Surround Modulation Properties of Tectal Neurons in Pigeons Characterized by Moving and Flashed Stimuli
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
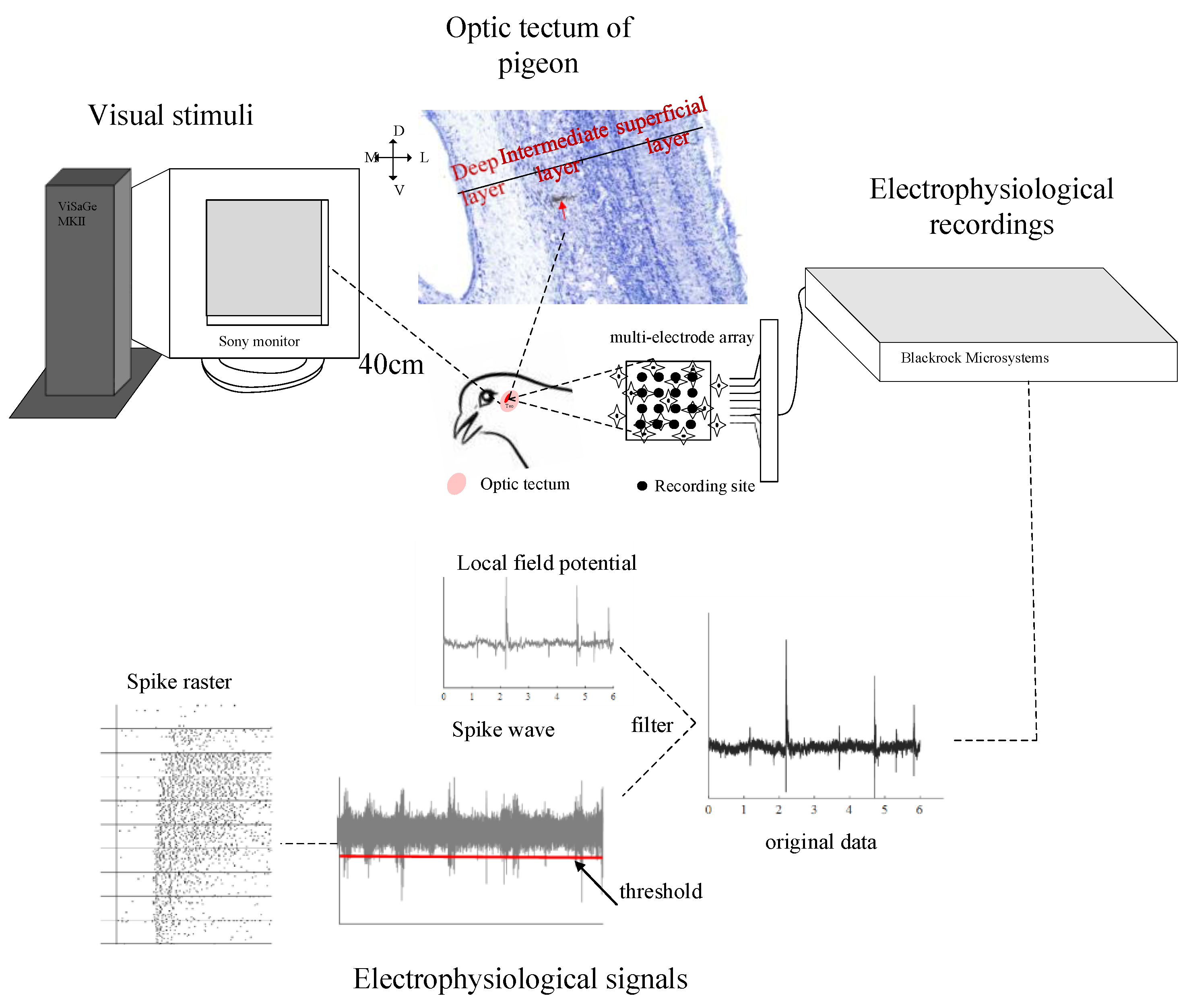
2.1. Animal Preparation
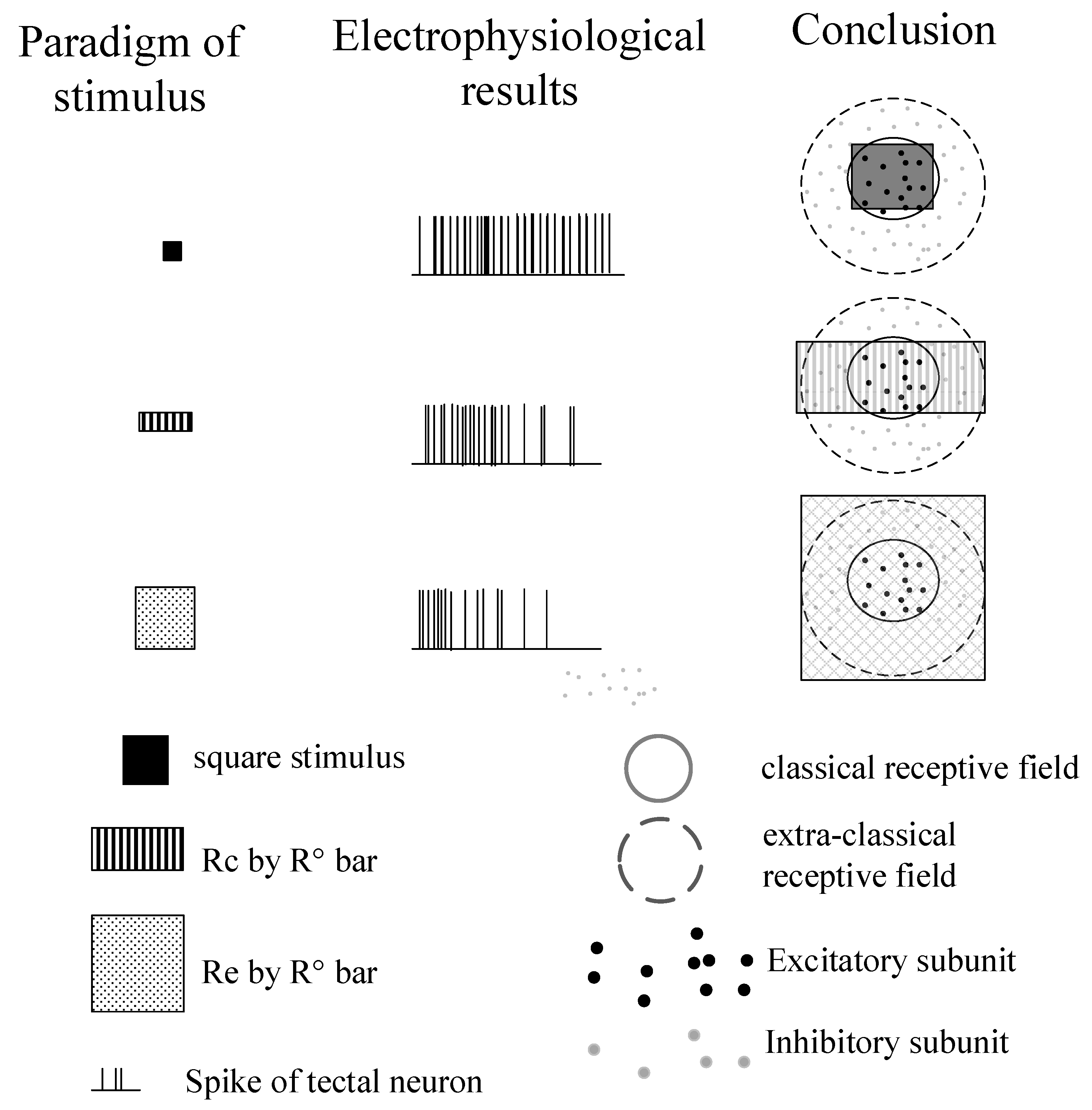
2.2. Visual Stimuli and Electrophysiological Recordings
2.3. Data Analysis
3. Results
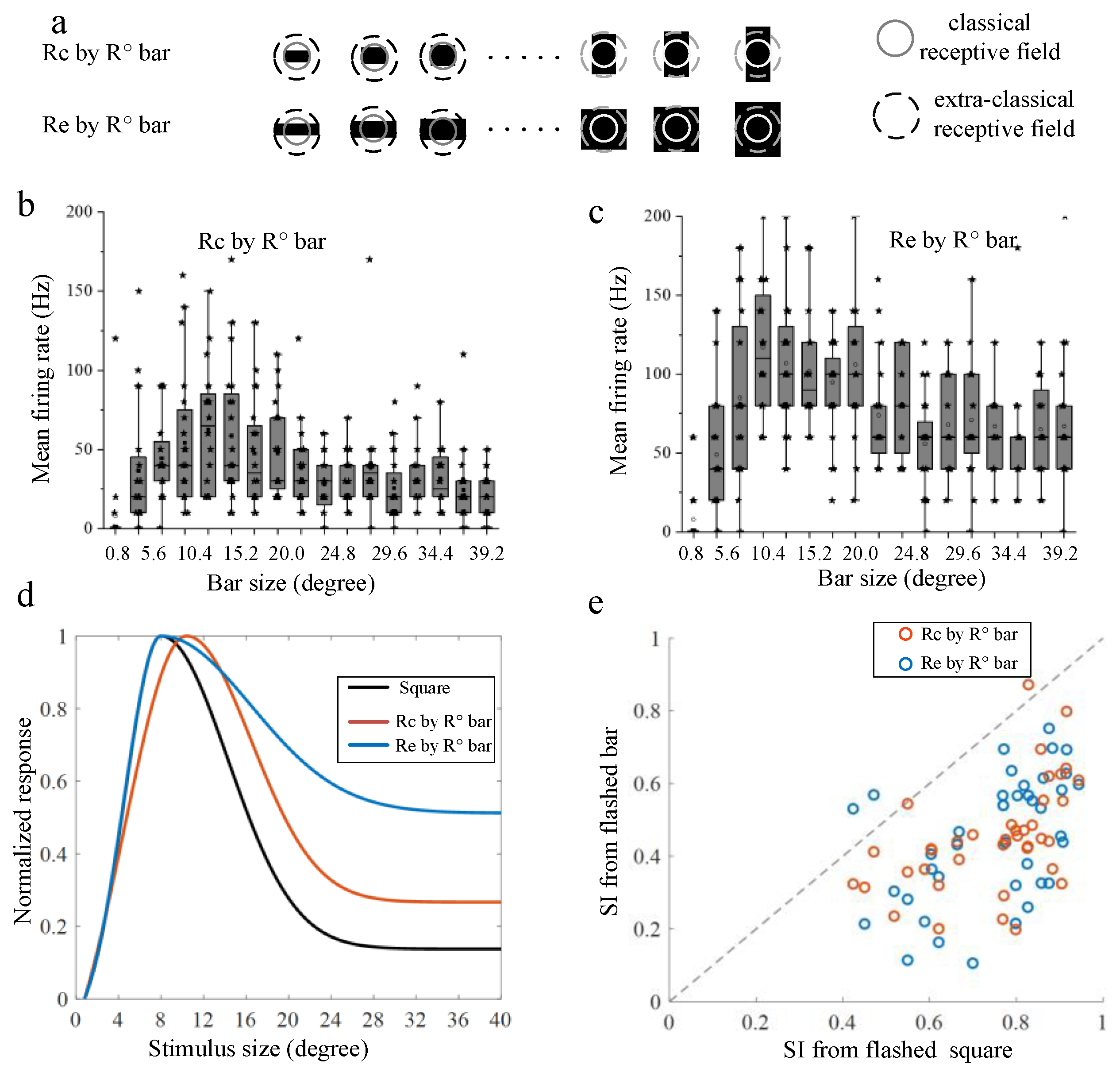
3.1. Surround Modulation Properties by Flashed Stimuli
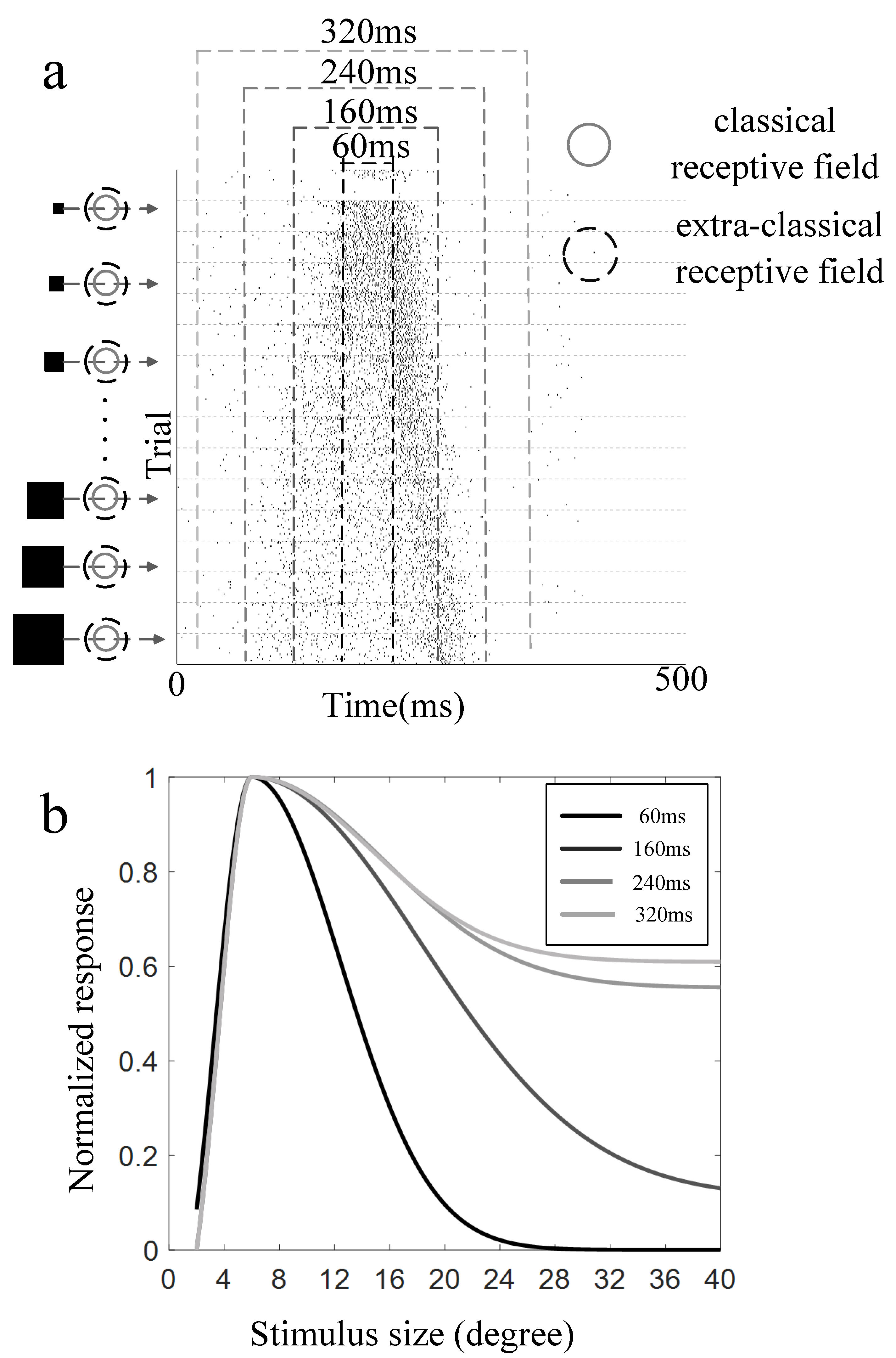
3.2. Surround Modulation Properties by Moving Stimuli
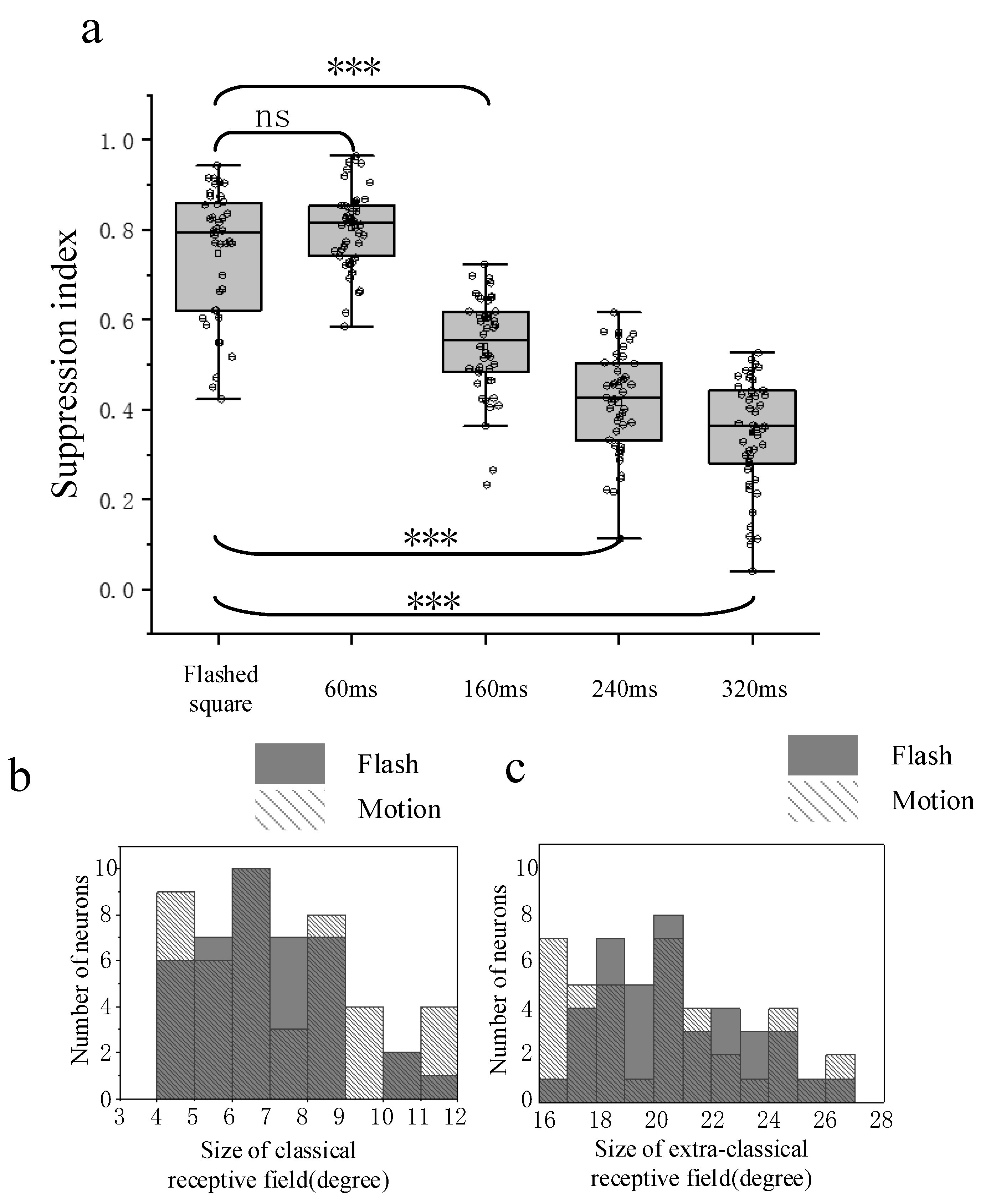
3.3. Comparison of Surround Suppression by Moving and Flashed Stimuli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlow, H.B. Summation and inhibition in the frog’s retina. J. Physiol. 1953, 119, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H.; Wiesel, T.N. Receptive Fields and Functional Architecture in Two Nonstriate Visual Areas (18 and 19) of the Cat. J. Neurophysiol. 1965, 28, 229–289. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.R.; Bair, W.; Movshon, J.A. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J. Neurophysiol. 2002, 88, 2530–2546. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, C.; Tobin, E.A. Lateral inhibition between orientation detectors in the cat’s visual cortex. Exp. Brain Res. 1972, 15, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.I.; Frost, B.J. Orientation-selective inhibition from beyond the classic visual receptive field. Brain Res. 1978, 139, 359–365. [Google Scholar] [CrossRef]
- Knierim, J.J.; van Essen, D.C. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. J. Neurophysiol. 1992, 67, 961–980. [Google Scholar] [CrossRef]
- DeAngelis, G.C.; Freeman, R.D.; Ohzawa, I. Length and width tuning of neurons in the cat’s primary visual cortex. J. Neurophysiol. 1994, 71, 347–374. [Google Scholar] [CrossRef]
- Sengpiel, F.; Sen, A.; Blakemore, C. Characteristics of surround inhibition in cat area 17. Exp. Brain Res. 1997, 116, 216–228. [Google Scholar] [CrossRef]
- Series, P.; Lorenceau, J.; Fregnac, Y. The "silent" surround of V1 receptive fields: Theory and experiments. J. Physiol. Paris. 2003, 97, 453–474. [Google Scholar] [CrossRef]
- Sceniak, M.P.; Ringach, D.L.; Hawken, M.J.; Shapley, R. Contrast’s effect on spatial summation by macaque V1 neurons. Nat. Neurosci. 1999, 2, 733–739. [Google Scholar] [CrossRef]
- Walker, G.A.; Ohzawa, I.; Freeman, R.D. Asymmetric suppression outside the classical receptive field of the visual cortex. J. Neurosci. 1999, 19, 10536–10553. [Google Scholar] [CrossRef]
- Walker, G.A.; Ohzawa, I.; Freeman, R.D. Suppression outside the classical cortical receptive field. Vis. Neurosci. 2000, 17, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, T.; Sato, H.; Yoshimura, Y.; Ozeki, H.; Shimegi, S. Suppressive effects of receptive field surround on neuronal activity in the cat primary visual cortex. Neurosci. Res. 2002, 43, 207–220. [Google Scholar] [CrossRef]
- Maffei, L.; Fiorentini, A. The unresponsive regions of visual cortical receptive fields. Vision. Res. 1976, 16, 1131–1139. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, W. Extensive integration field beyond the classical receptive field of cat’s striate cortical neurons--classification and tuning properties. Vision. Res. 1994, 34, 2337–2355. [Google Scholar] [CrossRef]
- Sillito, A.M.; Grieve, K.L.; Jones, H.E.; Cudeiro, J.; Davis, J. Visual cortical mechanisms detecting focal orientation discontinuities. Nature 1995, 378, 492–496. [Google Scholar] [CrossRef]
- Levitt, J.B.; Lund, J.S. Contrast dependence of contextual effects in primate visual cortex. Nature 1997, 387, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Polat, U.; Mizobe, K.; Pettet, M.W.; Kasamatsu, T.; Norcia, A.M. Collinear stimuli regulate visual responses depending on cell’s contrast threshold. Nature 1998, 391, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Albright, T.D.; Stoner, G.R. Contextual influences on visual processing. Annu. Rev. Neurosci. 2002, 25, 339–379. [Google Scholar] [CrossRef]
- Cavanaugh, J.R.; Bair, W.; Movshon, J.A. Selectivity and spatial distribution of signals from the receptive field surround in macaque V1 neurons. J. Neurophysiol. 2002, 88, 2547–2556. [Google Scholar] [CrossRef]
- Webb, B.S.; Tinsley, C.J.; Vincent, C.J.; Derrington, A.M. Spatial distribution of suppressive signals outside the classical receptive field in lateral geniculate nucleus. J. Neurophysiol. 2005, 94, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Barchini, J.; Shi, X.; Chen, H.; Cang, J. Bidirectional encoding of motion contrast in the mouse superior colliculus. Elife 2018, 7, e35261. [Google Scholar] [CrossRef]
- Okamoto, M.; Naito, T.; Sadakane, O.; Osaki, H.; Sato, H. Surround suppression sharpens orientation tuning in the cat primary visual cortex. Eur. J. Neurosci. 2009, 29, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Dan, Y.; Li, C.Y. Stimulation of non-classical receptive field enhances orientation selectivity in the cat. J. Physiol. 2005, 564, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Shapley, R.M.; Hawken, M.J.; Ringach, D.L. Effect of stimulus size on the dynamics of orientation selectivity in Macaque V1. J. Neurophysiol. 2005, 94, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Shi, L.; Wan, H.; Wang, Z.; Shang, Z.; Li, Z. Dynamic functional connectivity among neuronal population during modulation of extra-classical receptive field in primary visual cortex. Brain Res. Bull. 2015, 117, 45–53. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, G.; Solomon, S.G. Dynamic Contextual Modulation in Superior Colliculus of Awake Mouse. eNeuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Bowers, A.N. Visual circuits of the avian telencephalon: Evolutionary implications. Behav. Brain Res. 1999, 98, 183–191. [Google Scholar] [CrossRef]
- Soto, F.A.; Wasserman, E.A. Mechanisms of object recognition: What we have learned from pigeons. Front. Neural. Circuits. 2014, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.J.; Colombo, M. The functional architecture, receptive field characteristics, and representation of objects in the visual network of the pigeon brain. Prog. Neurobiol. 2020, 195, 101781. [Google Scholar] [CrossRef]
- Güntürkün, O. Sensory Physiology: Vision. In Sturkie’s Avian Physiology (Fifth Edition); Whittow, G.C., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 1–19. [Google Scholar]
- Henry, C.A.; Jazayeri, M.; Shapley, R.M.; Hawken, M.J. Distinct spatiotemporal mechanisms underlie extra-classical receptive field modulation in macaque V1 microcircuits. Elife 2020, 9, e54264. [Google Scholar] [CrossRef]
- Li, Y.; Young, L.S. Unraveling the mechanisms of surround suppression in early visual processing. PLoS Comput. Biol. 2021, 17, e1008916. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.J.; DiFranco, D.E. Motion characteristics of single units in the pigeon optic tectum. Vision. Res. 1976, 16, 1229–1234. [Google Scholar] [CrossRef]
- Verhaal, J.; Luksch, H. Neuronal responses to motion and apparent motion in the optic tectum of chickens. Brain Res. 2016, 1635, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, L.; Wang, Z.; Niu, X.; Hu, Y.; Shi, L. Luminance information decoding on the basis of local field potential signals of pigeon optic tectum neurons. Neuroreport 2017, 28, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, M.; Wang, Z.; Shi, L. First spike latency of ON/OFF neurons in the optic tectum of pigeons. Integr. Zool. 2019, 14, 479–493. [Google Scholar] [CrossRef]
- Jassik-Gerschenfeld, D.; Guichard, J. Visual receptive fields of single cells in the pigeon’s optic tectum. Brain Res. 1972, 40, 303–317. [Google Scholar] [CrossRef]
- Hardy, O.; Leresche, N.; Jassik-Gerschenfeld, D. The spatial organization of the excitatory regions in the visual receptive fields of the pigeon’s optic tectum. Exp. Brain Res. 1982, 46, 59–68. [Google Scholar] [CrossRef]
- Leresche, N.; Hardy, O.; Jassik-Gerschenfeld, D. Suppressive regions in the visual receptive fields of single cells of the pigeon’s optic tectum. Exp. Brain Res. 1984, 53, 327–334. [Google Scholar] [CrossRef]
- Frost, B.J.; Scilley, P.L.; Wong, S.C. Moving background patterns reveal double-opponency of directionally specific pigeon tectal neurons. Exp. Brain Res. 1981, 43, 173–185. [Google Scholar] [CrossRef]
- Frost, B.J.; Cavanagh, P.; Morgan, B. Deep tectal cells in pigeons respond to kinematograms. J. Comp. Physiol. A 1988, 162, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Zhao, J.; Southall, T.L.; Xu, B. Contextual influences on the directional responses of tectal cells in pigeons. Vis. Neurosci. 2002, 19, 133–144. [Google Scholar] [CrossRef]
- Zahar, Y.; Wagner, H.; Gutfreund, Y. Responses of tectal neurons to contrasting stimuli: An electrophysiological study in the barn owl. PLoS One 2012, 7, e39559. [Google Scholar] [CrossRef] [PubMed]
- Zahar, Y.; Lev-Ari, T.; Wagner, H.; Gutfreund, Y. Behavioral Evidence and Neural Correlates of Perceptual Grouping by Motion in the Barn Owl. J. Neurosci. 2018, 38, 6653–6664. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Huang, S.; Yang, S.; Wang, Z.; Li, Z.; Shi, L. Comparison of pop-out responses to luminance and motion contrasting stimuli of tectal neurons in pigeons. Brain Res. 2020, 1747, 147068. [Google Scholar] [CrossRef] [PubMed]
- Letelier, J.C.; Marin, G.; Sentis, E.; Tenreiro, A.; Fredes, F.; Mpodozis, J. The mapping of the visual field onto the dorso-lateral tectum of the pigeon (Columba livia) and its relations with retinal specializations. J. Neurosci. Methods 2004, 132, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.Q.; Xiao, Q.; Liu, R.F.; Wu, L.Q.; Wang, S.R. Response characteristics of the pigeon’s pretectal neurons to illusory contours and motion. J. Physiol. 2006, 577, 805–813. [Google Scholar] [CrossRef]
- Ozeki, H.; Sadakane, O.; Akasaki, T.; Naito, T.; Shimegi, S.; Sato, H. Relationship between excitation and inhibition underlying size tuning and contextual response modulation in the cat primary visual cortex. J. Neurosci. 2004, 24, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Sceniak, M.P.; Hawken, M.J.; Shapley, R. Visual spatial characterization of macaque V1 neurons. J. Neurophysiol. 2001, 85, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Osaki, H.; Naito, T.; Sadakane, O.; Okamoto, M.; Sato, H. Surround suppression by high spatial frequency stimuli in the cat primary visual cortex. Eur. J. Neurosci. 2011, 33, 923–932. [Google Scholar] [CrossRef]
- Jassik-Gerschenfeld, D.; Minois, F.; Conde-Courtine, F. Receptive field properties of directionally selective units in the pigeon’s optic tectum. Brain Res. 1970, 24, 407–421. [Google Scholar] [CrossRef]
- Hughes, C.P.; Pearlman, A.L. Single unit receptive fields and the cellular layers of the pigeon optic tectum. Brain Res. 1974, 80, 365–377. [Google Scholar] [CrossRef]
- Hardy, O.; Jassik-Gerschenfeld, D. Spatial organization of the excitatory regions in the receptive fields of the avian optic tectum and their spatial frequency selectivity. Brain Res. 1980, 199, 452–458. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Wang, S. Regional variation in receptive field properties of tectal neurons in pigeons. Brain Behav. Evol. 2000, 55, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Leresche, N.; Hardy, O.; Audinat, E.; Jassik-Gerschenfeld, D. Synaptic organization of inhibitory circuits in the pigeon’s optic tectum. Brain Res. 1986, 365, 383–387. [Google Scholar] [CrossRef]
- Winkowski, D.E.; Knudsen, E.I. Top-down gain control of the auditory space map by gaze control circuitry in the barn owl. Nature 2006, 439, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Winkowski, D.E.; Knudsen, E.I. Top-down control of multimodal sensitivity in the barn owl optic tectum. J. Neurosci. 2007, 27, 13279–13291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Wang, S.R. Excitatory and inhibitory receptive fields of tectal cells are differentially modified by magnocellular and parvocellular divisions of the pigeon nucleus isthmi. J. Comp. Physiol. A 2000, 186, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Faunes, M.; Fernandez, S.; Gutierrez-Ibanez, C.; Iwaniuk, A.N.; Wylie, D.R.; Mpodozis, J.; Karten, H.J.; Marin, G. Laminar segregation of GABAergic neurons in the avian nucleus isthmi pars magnocellularis: A retrograde tracer and comparative study. J. Comp. Neurol. 2013, 521, 1727–1742. [Google Scholar] [CrossRef]
- Wang, Y.; Major, D.E.; Karten, H.J. Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus). J. Comp. Neurol. 2004, 469, 275–297. [Google Scholar] [CrossRef]
- Frost, B.J. Moving background patterns alter directionally specific responses of pigeon tectal neurons. Brain Res. 1978, 151, 599–603. [Google Scholar] [CrossRef]
- McIlwain, J.T. Receptive Fields of Optic Tract Axons and Lateral Geniculate Cells: Peripheral Extent and Barbiturate Sensitivity. J. Neurophysiol. 1964, 27, 1154–1173. [Google Scholar] [CrossRef] [PubMed]
- Olveczky, B.P.; Baccus, S.A.; Meister, M. Segregation of object and background motion in the retina. Nature 2003, 423, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Tikidji-Hamburyan, A.; Reinhard, K.; Seitter, H.; Hovhannisyan, A.; Procyk, C.A.; Allen, A.E.; Schenk, M.; Lucas, R.J.; Munch, T.A. Retinal output changes qualitatively with every change in ambient illuminance. Nat. Neurosci. 2015, 18, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Y.V.; Park, S.J.; Demb, J.B.; Butts, D.A. Divisive suppression explains high-precision firing and contrast adaptation in retinal ganglion cells. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.D.; Murphy, G.J. Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. J. Neurosci. 2014, 34, 13458–13471. [Google Scholar] [CrossRef]
- Fisher, T.G.; Alitto, H.J.; Usrey, W.M. Retinal and Nonretinal Contributions to Extraclassical Surround Suppression in the Lateral Geniculate Nucleus. J. Neurosci. 2017, 37, 226–235. [Google Scholar] [CrossRef]
- Angelucci, A.; Bijanzadeh, M.; Nurminen, L.; Federer, F.; Merlin, S.; Bressloff, P.C. Circuits and Mechanisms for Surround Modulation in Visual Cortex. Annu. Rev. Neurosci. 2017, 40, 425–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Yang, G.; Dai, W.; Yang, Y.; Han, C.; Wang, X.; Zhang, Y.; Xing, D. Laminar Subnetworks of Response Suppression in Macaque Primary Visual Cortex. J. Neurosci. 2020, 40, 7436–7450. [Google Scholar] [CrossRef] [PubMed]
- Gur, M.; Kagan, I.; Snodderly, D.M. Orientation and direction selectivity of neurons in V1 of alert monkeys: Functional relationships and laminar distributions. Cereb. Cortex. 2005, 15, 1207–1221. [Google Scholar] [CrossRef]
- Schumacher, J.W.; Schneider, D.M.; Woolley, S.M. Anesthetic state modulates excitability but not spectral tuning or neural discrimination in single auditory midbrain neurons. J. Neurophysiol. 2011, 106, 500–514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sceniak, M.; Maciver, B. Cellular Actions of Urethane on Rat Visual Cortical Neurons In Vitro. J. Neurophysiol. 2006, 95, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- Mysore, S.; Asadollahi, A.; Knudsen, E. Global Inhibition and Stimulus Competition in the Owl Optic Tectum. J. Neurosci. 2010, 30, 1727–1738. [Google Scholar] [CrossRef] [PubMed]

| Pigeon ID | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | #11 |
| Effective recording sites | 14 | 15 | 15 | 16 | 12 | 14 | 14 | 12 | 15 | 13 | 16 |
| Carrying out further visual stimulation experiments | 6 | 9 | 5 | 10 | 4 | 5 | 6 | 4 | 8 | 3 | 5 |
| Pigeon ID | #12 | #13 | #14 | #15 | #16 | #17 | Total | ||||
| Effective recording sites | 14 | 15 | 16 | 16 | 15 | 12 | 244 | ||||
| Carrying out further visual stimulation experiments | 6 | 8 | 6 | 7 | 5 | 9 | 106 | ||||
| Stimulation Conditions | Carrying Out Further Visual Stimulation Experiments | Selected for Statistical Comparison under Each Stimulation Condition | Used for Statistical Comparison between Moving and Flashed Stimuli |
|---|---|---|---|
| Flashed stimuli | 57 | 40 | 40 |
| Motion stimuli | 64 | 46 | 46 |
| Both types | 15 | 15 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Huang, S.; Zhu, M.; Wang, Z.; Shi, L. Surround Modulation Properties of Tectal Neurons in Pigeons Characterized by Moving and Flashed Stimuli. Animals 2022, 12, 475. https://doi.org/10.3390/ani12040475
Niu X, Huang S, Zhu M, Wang Z, Shi L. Surround Modulation Properties of Tectal Neurons in Pigeons Characterized by Moving and Flashed Stimuli. Animals. 2022; 12(4):475. https://doi.org/10.3390/ani12040475
Chicago/Turabian StyleNiu, Xiaoke, Shuman Huang, Minjie Zhu, Zhizhong Wang, and Li Shi. 2022. "Surround Modulation Properties of Tectal Neurons in Pigeons Characterized by Moving and Flashed Stimuli" Animals 12, no. 4: 475. https://doi.org/10.3390/ani12040475
APA StyleNiu, X., Huang, S., Zhu, M., Wang, Z., & Shi, L. (2022). Surround Modulation Properties of Tectal Neurons in Pigeons Characterized by Moving and Flashed Stimuli. Animals, 12(4), 475. https://doi.org/10.3390/ani12040475






