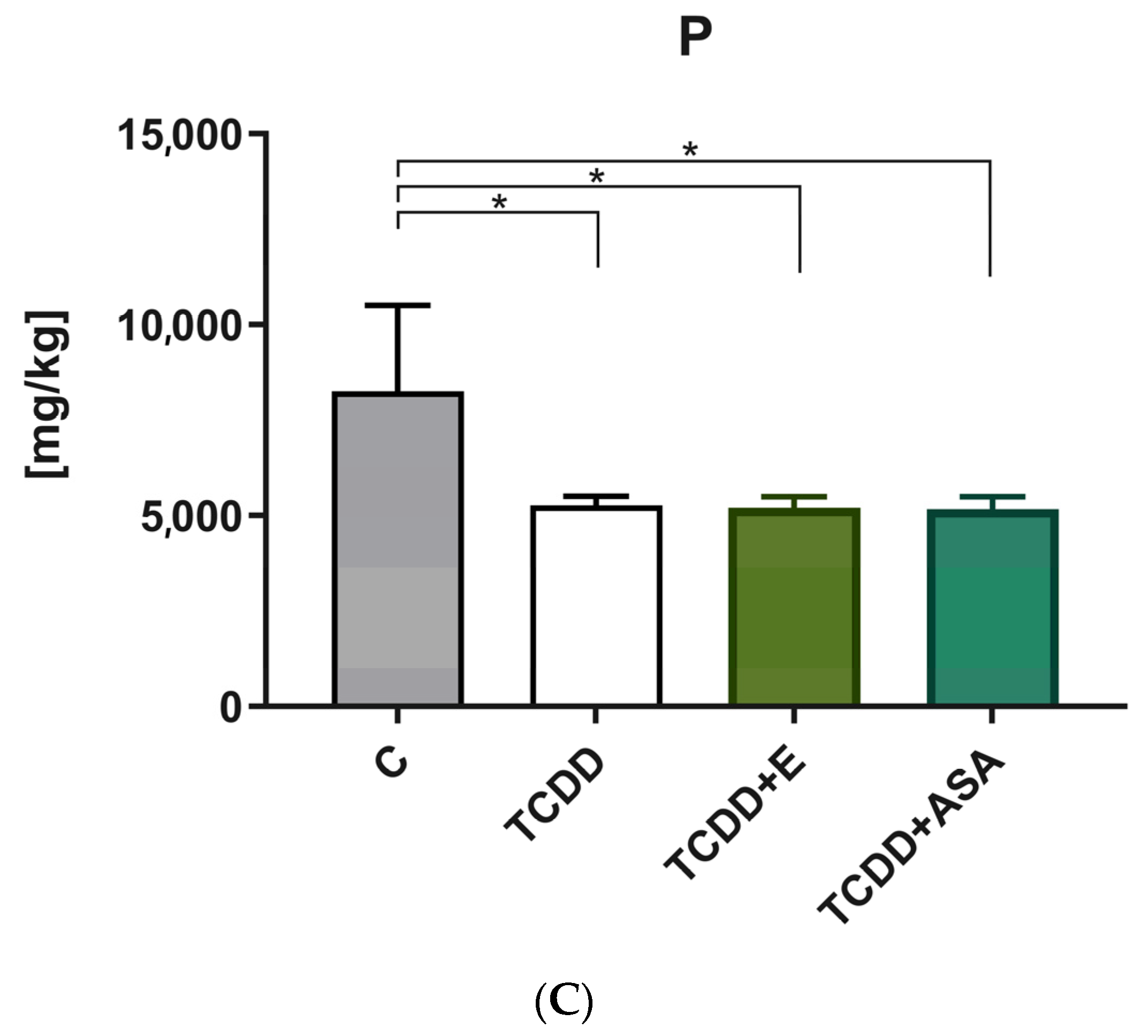
2.2.2. Determination of the Level of Ca, Mg and P
Mineralization of Research Material
Determination of Elements in the Research Material
- ‑
- Determination of calcium content by atomic emission spectrometry:
- ‑
- Determination of magnesium content by atomic absorption spectrometry:
- ‑
- Determination of phosphorus content by atomic emission spectrometry:
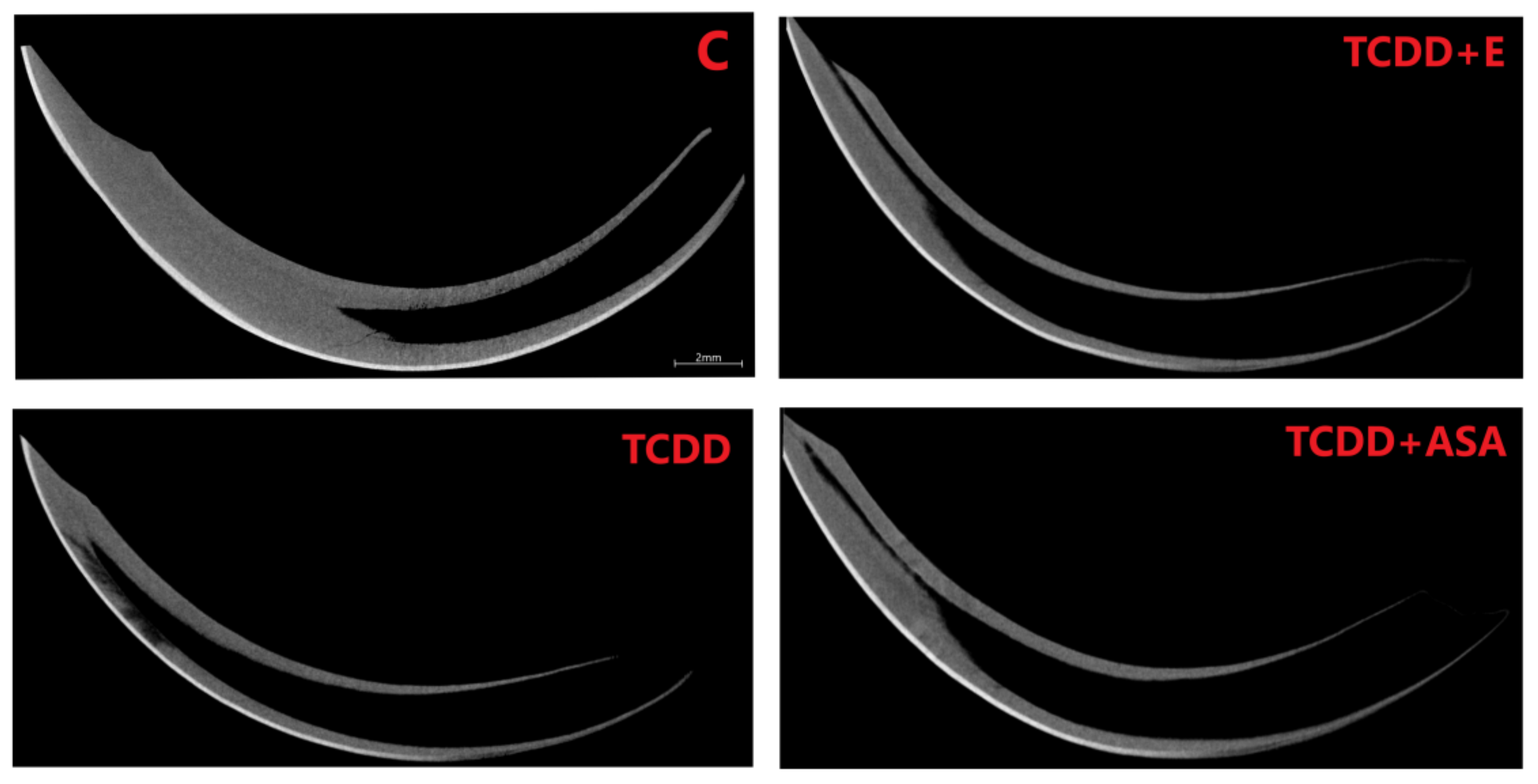
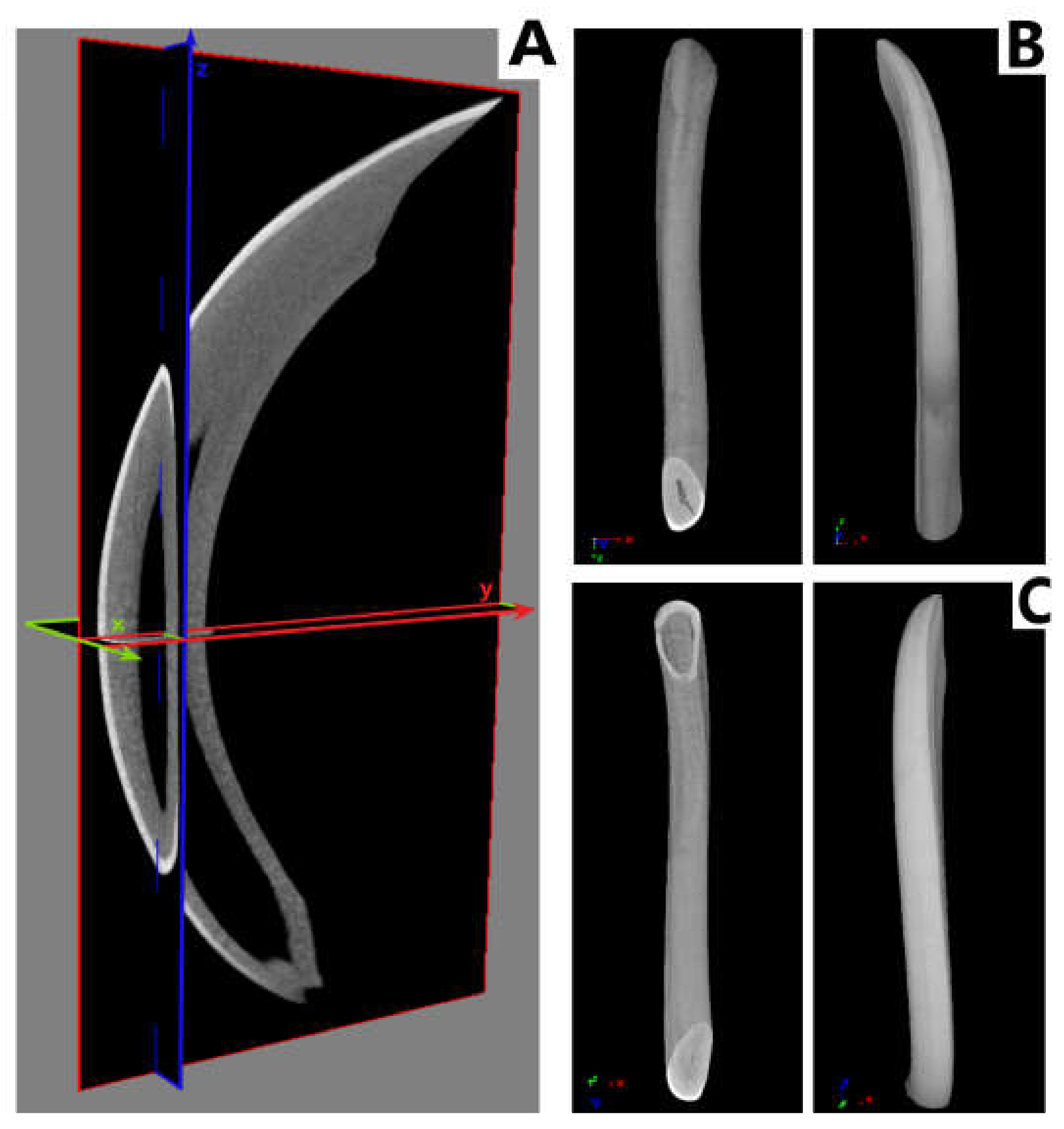
2.2.3. Measurement of Geometric Parameters and Tissue Density of Rats
2.2.4. Statistical Analysis
- ‑
- Estimating descriptive statistics (mean, standard deviation);
- ‑
- Checking the normality of the distribution of features using the Shapiro–Wilk test;
- ‑
- Verification of hypotheses about the equality of the level of measurable features with a normal distribution in more than two groups using the analysis of variance (ANOVA).
3. Results
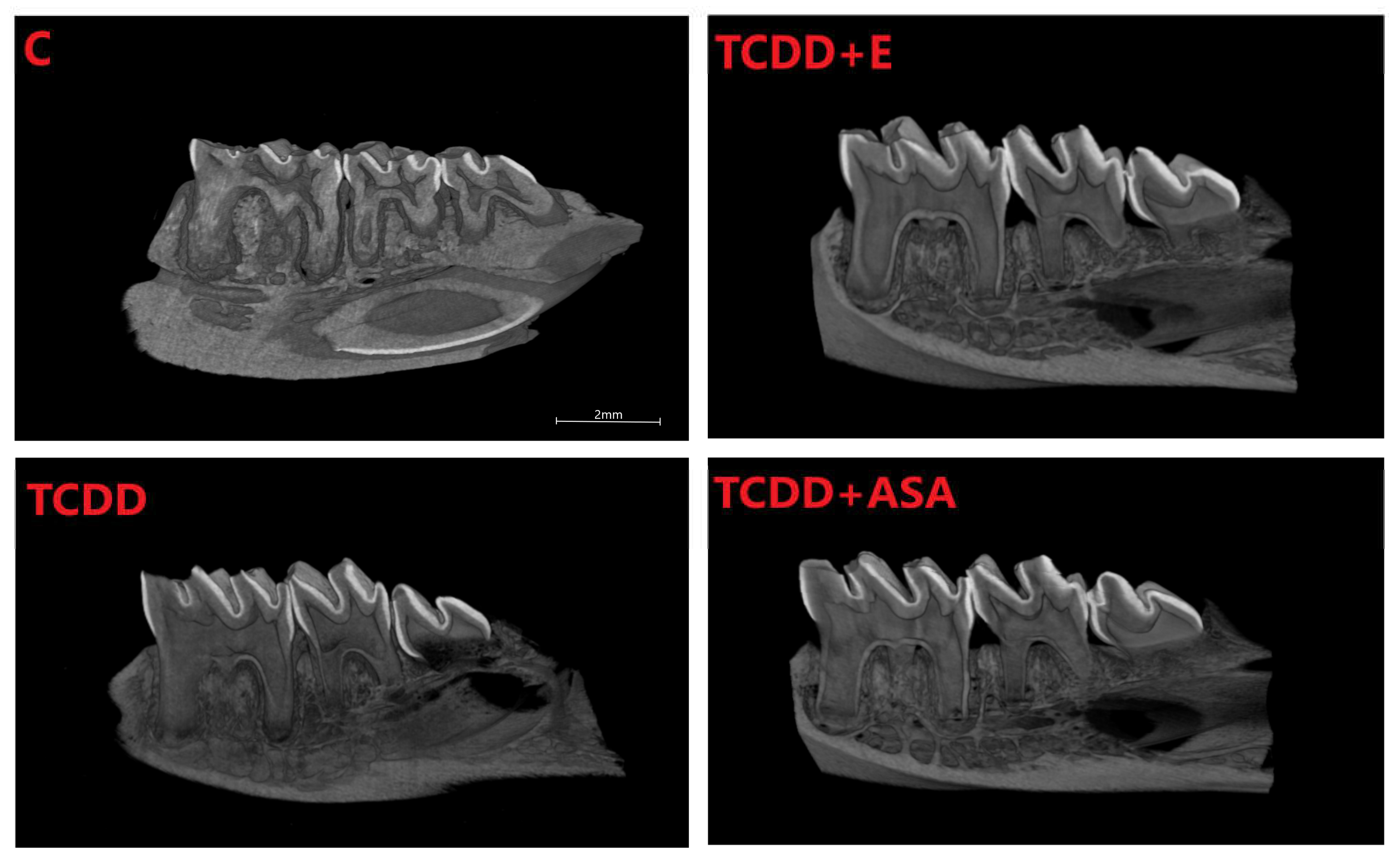
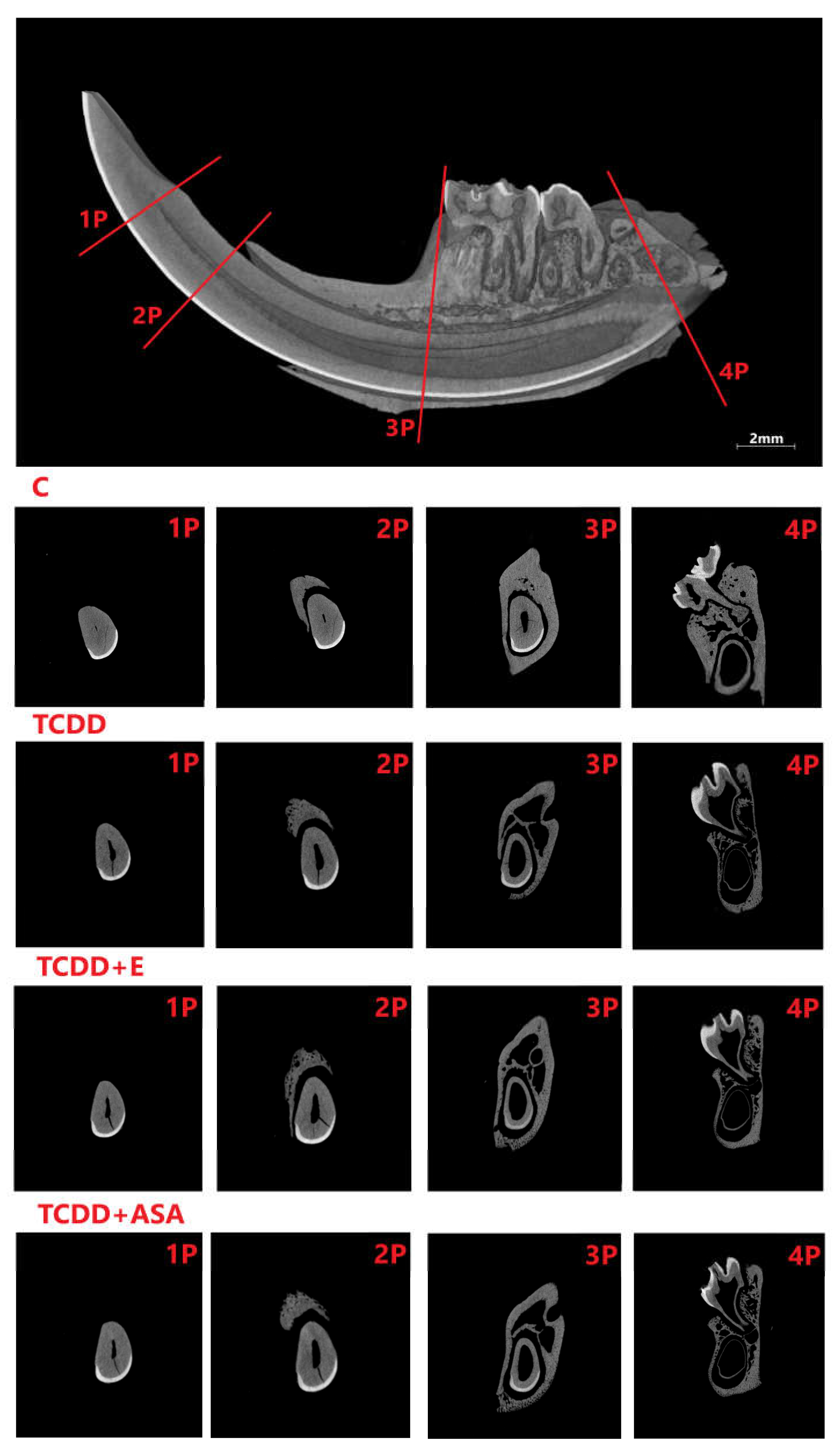
3.1. Histological Research
3.2. Elements’ Composition Analysis
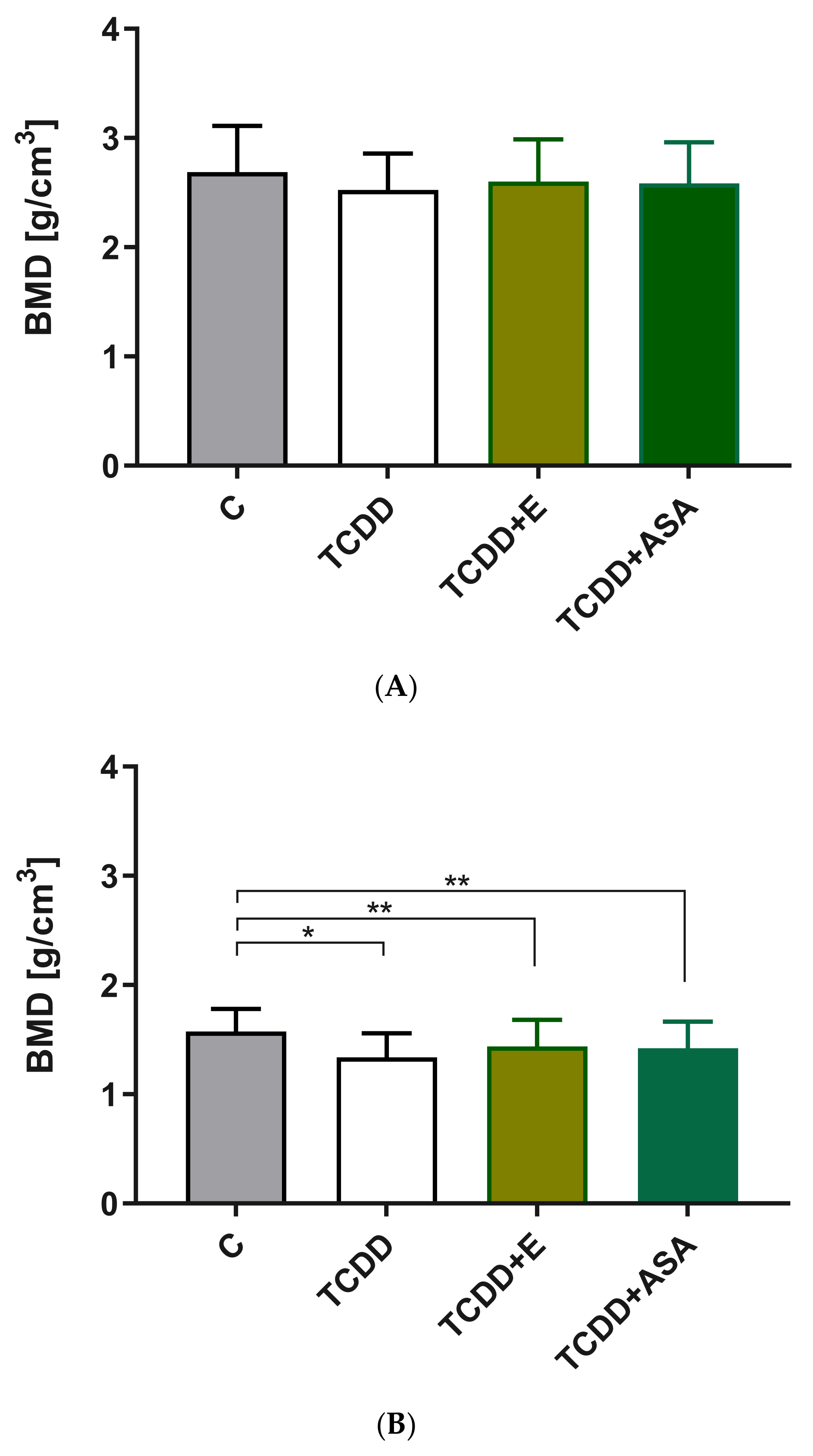
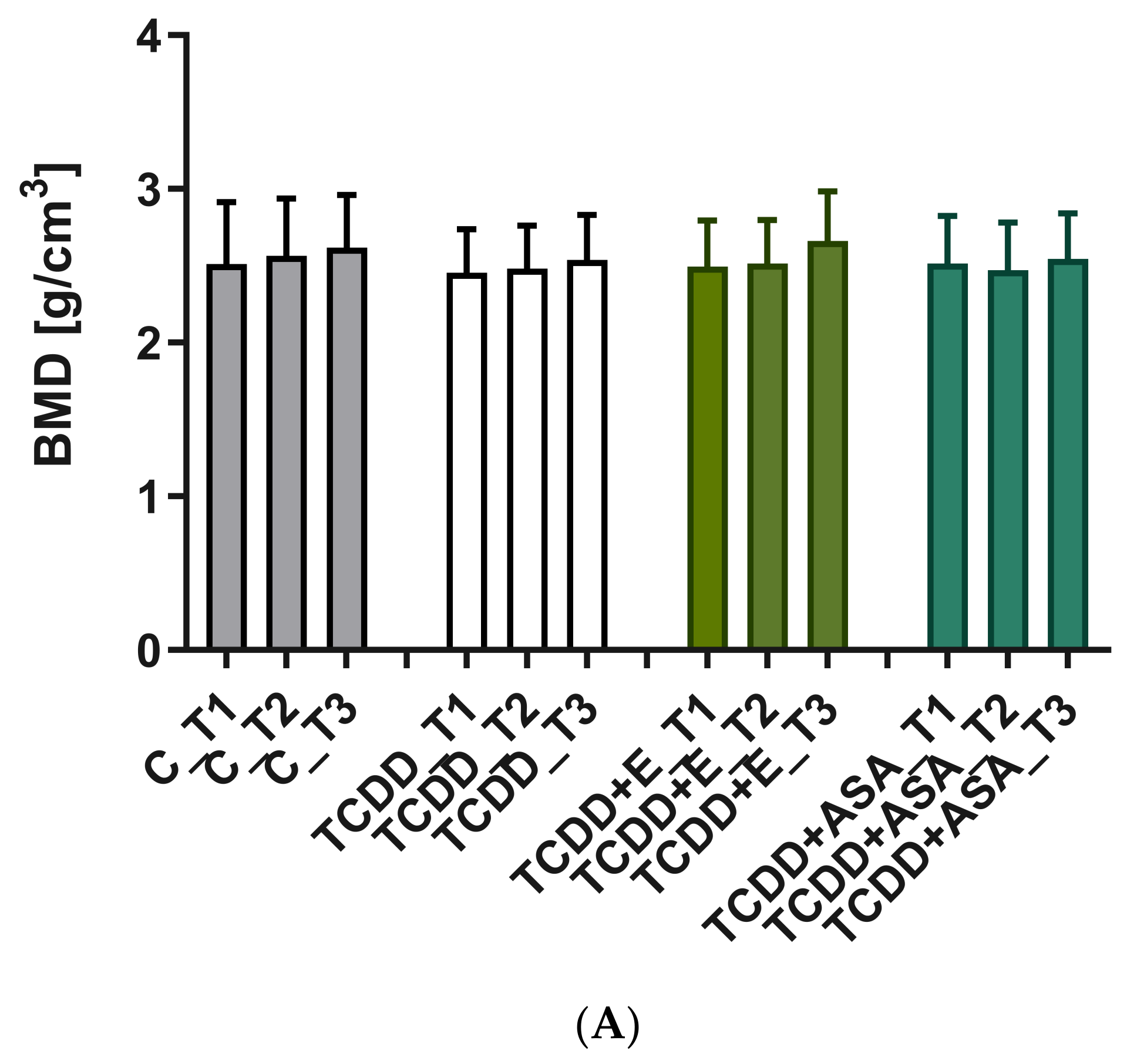
3.3. Measurement of Geometric Parameters and Tissue Density of Rats with Histological Picture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Bazan, J.; Dobrzyński, M.; Bronowicka-Szydełko, A.; Dzierzba, K. Influence of dioxin intoxication on the human system and possibilities of limiting its negative effects on the environment and living organisms. Ann. Agric. Environ. Med. 2014, 21, 518–524. [Google Scholar] [CrossRef]
- Całkosiński, I.; Borodulin-Nadzieja, L.; Stańda, M.; Wasilewska, U.; Pietraszkiewicz, T. Wpływ magnetostymulacji leczniczej na stężenie pochodnych kolagenu w przebiegu doświadczalnego zapalenia opłucnej. Med. Weter. 2003, 59, 161–164. [Google Scholar]
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Szopa, M.; Dobrzyński, M.; Gamian, A. High doses of tocopherol in the prevention and potentiation of dioxin in experimental inflammation-potential application. Postepy Hig. I Med. Dosw. (Online) 2011, 65, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, E.A.; Mathew, L.K.; Lohr, C.V.; Hasson, R.; Tanguay, R.L. Aryl hydrocarbon receptor activation impairs extracellular matrix remodeling during zebra fish fin regeneration. Toxicol. Sci. 2007, 95, 215–226. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.S.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (3,5,40-trihydroxystilbene) protects pregnant mother and fetus from the immunotoxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol. Nutr. Food Res. 2011, 55, 209–219. [Google Scholar] [CrossRef]
- Kiukkonen, A.; Sahlberg, C.; Lukinmaa, P.L.; Alaluusua, S.; Peltonen, E.; Partanen, A.M. 2,3,7,8-tetrachlorodibenzo-p-dioxin specifically reduces mRNA for the mineralization-related dentin sialophosphoprotein in cultured mouse embryonic molar teeth. Toxicol. Appl. Pharmacol. 2006, 216, 399–406. [Google Scholar] [CrossRef]
- Całkosiński, I.; Borodulin-Nadzieja, L.; Stańda, M.; Wasilewska, U.; Cegielski, M. Wpływ jednorazowej dawki TCDD na poziom estrogenów i reprodukcję u samic szczurów. Med. Weter. 2003, 59, 536–538. [Google Scholar]
- Całkosiński, I.; Dobrzyński, M.; Cegielski, M.; Sieja, A.; Całkosińska, M. The multifaceted effect of 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) in organisms, especially dentition changes. Postepy Hig. I Med. Dosw. (Online) 2006, 60, 237–240. [Google Scholar]
- Nishimura, N.; Nishimura, H.; Ito, T.; Miyata, C.; Izumi, K.; Fujimaki, H.; Matsumura, F. Dioxin-induced up-regulation of the active form of vitamin D is the main cause for its inhibitory action on osteoblast activities, leading to developmental bone toxicity. Toxicol. Appl. Pharmacol. 2009, 236, 301–309. [Google Scholar] [CrossRef]
- Singh, S.U.; Casper, R.F.; Fritz, P.C.; Sukhu, B.; Ganss, B.; Girard, B., Jr.; Savouret, J.F.; Tenenbaum, H.C. Inhibition of dioxin effects on bone formation in vitro by a newly described aryl hydrocarbon receptor antagonist, resveratrol. J. Endocrinol. 2000, 167, 183–195. [Google Scholar] [CrossRef]
- Kloser, E.; Böhmdorfer, S.; Brecker, L.; Kählig, H.; Netscher, T.; Mereiter, K. Synthesis of 5- (Fluorophenyl) tocopherols as Novel Dioxin Receptor Antagonists. Eur. J. Org. Chem. 2011, 13, 2450–2457. [Google Scholar] [CrossRef]
- MacDonald, C.J.; Ciolino, H.P.; Yeh, G.C. The drug salicylamide is an antagonist of the aryl hydrocarbon receptor that inhibits signal transduction induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Res. 2004, 64, 429–434. [Google Scholar] [CrossRef]
- Kmieć, Z. Histologia i Cytofizjologia Zęba i Jamy Ustnej; Elsevier Urban & Partner: Wrocław, Poland, 2007. [Google Scholar]
- Hagermann, E.; Schmidt, G. Ratte und Maus; Walter de Gruyter & Co: Berlin, Germany, 1960. [Google Scholar]
- Griffith, J.Q.; Farris, E.J. The Rat Laboratory Investigation; Hafner Publishing Company: New York, NY, USA, 1962. [Google Scholar]
- Krzanowska, H.; Preibisch, J.; Korda, P. Zwierzęta Laboratoryjne Hodowla i Użytkowanie; PZWL: Warszawa, Poland, 1974. [Google Scholar]
- Maciejewska, I.; Adamowicz-Klepalska, B.; Kmieć, Z. Wpływ niskiego i wysokiego stężenia fluorku sodowego podawanego w wodzie pitnej ciężarnym samicom szczura na odontogenezę potomstwa. Czas. Stomatol. 2000, 53, 3–10. [Google Scholar]
- Maciejewska, I.; Bereznowski, M. The aspects of the formation of extracellular matrix in mineralized tissues including the disturbances caused by fluoride. Part I Enamel. 1998, 35, 661–669. [Google Scholar]
- Królikowska-Prasał, I.; Czerny, K.; Majewska, T. Histomorfologia Narządu Zębowego; Wyd. Delfin: Słupsk, Poland, 1993. [Google Scholar]
- Polska Norma PN-EN 14084: 2004; Artykuły Żywnościowe. Oznaczanie Pierwiastków Śladowych. Oznaczanie Zawartości Sodu i Magnezu Metodą Atomowej Spektrometrii Absorpcyjnej (AAS) po Mineralizacji Mikrofalowej; Polski Komitet Normalizacyjny: Warsaw, Poland, 2004.
- Dobrzyński, M.; Kaczmarek, U.; Kuropka, P.; Reichert, P.; Grzech-Leśniak, K.; Całkosiński, I. Tooth development disorders in infants of rat dams exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin and protective role of tocopherol and acetylsalicylic acid. Pol. J. Vet. Sci. 2017, 20, 769–778. [Google Scholar]
- Dobrzyński, M.; Całkosiński, I.; Przywitowska, I.; Kobierska-Brzoza, J.; Czajczyńska-Waszkiewicz, A.; Sołtan, E.; Parulska, O. Effects of dioxins in environmental pollution on development of tooth disorders. Pol. J. Vet. Sci. 2009, 18, 319–323. [Google Scholar]
- Dobrzyński, M.; Korczyński, M.; Herman, K.; Całkosiński, I. Evaluation of the protective effect of different doses of alpha-tocopherol on calcium and magnesium content in bone tissue of rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Przem. Chem. 2016, 95, 1794–1796. [Google Scholar]
- Dobrzyński, M.; Kuropka, P.; Leśków, A.; Herman, K.; Tarnowska, M.; Wiglusz, R.J. Co-expression of thearyl hydrocarbon receptor and estrogen receptor in the developing teeth of rat offspring after rat mothers’ exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and the protective action of α-tocopherol and acetylsalicylic acid. Adv. Clin. Exp. Med. 2019, 28, 973–980. [Google Scholar] [CrossRef]
- Dobrzyński, M.; Kuropka, P.; Tarnowska, M.; Styczynska, M.; Dudek, K.; Leskow, A.; Targonska, S.; Wiglusz, R.J. The protective effect of α-tocopherol on the content of selected elements in the calvaria for exposed hens to TCDD in the early embryonic period. Biol. Trace Elem. Res. 2019, 190, 517–525. [Google Scholar] [CrossRef]
- Dobrzyński, M.; Kuropka, P.; Tarnowska, M.; Dudek, K.; Styczynska, M.; Leskow, A.; Targonska, S.; Wiglusz, R.J. Indirect study of the effect of a-tocopherol and acetylsalicylic acid on the mineral composition of bone tissue in the offspring of female rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: Longterm observations. RSC Adv. 2019, 9, 8016–8024. [Google Scholar] [CrossRef]
- Dobrzyński, M.; Pezowicz, C.; Tomanik, M.; Kuropka, P.; Dudek, K.; Fita, K.; Styczynska, M.; Wiglusz, R.J. Modulating effect of selected pharmaceuticals on bone in female rats exposed to 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD). RSC Adv. 2018, 8, 27537–27545. [Google Scholar] [CrossRef]
- Geng, H.O.; Zhang, J.C.; Hu, B.; Wang, J.B. Effects of lactational dioxin exposure to development of alveolar bone in SD rat offspring. Zhonghua Kou Qiang Yi Xue Za Zhi 2008, 43, 278–280. (In Chinese) [Google Scholar] [PubMed]
- Alaluusua, S.; Lukinmaa, P.L. Developmental dental toxicity of dioxin and related compounds—A review. Int. Dent. J. 2006, 56, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, H.M.; Sorvari, R.; Alaluusua, S.; Murtomaa, M.; Tuukkanen, J.; Viluksela, M. The effect of perinatal TCDD exposure on caries susceptibility in rats. Toxicol. Sci. 2006, 91, 568–575. [Google Scholar] [CrossRef][Green Version]
- Partanen, A.M.; Kiukkonen, A.; Sahlberg, C.; Alaluusua, S.; Thesleff, I.; Pohjanvirta, R.; Lukinmaa, P.L. Developmental toxicity of dioxin to mouse embryonic teeth in vitro: Arrest of tooth morphogenesis involves stimulation of apoptotic program in the dental epithelium. Toxicol. Appl. Pharmacol. 2004, 194, 24–33, Erratum in Toxicol. Appl. Pharmacol. 2005, 202, 212–214. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Walker, N.J.; Jokinen, M.P.; Brix, A.E.; Sells, D.M.; Marsh, T.; Wyde, M.E.; Orzech, D.; Haseman, J.K.; Nyska, A. Gingival carcinogenicity in female Harlan Sprague-Dawley rats following two-year oral treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like compounds. Toxicol. Sci. 2005, 83, 64–77, Erratum in Toxicol. Sci. 2005, 83, 405–406.. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, L.; Wei, K.; Zhao, J.; Wang, Y.; Jin, F.; Xuan, K. Exposure to a continuous low dose of tetrachlorodibenzo-p-dioxin impairs the development of the tooth root in lactational rats and alters the function of apical papilla-derived stem cells. Arch. Oral Biol. 2015, 60, 199–207. [Google Scholar] [CrossRef]
- Gao, Y.; Sahlberg, C.; Kiukkonen, A.; Alaluusua, S.; Pohjanvirta, R.; Tuomisto, J.; Lukinmaa, P.L. Lactational exposure of Han/Wistar rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin interferes with enamel maturation and retards dentin mineralization. J. Dent. Res. 2004, 83, 139–144. [Google Scholar] [CrossRef]
- Simanainen, U.; Tuomisto, J.T.; Tuomisto, J.; Viluksela, M. Dose-response analysis of short-term effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in three differentially susceptible rat lines. Toxicol. Appl. Pharmacol. 2003, 187, 128–136. [Google Scholar] [CrossRef]
- Miettinen, H.M.; Alaluusua, S.; Tuomisto, J.; Viluksela, M. Effect of in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on rat molar development: The role of exposure time. Toxicol. Appl. Pharmacol. 2002, 184, 57–66. [Google Scholar] [CrossRef]
- Kiukkonen, A.; Viluksel, M.; Sahlberg, C.; Alaluusua, S.; Tuomisto, J.T.; Tuomisto, J.; Lukinmaa, P.L. Response of the incisor tooth to 2,3,7,8-tetrachlorodibenzo-p-dioxin in a dioxin-resistant and a dioxin-sensitive rat strain. Toxicol. Sci. 2002, 69, 482–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kattainen, H.; Tuukkanen, J.; Simanainen, U.; Tuomisto, J.T.; Kovero, O.; Lukinmaa, P.L.; Alaluusua, S.; Tuomisto, J.; Viluksela, M. In utero/lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure impairs molar tooth development in rats. Toxicol. Appl. Pharmacol. 2001, 174, 216–224. [Google Scholar] [CrossRef]
- Aulerich, R.J.; Yamini, B.; Bursian, S.J. Dietary exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) does not induce proliferation of squamous epithelium or osteolysis in the jaws of weanling rats. Vet. Hum. Toxicol. 2001, 43, 170–171. [Google Scholar] [PubMed]
- Alaluusua, S.; Lukinmaa, P.L.; Pohjanvirta, R.; Unkila, M.; Tuomisto, J. Exposure to 2,3,7,8-tetrachlorodibenzo-para-dioxin leads to defective dentin formation and pulpal perforation in rat incisor tooth. Toxicology 1993, 81, 1–13. [Google Scholar] [CrossRef]


| Transaxial Plane (XY) | Coronary Plane (XZ) | |
|---|---|---|
| T.Or (°) | difference | difference |
| C | 25.354 | 14.606 |
| TCDD | 21.553 | 30.748 |
| TCDD+E | 24.225 | 33.941 |
| TCDD+ASA | 21.879 | 30.008 |
| E.Th (mm) | ||||
|---|---|---|---|---|
| Group | IN | T1 | T2 | T3 |
| C | 0.150 ± 0.04 | 0.103 ± 0.02 | 0.118 ± 0.02 | 0.127 ± 0.02 |
| TCDD | 0.092 ± 0.02 | 0.106 ± 0.02 | 0.115 ± 0.02 | 0.120 ± 0.03 |
| TCDD+E | 0.099 ± 0.03 | 0.103 ± 0.02 | 0.112 ± 0.03 | 0.121 ± 0.03 |
| TCDD+ASA | 0.099 ± 0.02 | 0.101 ± 0.02 | 0.110 ± 0.02 | 0.115 ± 0.03 |
| EV/TV (%) | ||||
| Group | IN | T1 | T2 | T3 |
| C | 15.074 ± 2.2 | 25.523 ± 4.1 | 27.561 ± 5.1 a | 28.851 ± 4.3 b |
| TCDD | 14.030 ± 1.5 | 28.718 ± 3.4 | 35.331 ± 4.2 a | 44.009 ± 5.2 b |
| TCDD+E | 13.686 ± 2.2 | 30.031 ± 4.5 | 35.966 ± 5.3 | 43.808 ± 5.5 |
| TCDD+ASA | 12.506 ± 2.1 | 28.836 ± 4.8 | 33.139 ± 5.6 | 43.095 ± 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyński, M.; Nikodem, A.; Klećkowska-Nawrot, J.; Goździewska-Harłajczuk, K.; Janeczek, M.; Styczyńska, M.; Kuropka, P. Assessment of Selected Morphological, Physical and Chemical Parameters of the Teeth of the Offspring of Female Rats Exposed to 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), Taking into Account the Protective Role of Selected Antioxidants—Preliminary Study. Animals 2022, 12, 484. https://doi.org/10.3390/ani12040484
Dobrzyński M, Nikodem A, Klećkowska-Nawrot J, Goździewska-Harłajczuk K, Janeczek M, Styczyńska M, Kuropka P. Assessment of Selected Morphological, Physical and Chemical Parameters of the Teeth of the Offspring of Female Rats Exposed to 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), Taking into Account the Protective Role of Selected Antioxidants—Preliminary Study. Animals. 2022; 12(4):484. https://doi.org/10.3390/ani12040484
Chicago/Turabian StyleDobrzyński, Maciej, Anna Nikodem, Joanna Klećkowska-Nawrot, Karolina Goździewska-Harłajczuk, Maciej Janeczek, Marzena Styczyńska, and Piotr Kuropka. 2022. "Assessment of Selected Morphological, Physical and Chemical Parameters of the Teeth of the Offspring of Female Rats Exposed to 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), Taking into Account the Protective Role of Selected Antioxidants—Preliminary Study" Animals 12, no. 4: 484. https://doi.org/10.3390/ani12040484
APA StyleDobrzyński, M., Nikodem, A., Klećkowska-Nawrot, J., Goździewska-Harłajczuk, K., Janeczek, M., Styczyńska, M., & Kuropka, P. (2022). Assessment of Selected Morphological, Physical and Chemical Parameters of the Teeth of the Offspring of Female Rats Exposed to 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), Taking into Account the Protective Role of Selected Antioxidants—Preliminary Study. Animals, 12(4), 484. https://doi.org/10.3390/ani12040484









