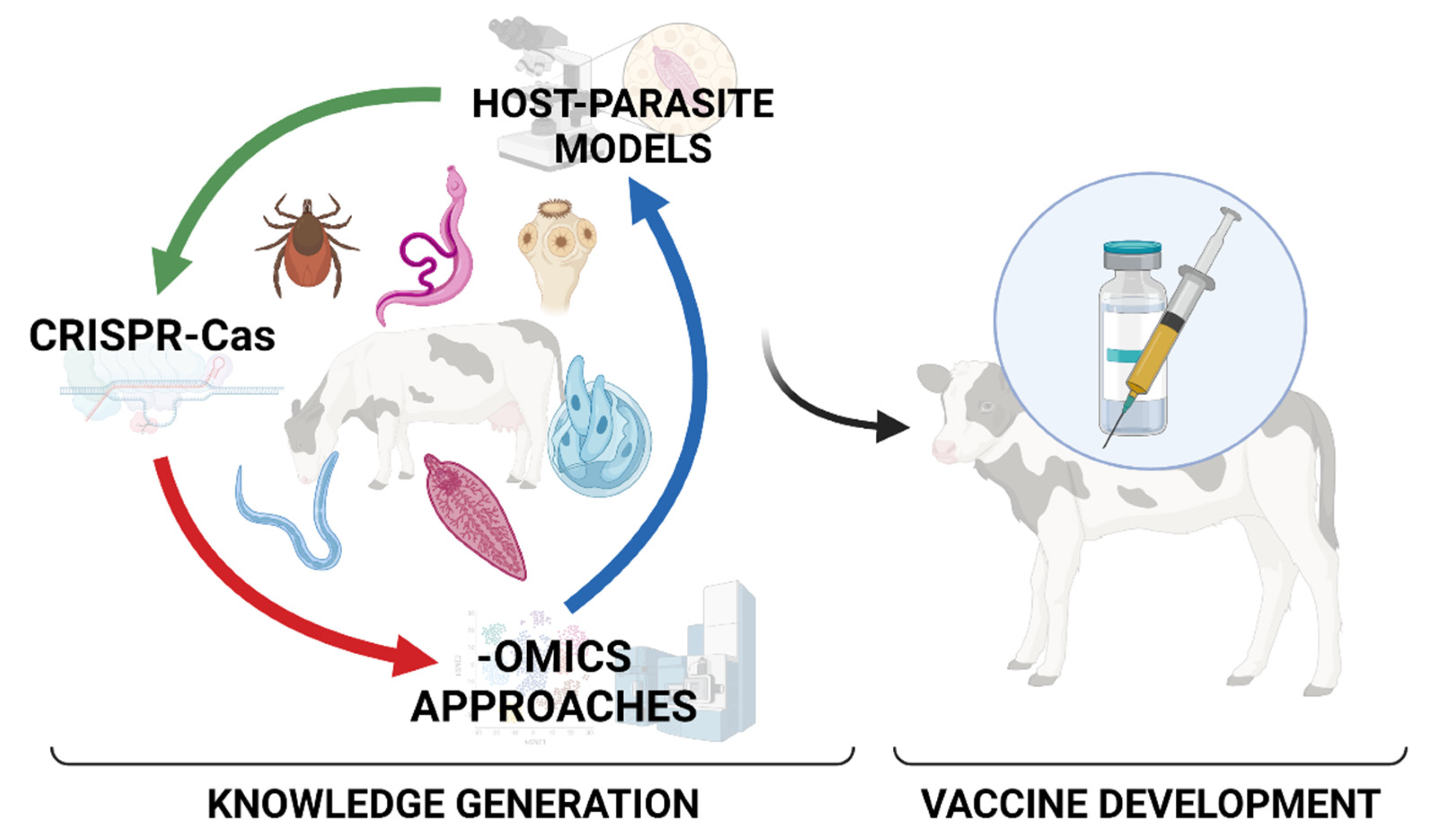
Host–Parasite Relationships in Veterinary Parasitology: Get to Know Your Enemy before Fighting It
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulin, R. Evolutionary Ecology of Parasites, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Abongwa, M.; Martin, R.J.; Robertson, A.P. A Brief Review on the Mode of Action of Antinematodal Drugs. Acta Vet. (Beogr). 2017, 67, 137–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stutzer, C.; Richards, S.A.; Ferreira, M.; Baron, S.; Maritz-Olivier, C. Metazoan Parasite Vaccines: Present Status and Future Prospects. Front. Cell. Infect. Microbiol. 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piegari, G.; Pepe, P.; De Biase, D.; d’Aquino, I.; Bosco, A.; Cringoli, G.; Papparella, S.; Rinaldi, L.; Paciello, O. Immunopathological Response, Histological Changes, Parasitic Burden, and Egg Output in Sheep Naturally Infected by Dicrocoelium dendriticum. Animals 2021, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Ptáček, M.; Kyriánová, I.A.; Nápravníková, J.; Ducháček, J.; Husák, T.; Chay-Canul, A.J.; Zaragoza-Vera, C.; Cruz-Bacab, L.; Vadlejch, J. Do Live Weight, Body Condition Score, Back Muscle or Back-Fat Reserves Create the Suspicion of Goats Infected with Eimeria or Trichostrongylids? Animals 2021, 11, 3591. [Google Scholar] [CrossRef] [PubMed]
- Diosdado, A.; Simón, F.; Morchón, R.; González-Miguel, J. Host-Parasite Relationships in Porcine Ascariosis: Anticoagulant Potential of the Third Larval Stage of Ascaris suum as a Possible Survival Mechanism. Animals 2021, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Cafrune, M.M.; Funatsu, I.R.; Mangold, A.J.; Angles, R.; Buchon, P.; Fantozzi, M.C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Fascioliasis in Llama, Lama glama, in Andean Endemic Areas: Experimental Transmission Capacity by the High Altitude Snail Vector Galba truncatula and Epidemiological Analysis of Its Reservoir Role. Animals 2021, 11, 2693. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Valero, M.A.; Trueba, G.A.; Fornasini, M.; Villavicencio, A.F.; Guamán, R.; De Elías-Escribano, A.; Pérez-Crespo, I.; Artigas, P.; Mas-Coma, S. DNA Multi-Marker Genotyping and CIAS Morphometric Phenotyping of Fasciola gigantica-Sized Flukes from Ecuador, with an Analysis of the Radix Absence in the New World and the Evolutionary Lymnaeid Snail Vector Filter. Animals 2021, 11, 2495. [Google Scholar] [CrossRef] [PubMed]
- Barbour, T.; Cwiklinski, K.; Lalor, R.; Dalton, J.P.; De Marco Verissimo, C. The Zoonotic Helminth Parasite Fasciola hepatica: Virulence-Associated Cathepsin B and Cathepsin L Cysteine Peptidases Secreted by Infective Newly Excysted Juveniles (NEJ). Animals 2021, 11, 3495. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hernández, V.; Ruiz-Campillo, M.T.; Martínez-Moreno, F.J.; Buffoni, L.; Martínez-Moreno, Á.; Zafra, R.; Bautista, M.J.; Escamilla, A.; Pérez-Caballero, R.; Pérez, J. A Partially Protective Vaccine for Fasciola hepatica Induced Degeneration of Adult Flukes Associated to a Severe Granulomatous Reaction in Sheep. Animals 2021, 11, 2869. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Miguel, J. Host–Parasite Relationships in Veterinary Parasitology: Get to Know Your Enemy before Fighting It. Animals 2022, 12, 448. https://doi.org/10.3390/ani12040448
González-Miguel J. Host–Parasite Relationships in Veterinary Parasitology: Get to Know Your Enemy before Fighting It. Animals. 2022; 12(4):448. https://doi.org/10.3390/ani12040448
Chicago/Turabian StyleGonzález-Miguel, Javier. 2022. "Host–Parasite Relationships in Veterinary Parasitology: Get to Know Your Enemy before Fighting It" Animals 12, no. 4: 448. https://doi.org/10.3390/ani12040448
APA StyleGonzález-Miguel, J. (2022). Host–Parasite Relationships in Veterinary Parasitology: Get to Know Your Enemy before Fighting It. Animals, 12(4), 448. https://doi.org/10.3390/ani12040448




