Epidemiology of Porcine Circovirus Type 2 Circulating in Wild Boars of Portugal during the 2018–2020 Hunting Seasons Suggests the Emergence of Genotype 2d
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Selection
2.2. Nucleic Acid Extraction
2.3. PCV-2 Detection
2.4. Sequencing and Phylogenetic Analysis
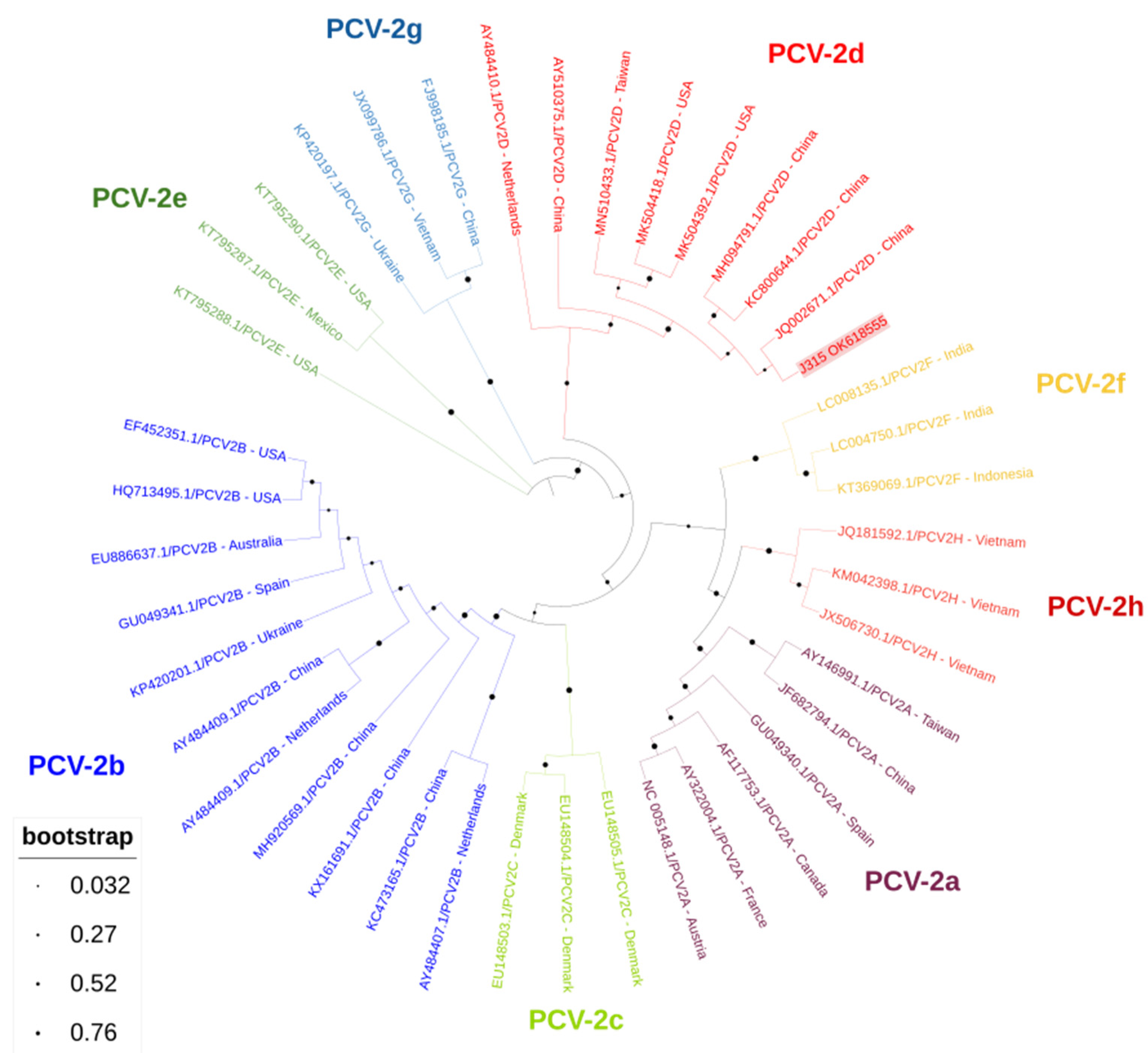
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dei Giudici, S.; Lo Presti, A.; Bonelli, P.; Angioi, P.P.; Sanna, G.; Zinellu, S.; Balzano, F.; Salis, F.; Ciccozzi, M.; Oggiano, A. Phylogenetic analysis of porcine circovirus type 2 in Sardinia, Italy, shows genotype 2d circulation among domestic pigs and wild boars. Infect. Genet. Evol. 2019, 71, 189–196. [Google Scholar] [CrossRef]
- Opriessnig, T.; Karuppannan, A.K.; Castro AM, M.G.; Xiao, C.T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Hu, W.Q.; Li, J.Y.; Liu, T.N.; Zhou, J.Y.; Opriessnig, T.; Xiao, C.T. Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transbound. Emerg. Dis. 2020, 67, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Allan, G.M.; Domingo, M. Porcine circovirus diseases. Anim. Health Res. Rev. 2005, 6, 119–142. [Google Scholar] [CrossRef]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.W. Porcine Multi-Systemic Wasting Syndrome (PMWS): A Review. 10 April 2006. Available online: https://www.thepigsite.com/articles/porcine-multisystemic-wasting-syndrome-pmws-a-review (accessed on 10 January 2022).
- Alarcon, P.; Rushton, J.; Wieland, B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England—An economic disease model. Prev. Vet. Med. 2013, 110, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.-T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef]
- Nawagitgul, P.; Morozov, I.; Bolin, S.R.; Harms, P.A.; Sorden, S.D.; Paul, P.S. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 2000, 81 Pt 9, 2281–2287. [Google Scholar] [CrossRef]
- Knell, S.; Willems, H.; Hertrampf, B.; Reiner, G. Comparative genetic characterization of Porcine Circovirus type 2 samples from German wild boar populations. Vet. Microbiol. 2005, 109, 169–177. [Google Scholar] [CrossRef]
- Sanchez, R.; Nauwynck, H.; Pensaert, M. Serological survey of porcine circovirus 2 antibodies in domestic and feral pig populations in Belgium. In Proceedings of the 1st ssDNA Viruses of Plants, Birds, Pigs and Primates Meeting, Saint-Malo, France, 24–27 September 2001. [Google Scholar]
- Toplak, I.; Grom, J.; Hostnik, P.; Barlic-Maganja, D. Phylogenetic analysis of type 2 porcine circoviruses identified in wild boar in Slovenia. Vet. Rec. 2004, 155, 178–180. [Google Scholar] [CrossRef]
- Sedlak, K.; Bartova, E.; Machova, J. Antibodies to selected viral disease agents in wild boars from the Czech Republic. J. Wildl. Dis. 2008, 44, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Morandi, F.; Verin, R.; Sarli, G.; Canetti, N.; Scacco, M.; Panarese, S.; Poli, A. Porcine circovirus type 2 (PCV2) antigen localisation and post-weaning multisystemic wasting syndrome (PMWS) in free-ranging wild boar (Sus scrofa ssp. scrofa) in Italy. Eur. J. Wildl. Res. 2010, 56, 717–724. [Google Scholar] [CrossRef]
- Vicente, J.; Segalés, J.; Höfle, U.; Balasch, M.; Plana-Durán, J.; Domingo, M.; Gortázar, C. Epidemiological study on porcine circovirus type 2 (PCV2) infection in the European wild boar (Sus scrofa). Vet. Res. 2004, 35, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cságola, A.; Kecskeméti, S.; Kardos, G.; Kiss, I.; Tuboly, T. Genetic characterization of type 2 porcine circoviruses detected in Hungarian wild boars. Arch. Virol. 2006, 151, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.M.; Duarte, M.; Fagulha, T.; Ramos, F.; Barros, S.C.; Luís, T.; Fevereiro, M. Molecular study of porcine circovirus type 2 circulating in Portugal. Infect. Genet. Evol. 2011, 11, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef] [Green Version]
- Franzo, G.; Cortey, M.; Segalés, J.; Hughes, J.; Drigo, M. Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol. Phylogenetics Evol. 2016, 100, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Vidigal PM, P.; Mafra, C.L.; Silva FM, F.; Fietto JL, R.; Silva Júnior, A.; Almeida, M.R. Tripping over emerging pathogens around the world: A phylogeographical approach for determining the epidemiology of Porcine circovirus-2 (PCV-2), considering global trading. Virus Res. 2012, 163, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mur, L.; Atzeni, M.; Martínez-López, B.; Feliziani, F.; Rolesu, S.; Sanchez-Vizcaino, J.M. Thirty-Five-Year Presence of African Swine Fever in Sardinia: History, Evolution and Risk Factors for Disease Maintenance. Transbound. Emerg. Dis. 2016, 63, e165–e177. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, A.; Rolesu, S.; Loi, F.; Cappai, S.; Oggiano, A.; Madrau, M.P.; Sanna, M.L.; Pilo, G.; Bandino, E.; Brundu, D.; et al. Surveillance and control of African Swine Fever in free-ranging pigs in Sardinia. Transbound. Emerg. Dis. 2019, 66, 1114–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurado, C.; Fernández-Carrión, E.; Mur, L.; Rolesu, S.; Laddomada, A.; Sánchez-Vizcaíno, J.M. Why is African swine fever still present in Sardinia? Transbound. Emerg. Dis. 2018, 65, 557–566. [Google Scholar] [CrossRef]
- Karuppannan, A.K.; Opriessnig, T.; Dixon, L.; Graham, S. Porcine Circovirus Type 2 (PCV2) Vaccines in the Context of Current Molecular Epidemiology. Viruses 2017, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Torres, R.T.; Fernandes, J.; Carvalho, J.; Cunha, M.V.; Caetano, T.; Mendo, S.; Serrano, E.; Fonseca, C. Wild boar as a reservoir of antimicrobial resistance. Sci. Total Environ. 2020, 717, 135001. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa Moreira, A.; Santos-Silva, S.; Mega, J.; Palmeira, J.D.; Torres, R.T.; Mesquita, J.R. Epidemiology of Porcine Circovirus Type 2 Circulating in Wild Boars of Portugal during the 2018–2020 Hunting Seasons Suggests the Emergence of Genotype 2d. Animals 2022, 12, 451. https://doi.org/10.3390/ani12040451
de Sousa Moreira A, Santos-Silva S, Mega J, Palmeira JD, Torres RT, Mesquita JR. Epidemiology of Porcine Circovirus Type 2 Circulating in Wild Boars of Portugal during the 2018–2020 Hunting Seasons Suggests the Emergence of Genotype 2d. Animals. 2022; 12(4):451. https://doi.org/10.3390/ani12040451
Chicago/Turabian Stylede Sousa Moreira, Alícia, Sérgio Santos-Silva, João Mega, Josman D. Palmeira, Rita T. Torres, and João R. Mesquita. 2022. "Epidemiology of Porcine Circovirus Type 2 Circulating in Wild Boars of Portugal during the 2018–2020 Hunting Seasons Suggests the Emergence of Genotype 2d" Animals 12, no. 4: 451. https://doi.org/10.3390/ani12040451
APA Stylede Sousa Moreira, A., Santos-Silva, S., Mega, J., Palmeira, J. D., Torres, R. T., & Mesquita, J. R. (2022). Epidemiology of Porcine Circovirus Type 2 Circulating in Wild Boars of Portugal during the 2018–2020 Hunting Seasons Suggests the Emergence of Genotype 2d. Animals, 12(4), 451. https://doi.org/10.3390/ani12040451






