Genetic Diversity of Creole Sheep Managed by Indigenous Communities of the Central Region of Veracruz, Mexico
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
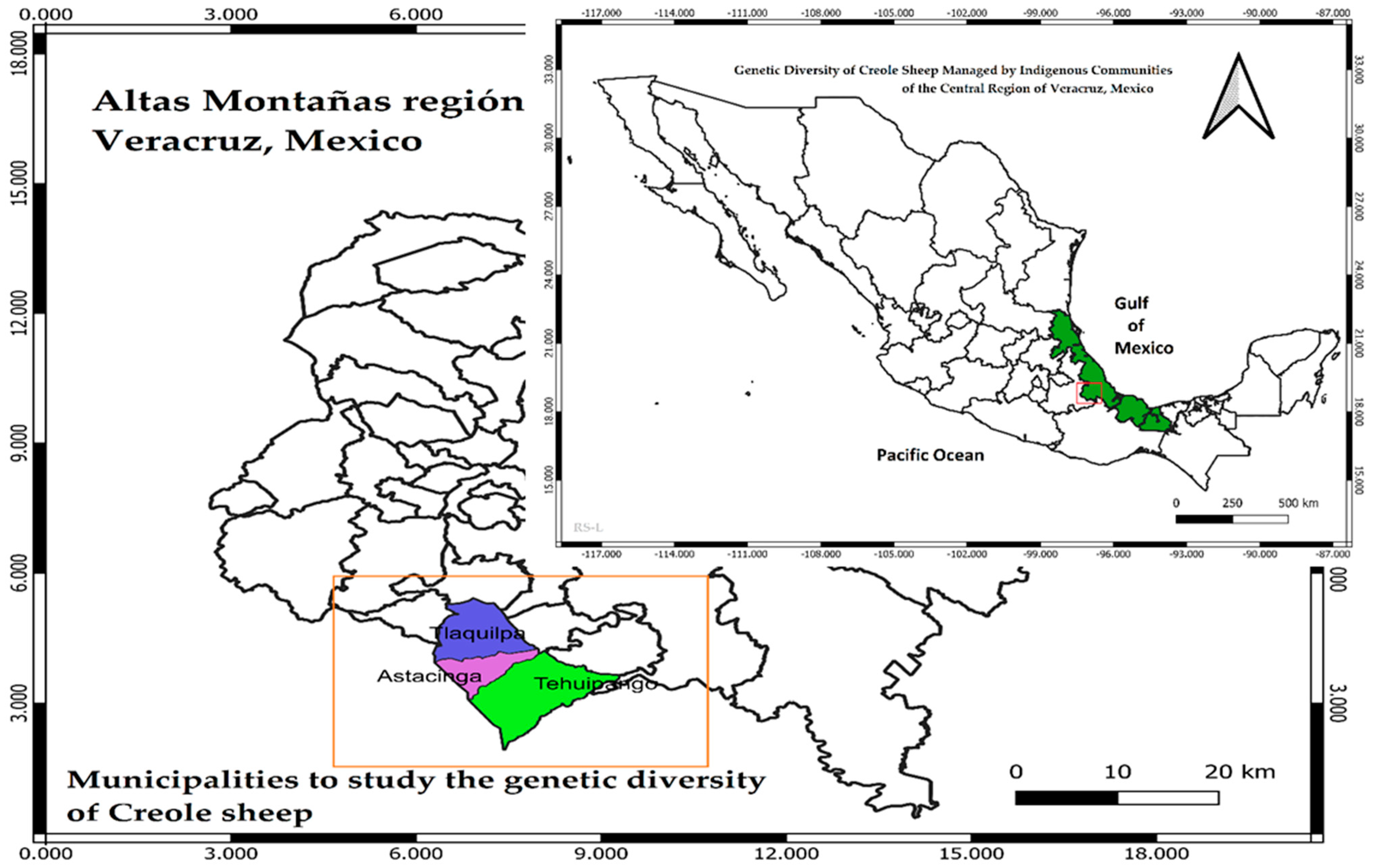
2.1. Characteristics of Populations of Sheep Evaluated and Blood Sample Collection
2.2. DNA Extraction and PCR
2.3. Electrophoresis of the Microsatellites
2.4. Analysis of Genetic Data
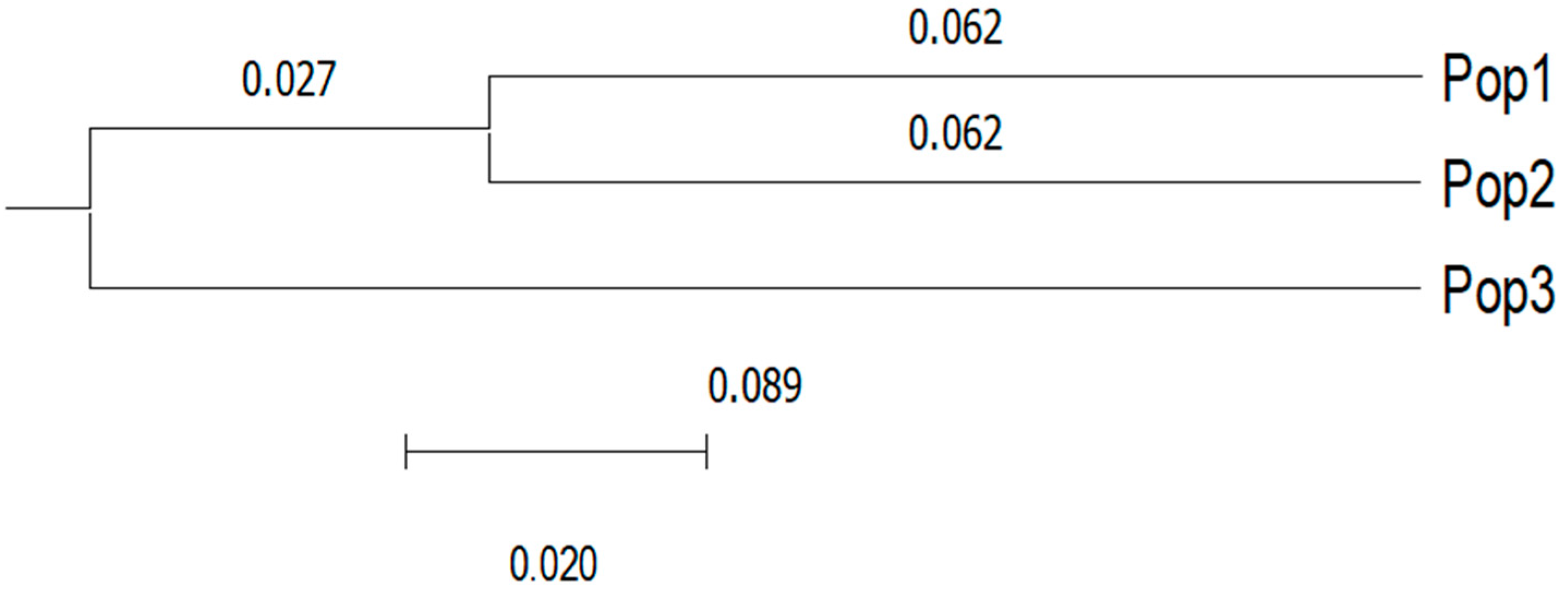
3. Results
4. Discussion
4.1. Polymorphism of the Loci
4.2. Allelic Diversity and Number of Alleles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saucedo, P. Industria Ganadera. En Historia de la Ganadería en México; UNAM: Mexico City, Mexico, 1987; pp. 31–66. [Google Scholar]
- Pablo, D.N. Importancia Económica, Ambiental y Social de la Producción Ovina en el Área de Protección de Flora y Fauna Nevado de Toluca. Ph.D. Thesis, Universidad Autónoma Del Estado De México, Toluca de Lerdo, México, 2017. [Google Scholar]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA). Programas SAGARPA 2017. 2009. Available online: http://www.gob.mx/sagarpa/acciones-y-programas/programas-sagarpa-2017 (accessed on 17 December 2021).
- Hernández, M.P.M. Dinámica Espacio-Temporal del Venado Cola Blanca (Odocoileus virgininanus) en Mexico. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, México, 2010. [Google Scholar]
- Ulloa-Arvizu, R.; Gayosso-Vázquez, A.; Alonso-Morales, R.A. Origen genético del ovino criollo mexicano (Ovis aries) por el análisis del gen del Citocromo C Oxidasa subunidad I. Rev. Tec. Pecu. México 2009, 47, 323–328. [Google Scholar]
- Peña, S.; Lopez, G.; Martínez, R.; Abbiati, N.; Castagnasso, E.; Giovambattista, G.; Genero, E. Características zoométricas de ovinos criollos de cuatro regiones de la Argentina. Actas Iberoam. Conserv. Anim. 2013, 3, 174–181. [Google Scholar]
- Candelaria-Martínez, B.; Ramírez-Mella, M.; Flota-Bañuelos, C.; Dorantes-Jiménez, J. Recursos genéticos “criollos” de zonas rurales de Campeche, México. Agroproductividad 2016, 9, 29–32. [Google Scholar]
- Rimieri, P. Genetic diversity and genetic variability: Two different concepts associated with germplasm and plant genetic improvement. J. Basic Appl. Genet. 2017, 28, 7–13. [Google Scholar]
- Herrera, J.; Jordán, H.; Senra, A.F. Aspectos del manejo y alimentación de la reproductora ovina Pelibuey en Cuba. Rev. Cuba. Cienc. Agríc. 2010, 44, 211–219. [Google Scholar]
- Tamqmone, N.M. Pérdida de Diversidad Genética: Implicaciones para la Evolución y la Conservación de dos Especies de Ctenomys (Rodentia: Ctenomyidae) en Patagonia Norte. Available online: http://www.secheresse.info/spip.php?article95837 (accessed on 17 December 2021).
- Espinosa-García, J.A.; Quiroz-Valiente, J.; Moctezuma-López, G.; Oliva-Hernández, J.; Granados-Zurita, L.; Berumen-Alatorre, A.C. Prospección tecnológica y estrategias de innovación para producción ovina en Tabasco, México. Rev. Científica 2015, 15, 107–115. [Google Scholar]
- Mariscal, J.C.; Mathez-Stiefel, S.L. Fortaleciendo la soberanía alimentaria mediante la revalorización de saberes ecológicos locales: Experiencia en los Andes bolivianos. Etnobiología 2010, 8, 75–89. [Google Scholar]
- Cardozo de Martínez, C.A.; de Osorio, A.M. Etica en investigación con animales: Una actitud responsable y respetuosa del investigador con rigor y calidad científica. Rev. Latinoam. Bioética 2008, 8, 46–71. [Google Scholar]
- Burgos-Paz, W.; Rosero-Galindo, C.; Cárdenas-Henao, H.; Solarte-Portilla, C. Polimorfismos en la longitud de fragmentos amplificados (AFLP´s) a partir de muestras de sangre almacenadas en tarjetas FTA® para la especie Cavia porcellus Lin (Rodentia: Caviidae). Rev. Colomb. Cienc. Pecu. 2007, 20, 67–72. [Google Scholar]
- Food and Agriculture Organization. Molecular genetic characterization of animal genetic resources. In FAO Animal Production and Health Guidelines; Food and Agriculture Organization: Rome, Italy, 2011. [Google Scholar]
- Agaviezor, B.O.; Peters, S.O.; Adefenwa, M.A. Morphological DNA and microsatellite diversity of indigenous Nigerian sheep. J. Anim. Sci. Biotechnol. 2017, 3, 38. [Google Scholar] [CrossRef]
- Luna-González, A.; Hernández-Arteaga, S.; Sánchez-Garza, M.; López-Revilla, R.; Medina-Esparza, L.; Cruz-Vázquez, C. Microsatellite loci and paternity analysis in Nubia and Boer goats. Arch. Med. Vet. 2012, 44, 123–127. [Google Scholar] [CrossRef][Green Version]
- Aguilar Martínez, C.U.; Espinoza Gutiérrez, B.; Segura Correa, J.C.; Berruecos Villalobos, J.M.; Valencia Méndez, J.; Roldán Roldán, A. Caracterización genética de la oveja Pelibuey de México usando marcadores microsatélites. Rev. Mex. Cienc. Pecu. 2021, 12, 36–57. [Google Scholar] [CrossRef]
- Cardoso, C.; Da Silva, E.C.; Pimentel, C.; Caetano, A.; Faco, O.; Paiva, S. Estimativa Inicial de Erros de Paternidade em um Programa de Melhoramento Genético de Caprinos Leiteiros. 2012. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/163386/1/CNPC-2012-Estimativa.pdf (accessed on 17 December 2021).
- International Maize and Wheat Improvement Center (CIMMYT). Protocolos de Laboratorio: Laboratorio de Genética Molecular Aplicada, 3rd ed.; CIMMYT: El Batán, Mexico, 2006; 93p. [Google Scholar]
- Sanguinetti, C.; Dias, J.; Neto, E.; Simpson, A.J. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 1994, 17, 914–921. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar]
- Liu, K.; Muse, S. PowerMaker: An integrated analysis environment for genetic maker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J.P. DarWin Software. 2006. Available online: http://darwin.cirad.fr/ (accessed on 17 December 2021).
- Ortega, M.J.; Introducción al Mejoramiento Genético. Notas de Campus. 2017. Available online: https://hemeroteca.unad.edu.co/index.php/notas/article/view/1809 (accessed on 17 December 2021).
- Casado-Gonzalez, M.; Gonzalez-Duarte, R. Los Retos de la Genética en el Siglo XXI: Genética y Bioética; Edicions Universitat Barcelona: Barcelona, Spain, 1999. [Google Scholar]
- Botstein, D.; White, L.; Skolmick, H.; Davis, W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Ocampo-Gallego, R.J. Caracterizacion Genética de Ovinos en Colombia por Medio de Markers Microsatelites. Master’s Thesis, Universidad de Antioquia, Medellín, Colombia, 2014. [Google Scholar]
- Da Silva, R.C. Caracterização Zoométrica e Genética de Ovinos Morada Nova. Ph.D. Thesis, Universidade Federal Da Paraíba, João Pessoa, Brazil, 2012. [Google Scholar]
- Ocampo, R.J.; Martinez, R.A.; Rocha, J.F.; Cardona, H. 2017 Genetic characterization of Colombian indigenous sheep. Rev. Colomb. Cienc. Pecu. 2017, 30, 116–125. [Google Scholar] [CrossRef]
- Hussain, T.; Musthafa, M.M.; Babar, M.E.; Shaheen, M.; Marikar, F.M. Molecular genetic diversity and relationship of indigenous sheep breeds of Pakistan based on nuclear microsatellite loci. Rev. Vet. 2019, 30, 54–58. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ahmad, S.; Durrani, I.S.; Iqbal, A.; Munir, I.; Swati, Z.A. Genetic polymorphism and bottleneck analysis of Balkhi, Hashtnagri, and Michni sheep populations using microsatellite markers. Anim. Biotechnol. 2018, 29, 216–226. [Google Scholar] [CrossRef]
- Santos-Silva, F.; Ivo, R.S.; Sousa, M.C.O.; Carolino, M.I.; Ginja, C.; Gama, L.T. Assessing genetic diversity and differentiation in Portuguese coarse-wool sheep breeds with microsatellite markers. Small Rumin. Res. 2008, 78, 32–40. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, M.L.; Zhang, X.H.; Wu, D.J. Study on population genetic structure of Liangshan semi-wool sheep using microsatellite markers. Pak. J. Biol. Sci. 2009, 11, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Myles, S.; Tang, K.; Somel, M.E.H.M.E.T.; Green, R.E.; Kelso, J.; Stoneking, M. Identification and analysis of genomic regions with large between-population differentiation in humans. Ann. Hum. Genet. 2008, 72, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Vega-Hernández, D.M.; Gutiérrez-Velázquez, M.V. Efectos de la actividad antropogénica sobre la estructura genética de ungulados silvestres. Órgano Difusión Científica Tecnológica Cent. Interdiscip. Investig. Desarro. Integral Reg. Unidad Durango 2015, 7, 60–63. [Google Scholar]
- Brommer, J.E.; Kekkonen, J.; Wikström, M. Using heterozygosity-fitness correlations to study inbreeding depression in an isolated population of white-tailed deer founded by few individuals. Ecol. Evol. 2015, 5, 357–367. [Google Scholar] [CrossRef] [PubMed]


| Locus | Sequence 5′–3′ | Size (bp) | Source |
|---|---|---|---|
| OarFCB128-F | ATTAAAGCATCTTCTCTTTATTTCCTCGC | 96–136 | [16] |
| OarFCB128-R | CAGCTGAGCAACTAAGACATACATGCG | ||
| SRCRSP9-F | AGAGGATCTGGAAATGGAATC | 119–143 | [17] |
| SRCRSP9-R | GCACTCTTTTCAGCCCTAATG | ||
| ILSTS5-F | GGAAGCAATGAAATCTATAGCC | 174–221 | [18] |
| ILSTS5-R | TGTTCTGTGAGTTTGTAAGC | ||
| ILSTS11-F | GCTTGCTACATGGAAAGTGC | 256–294 | [19] |
| ILSTS11-R | CTAAAATGCAGAGCCCTACC |
| Population | Locus | df | X2 | p-Value | Significance |
|---|---|---|---|---|---|
| Pop1 | ILSTS11 | 36 | 23.833 | 0.940 | ns |
| Pop1 | ILSTS5 | 66 | 69.686 | 0.355 | ns |
| Pop1 | SRCRSP9 | 10 | 26.071 | 0.004 | ** |
| Pop1 | OarFCB128 | 28 | 52.308 | 0.004 | ** |
| Pop2 | ILSTS11 | 36 | 41.382 | 0.247 | ns |
| Pop2 | ILSTS5 | 66 | 148.901 | 0.000 | *** |
| Pop2 | SRCRSP9 | 45 | 56.012 | 0.126 | ns |
| Pop2 | OarFCB128 | 36 | 49.965 | 0.061 | ns |
| Pop3 | ILSTS11 | 36 | 41.348 | 0.248 | ns |
| Pop3 | ILSTS5 | 45 | 68.033 | 0.015 | * |
| Pop3 | SRCRSP9 | 55 | 88.053 | 0.003 | ** |
| Pop3 | OarFCB128 | 28 | 80.500 | 0.000 | *** |
| Marker | Allele | Pop 1 | Pop 2 | Pop 3 |
|---|---|---|---|---|
| ILSTS11 | 260 | 0.000 | 0.030 | 0.000 |
| ILSTS11 | 268 | 0.048 | 0.015 | 0.028 |
| ILSTS11 | 270 | 0.048 | 0.000 | 0.028 |
| ILSTS11 | 274 | 0.024 | 0.000 | 0.000 |
| ILSTS11 | 276 | 0.048 | 0.000 | 0.056 |
| ILSTS11 | 278 | 0.190 | 0.182 | 0.167 |
| ILSTS11 | 279 | 0.000 | 0.015 | 0.000 |
| ILSTS11 | 280 | 0.262 | 0.318 | 0.222 |
| ILSTS11 | 282 | 0.000 | 0.091 | 0.125 |
| ILSTS11 | 284 | 0.214 | 0.136 | 0.167 |
| ILSTS11 | 286 | 0.000 | 0.015 | 0.056 |
| ILSTS11 | 288 | 0.024 | 0.000 | 0.000 |
| ILSTS11 | 289 | 0.000 | 0.030 | 0.000 |
| ILSTS11 | 290 | 0.024 | 0.030 | 0.014 |
| ILSTS11 | 294 | 0.119 | 0.136 | 0.083 |
| ILSTS5 | 186 | 0.048 | 0.000 | 0.000 |
| ILSTS5 | 188 | 0.000 | 0.015 | 0.000 |
| ILSTS5 | 190 | 0.143 | 0.076 | 0.111 |
| ILSTS5 | 192 | 0.000 | 0.015 | 0.000 |
| ILSTS5 | 193 | 0.000 | 0.015 | 0.000 |
| ILSTS5 | 194 | 0.024 | 0.076 | 0.069 |
| ILSTS5 | 195 | 0.000 | 0.000 | 0.014 |
| ILSTS5 | 196 | 0.143 | 0.152 | 0.083 |
| ILSTS5 | 198 | 0.119 | 0.061 | 0.042 |
| ILSTS5 | 200 | 0.167 | 0.212 | 0.208 |
| ILSTS5 | 214 | 0.024 | 0.000 | 0.000 |
| ILSTS5 | 202 | 0.000 | 0.015 | 0.000 |
| ILSTS5 | 204 | 0.024 | 0.076 | 0.083 |
| ILSTS5 | 206 | 0.024 | 0.030 | 0.069 |
| ILSTS5 | 208 | 0.024 | 0.061 | 0.014 |
| ILSTS5 | 210 | 0.119 | 0.121 | 0.056 |
| ILSTS5 | 212 | 0.048 | 0.045 | 0.000 |
| Marker | Frequency of the Most Common Allele | No. of Genotypes | No. of Alleles | He | Ho | PIC |
|---|---|---|---|---|---|---|
| ILSTS11 | 0.27 | 34 | 16 | 0.85 | 0.60 | 0.83 |
| ILSTS5 | 0.20 | 33 | 20 | 0.89 | 0.81 | 0.88 |
| SRCRSP9 | 0.21 | 29 | 14 | 0.85 | 0.76 | 0.84 |
| OarFCB128 | 0.17 | 21 | 15 | 0.86 | 0.77 | 0.85 |
| Average | 0.22 | 29.25 | 16.25 | 0.86 | 0.73 | 0.85 |
| Locus | FIS | FIT | FST |
|---|---|---|---|
| ILSTS11 | −0.234 | −0.203 | 0.025 |
| ILSTS5 | −-0.150 | −0.137 | 0.011 |
| SRCRSP9 | −0.248 | −0.212 | 0.029 |
| OarFCB128 | −0.221 | −0.18 | 0.034 |
| Mean | −0.213 | −0.183 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Rodríguez, R.G.; Segura-León, O.L.; Hernández-Rodríguez, M.; Serna-Lagunes, R.; Salinas-Ruiz, J.; Salazar-Ortiz, J. Genetic Diversity of Creole Sheep Managed by Indigenous Communities of the Central Region of Veracruz, Mexico. Animals 2022, 12, 456. https://doi.org/10.3390/ani12040456
Castillo-Rodríguez RG, Segura-León OL, Hernández-Rodríguez M, Serna-Lagunes R, Salinas-Ruiz J, Salazar-Ortiz J. Genetic Diversity of Creole Sheep Managed by Indigenous Communities of the Central Region of Veracruz, Mexico. Animals. 2022; 12(4):456. https://doi.org/10.3390/ani12040456
Chicago/Turabian StyleCastillo-Rodríguez, Ruth Guadalupe, Obdulia Lourdes Segura-León, Martha Hernández-Rodríguez, Ricardo Serna-Lagunes, Josafhat Salinas-Ruiz, and Juan Salazar-Ortiz. 2022. "Genetic Diversity of Creole Sheep Managed by Indigenous Communities of the Central Region of Veracruz, Mexico" Animals 12, no. 4: 456. https://doi.org/10.3390/ani12040456
APA StyleCastillo-Rodríguez, R. G., Segura-León, O. L., Hernández-Rodríguez, M., Serna-Lagunes, R., Salinas-Ruiz, J., & Salazar-Ortiz, J. (2022). Genetic Diversity of Creole Sheep Managed by Indigenous Communities of the Central Region of Veracruz, Mexico. Animals, 12(4), 456. https://doi.org/10.3390/ani12040456






