Associations of Apoptotic and Anti-Apoptotic Factors with Beef Quality, Histochemical Characteristics, and Palatability of Hanwoo Longissimus thoracis Muscle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Muscle Samples
2.2. Meat Quality Characteristics
2.3. Histochemical Analysis
2.4. Sensory Evaluation
2.5. Quantitative RT-PCR
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Comparison of Meat Quality and Histochemical Characteristics
3.2. Comparison of Eating Quality Characteristics
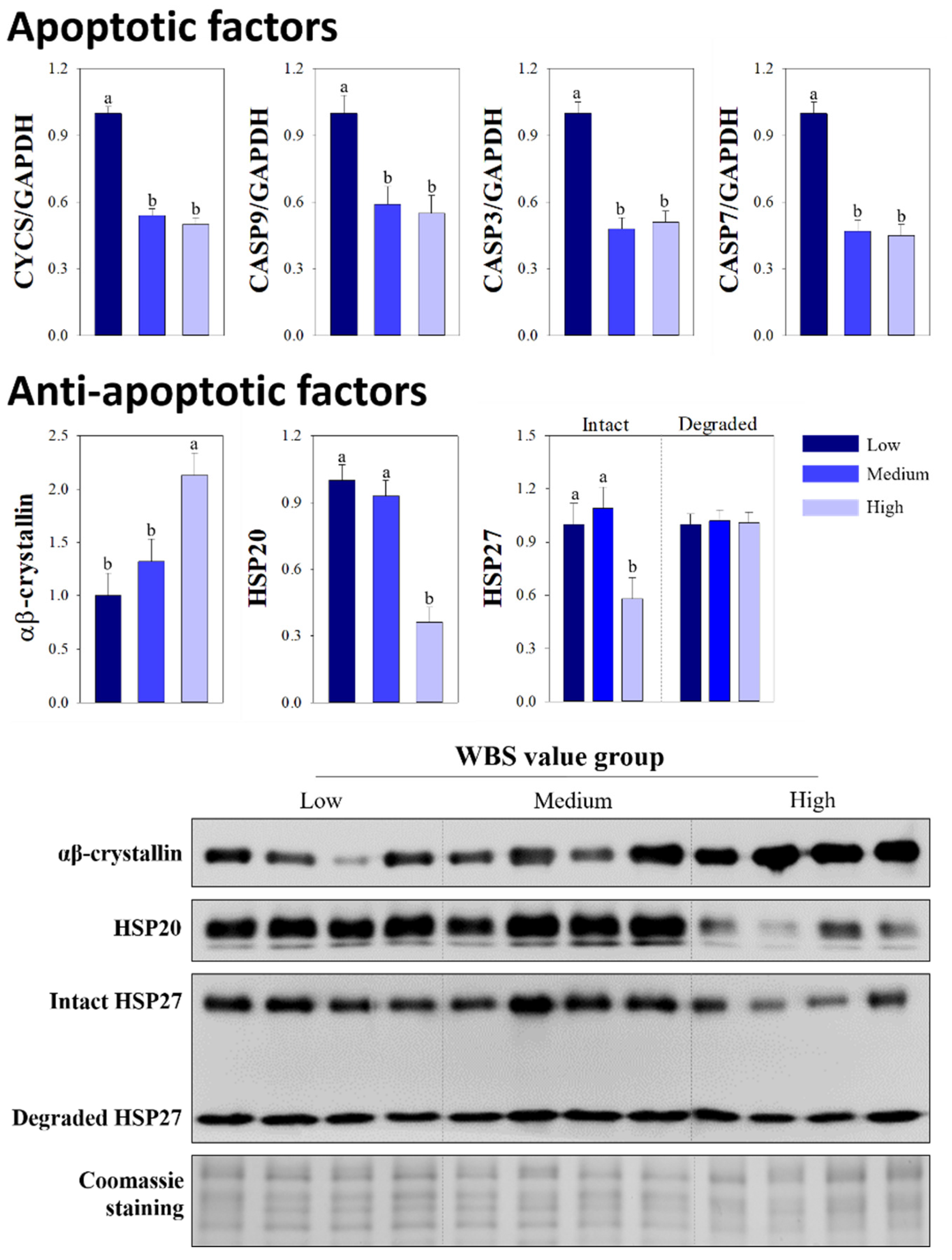
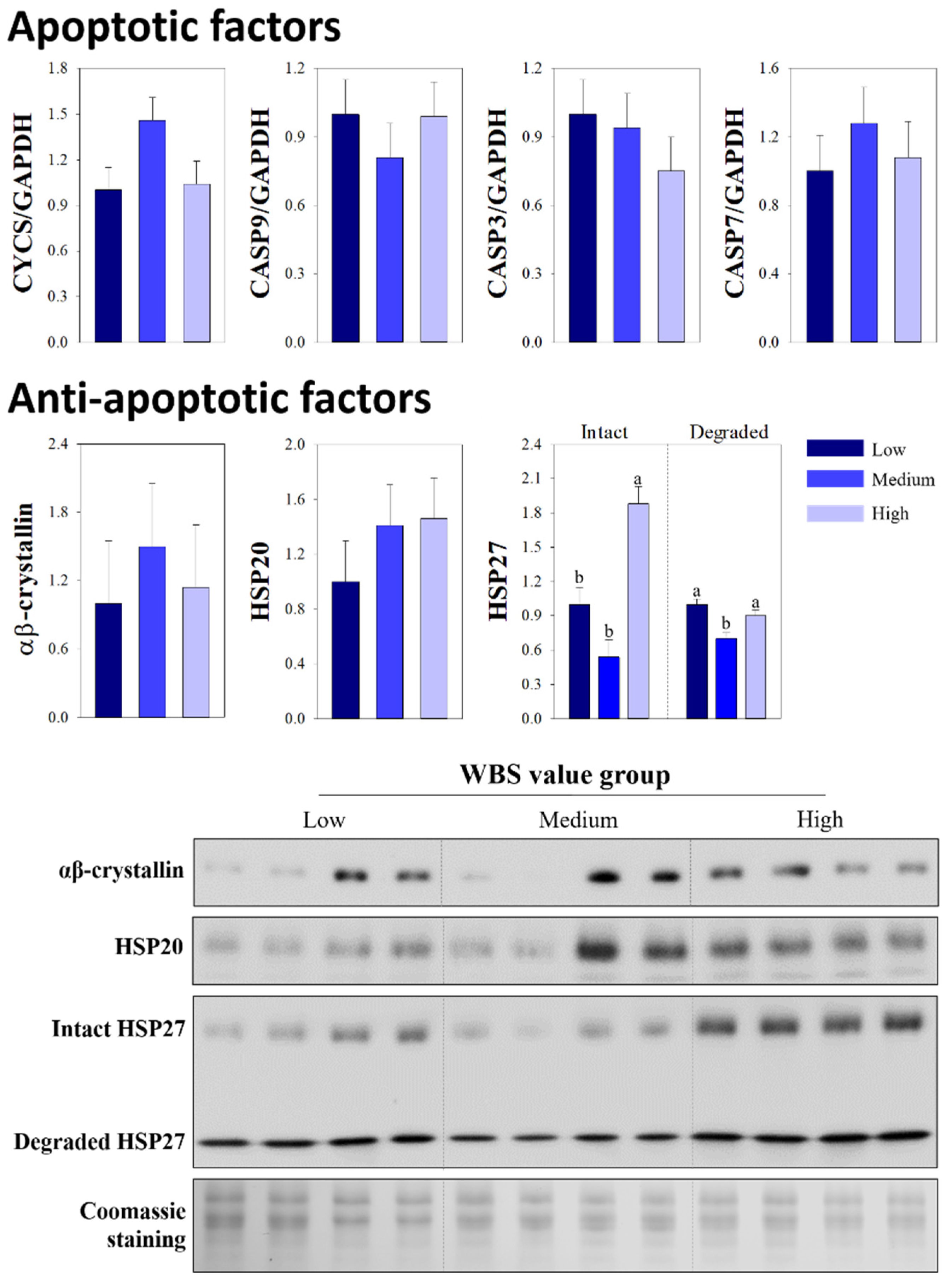
3.3. Comparison of Apoptotic and Anti-Apoptotic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ma, D.; Kim, Y.H.B. Proteolytic changes of myofibrillar and small heat shock proteins in different bovine muscles during aging: Their relevance to tenderness and water-holding capacity. Meat Sci. 2020, 163, 108090. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.F.; Ellies-Oury, M.P.; Legrand, I.; Pethick, D.; Gardner, G.; Wierzbicki, J.; Polkinghorne, R. Research in beef tenderness and palatability in the Era of big data. Meat Muscle Biol. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Proteomic investigations of beef tenderness. In Protemoics in Food Science: From Farm to Fork; Colgrave, M., Ed.; Elsevier and Academic Press: London, UK, 2017; pp. 177–196. [Google Scholar]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Role of caplain system in meat tenderness: A review. Food Sci. Hum. Wellness 2018, 7, 196–204. [Google Scholar] [CrossRef]
- Garcia-Macia, M.; Sierra, V.; Palanca, A.; Vega-Naredo, I.; De Gonzalo-Calvo, D.; Rodriguez-Gonzalez, S.; Olivan, M.; Coto-Montes, A. Autophagy during beef aging. Authophagy 2014, 10, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Kemp, C.M.; Parr, T. Advances in apoptotic mediated proteolysis in meat tenderization. Meat Sci. 2012, 92, 252–259. [Google Scholar] [CrossRef]
- Ouali, A.; Herrera-Mendez, C.H.; Coulis, G.; Becila, S.; Boudjellal, A.; Aubry, L.; Sentandreu, M.A. Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Sci. 2006, 74, 44–58. [Google Scholar] [CrossRef]
- Huang, F.; Huang, M.; Zhou, G.; Xu, X.; Xue, M. In vitro proteolysis of myofibrillar proteins from beef skeletal muscle by caspase-3 and caspase-6. J. Agric. Food Chem. 2011, 59, 9658–9663. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Zhou, G.; Liu, Y.; Zhang, Q.; Ye, K.; Pan, D.; Ou, C. Activation of caspase-9 and its influencing factors in beef during conditioning. Animal 2014, 8, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Kamradt, M.C.; Chen, F.; Cryns, V. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 2001, 276, 16059–16063. [Google Scholar] [CrossRef] [Green Version]
- Gopal-Srivastava, R.; Piatigorsky, J. The murine alpha B-crystallin/small heat shock protein enhancer: Identification of alpha BE-1, alpha BE-2, alpha BE-3, and MRF control elements. Mol. Cell. Biol. 1993, 13, 7144–7152. [Google Scholar] [PubMed] [Green Version]
- Waters, E.R.; Lee, G.J.; Vierling, E. Evolution, structure and function of the small heat shock proteins in plants. J. Exp. Bot. 1996, 47, 325–338. [Google Scholar] [CrossRef]
- Korea Institute of Animal Products Quality Evaluation (KAPE). The Beef Carcass Grading. Available online: http://www.ekape.or.kr/index.do (accessed on 1 June 2020).
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Commission Internationale de l’Eclairage (CIE). Recommendations on Uniform Color Spaces–Color Differences Equations, Psychrometic Color Terms; Central Bureau of the Commission Internationale de l’Eclairage: Vienna, Austria, 1978. [Google Scholar]
- Kauffman, R.G.; Eikelenboom, G.; Van der Wal, P.G.; Merkus, G.; Zaar, M. The use of filter paper to estimate drip loss of porcine musculature. Meat Sci. 1986, 18, 191–200. [Google Scholar] [CrossRef]
- Brooke, M.H.; Kaiser, K.K. Three “myosin adenosine triphosphatase” systems: The nature of their pH lability and sulfhydryl dependence. J. Histochem. Cytochem. 1970, 18, 670–672. [Google Scholar] [CrossRef]
- American Meat Science Association. Research Guideline for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Fresh Meat, 2nd ed.; American Meat Science Association: Champaign, IL, USA, 2015. [Google Scholar]
- Miller, M.F.; Carr, M.A.; Ramsey, C.B.; Crockett, K.L.; Hoover, L.C. Consumer thresholds for establishing the value of beef tenderness. J. Anim. Sci. 2001, 79, 3062–3068. [Google Scholar] [CrossRef]
- Hopkins, D.L. The Eating Quality of Meat: II-Tenderness. In Lawrie’s Meat Science, 8th ed.; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Lee, Y.; Lee, B.; Kim, H.K.; Yun, Y.K.; Kang, S.J.; Kim, K.T.; Kim, B.D.; Kim, E.J.; Choi, Y.M. Sensory quality characteristics with different beef quality grades and surface texture features assessed by dented area and firmness, and the relation to muscle fiber and bundle characteristics. Meat Sci. 2018, 145, 195–201. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baeza, E.; Medale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Choi, Y.M. Correlations of marbling characteristics with meat quality and histochemical characteristics in longissimus thoracis muscle of Hanwoo steers. Food Sci. Anim. Resour. 2019, 39, 151–161. [Google Scholar] [CrossRef]
- Choi, Y.M.; Garcia, L.G.; Lee, K. Correlation of sensory quality characteristics with intramuscular fat content and bundle characteristics in bovine longissimus thoracis muscle. Food Sci. Anim. Resour. 2019, 39, 197–208. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, B.; Kim, D.H.; Lee, K.; Kim, E.J.; Choi, Y.M. Sensory quality and histochemical characteristics of longissimus thoracis muscle between Hanwoo and Holstein steers of different quality grades. Food Sci. Anim. Resour. 2021, 41, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Sierra, V.; Olivan, M. Role of mitochondria on muscle cell death and meat tenderization. Recent. Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Lee, B.; Choi, Y.M. Associations of heat-shock protein expression with meat quality and sensory quality characteristics in highly marbled longissimus thoracis muscle from Hanwoo steers categorized by Warner-Bratzler shear force value. Foods 2019, 8, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Concannon, C.G.; Gorman, A.M.; Samali, A. On the role of Hsp27 in regulating apoptosis. Apoptosis 2003, 8, 61–70. [Google Scholar] [CrossRef]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Cramer, T.; Penick, M.L.; Waddell, J.N.; Bidwell, C.A.; Kim, Y.H.B. A new insight into meat toughness of callipyge lamb loins–The relevance of anti-apoptotic systems to decreased proteolysis. Meat Sci. 2018, 140, 66–71. [Google Scholar] [CrossRef]
- Laville, E.; Sayd, T.; Morzel, M.; Blinet, S.; Chambon, C.; Lepetit, J.; Renand, G.; Hocquette, J.F. Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J. Agric. Food Chem. 2009, 57, 10755–10764. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, D.; Kim, Y.H.B. Mitochondrial apoptosis and proteolytic changes of myofibrillar proteins in two different pork muscles during aging. Food Chem. 2020, 319, 126571. [Google Scholar] [CrossRef]
- Ding, Z.; Wei, Q.; Liu, C.; Zhang, H.; Huang, F. The quality changes and proteomic analysis of cattle muscle postmortem during rigor mortis. Foods 2022, 11, 217. [Google Scholar] [CrossRef]
- Underwood, K.R.; Means, W.J.; Du, M. Caspase 3 is not likely involved in the postmortem tenderization of beef muscle. J. Anim. Sci. 2008, 86, 960–966. [Google Scholar] [CrossRef]
- Lee, B.; Choi, Y.M. Research Note: Comparison of histochemical characteristics, chicken meat quality, and heat shock protein expressions between PSE-like condition and white-stripping features of pectoralis major muscle. Poult. Sci. 2021, 100, 10260. [Google Scholar] [CrossRef] [PubMed]
- Lomiwes, D.; Farouk, M.M.; Wiklund, E.; Young, O.A. Small heat shock proteins and their role in meat tenderness: A review. Meat Sci. 2014, 96, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, X.C.; Lu, F.; Xu, X.L.; Zhou, G.H.; Li, Q.Y.; Guo, X.Y. Effects of camptothecin, etoposide and Ca2+ on caspase-3 activity and myofibrillar disruption of chicken during postmortem ageing. Meat Sci. 2011, 87, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Pulford, D.J.; Dobbie, P.; Fraga-Vazquez, S.; Fraser-Smith, E.; Frost, D.A.; Morris, C.A. Variation in bull beef quality due to ultimate muscle pH is correlated to endopeptidase and small heat shock protein levels. Meat Sci. 2009, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Huang, M.; Zhang, H.; Zhang, C.; Zhang, D.; Zhou, G. Changes in apoptotic factors and caspase activation pathways during the postmortem aging of beef muscle. Food Chem. 2016, 190, 110–114. [Google Scholar] [CrossRef] [PubMed]
| WBS Value Group | p-Value | |||
|---|---|---|---|---|
| Low (n = 14) | Medium (n = 14) | High (n = 13) | ||
| WBS (N) | 50.9 c (0.94) 1 | 58.9 b (0.94) | 67.4 a (1.06) | <0.0001 |
| Marbling score | 3.93 (0.32) | 3.79 (0.32) | 3.67 (0.34) | 0.9082 |
| IMF content (%) | 7.98 (1.63) | 9.27 (1.63) | 9.12 (1.63) | 0.7642 |
| Muscle pH45 min | 6.14 (0.09) | 6.22 (0.09) | 6.12 (0.09) | 0.7893 |
| Muscle pH24 h | 5.45 (0.04) | 5.56 (0.04) | 5.45 (0.04) | 0.1467 |
| Lightness (L*) | 29.9 (1.07) | 30.0 (1.07) | 28.2 (1.16) | 0.8546 |
| Redness (a*) | 16.1 (0.78) | 16.3 (0.78) | 9.06 (1.45) | 0.9596 |
| Yellowness (b*) | 7.52 (1.34) | 7.71 (1.34) | 9.06 (1.45) | 0.6560 |
| Drip loss (%) | 1.07 (0.16) | 0.99 (0.16) | 0.80 (0.17) | 0.3913 |
| FFU (mg) | 3.12 (1.11) | 3.11 (1.11) | 5.57 (1.19) | 0.9995 |
| Cooking loss (%) | 22.6 (2.42) | 23.6 (2.42) | 25.1 (2.61) | 0.4044 |
| WBS Value Group | p-Value | |||
|---|---|---|---|---|
| Low | Medium | High | ||
| Muscle fiber area (μm2) | 4030 (236) 1 | 3995 (236) | 4190 (226) | 0.8304 |
| Total fiber number (×1000) | 2444 (185) | 2335 (185) | 2363 (151) | 0.8896 |
| Fiber area percentage (%) | ||||
| Type I | 24.8 (1.65) | 19.4 (1.65) | 22.4 (1.34) | 0.0610 |
| Type IIA | 23.7 (2.42) | 25.2 (2.42) | 25.7 (1.98) | 0.7780 |
| Type IIX | 51.4 (2.60) | 55.3 (2.60) | 51.8 (2.13) | 0.4837 |
| Muscle bundle characteristics | ||||
| Bundle area (mm2) | 0.37 (0.03) | 0.36 (0.03) | 0.42 (0.03) | 0.3117 |
| Fiber number per bundle | 92.8 (12.2) | 97.3 (12.2) | 107 (9.96) | 0.6051 |
| WBS Value Group | p-Value | |||
|---|---|---|---|---|
| Low | Medium | High | ||
| Softness | 6.02 a (0.23) 1 | 5.20 b (0.23) | 4.67 c (0.25) | 0.0012 |
| Initial tenderness | 5.92 a (0.24) | 5.00 b (0.24) | 4.55 b (0.26) | 0.0016 |
| Chewiness | 5.59 a (0.25) | 4.90 ab (0.25) | 4.24 b (0.26) | 0.0038 |
| Rate of breakdown | 5.50 a (0.22) | 4.96 ab (0.22) | 4.60 b (0.24) | 0.0315 |
| Amount of perceptible residue | 5.68 a (0.21) | 5.14 ab (0.21) | 4.91 b (0.22) | 0.0429 |
| Overall tenderness | 5.78 a (0.25) | 4.94 b (0.25) | 4.47 b (0.27) | 0.0037 |
| Juiciness | 5.19 (0.21) | 4.89 (0.21) | 4.67 (0.22) | 0.2365 |
| Flavor intensity | 5.62 (0.14) | 5.66 (0.14) | 5.49 (0.15) | 0.7169 |
| Off-flavor intensity | 6.36 (0.18) | 6.36 (0.18) | 6.03 (0.19) | 0.3641 |
| Overall acceptability | 5.71 a (0.25) | 4.93 b (0.25) | 4.49 b (0.27) | 0.0065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Kim, J.-Y.; Choi, Y.-M. Associations of Apoptotic and Anti-Apoptotic Factors with Beef Quality, Histochemical Characteristics, and Palatability of Hanwoo Longissimus thoracis Muscle. Animals 2022, 12, 467. https://doi.org/10.3390/ani12040467
Lee B, Kim J-Y, Choi Y-M. Associations of Apoptotic and Anti-Apoptotic Factors with Beef Quality, Histochemical Characteristics, and Palatability of Hanwoo Longissimus thoracis Muscle. Animals. 2022; 12(4):467. https://doi.org/10.3390/ani12040467
Chicago/Turabian StyleLee, Boin, Jae-Yeong Kim, and Young-Min Choi. 2022. "Associations of Apoptotic and Anti-Apoptotic Factors with Beef Quality, Histochemical Characteristics, and Palatability of Hanwoo Longissimus thoracis Muscle" Animals 12, no. 4: 467. https://doi.org/10.3390/ani12040467
APA StyleLee, B., Kim, J.-Y., & Choi, Y.-M. (2022). Associations of Apoptotic and Anti-Apoptotic Factors with Beef Quality, Histochemical Characteristics, and Palatability of Hanwoo Longissimus thoracis Muscle. Animals, 12(4), 467. https://doi.org/10.3390/ani12040467






