The Effects of Castration, Implant Protocol, and Supplementation of Bos indicus-Influenced Beef Cattle under Tropical Savanna Conditions on Growth Performance, Carcass Characteristics, and Meat Quality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Pastures
2.2. Animal Management
2.2.1. Experiment I
2.2.2. Experiment II
2.3. Harvest and Carcass Evaluation
2.3.1. Experiment I
2.3.2. Experiment II
2.4. Carcass Fabrication
2.5. Culinary, Sensory Evaluation, and Shear Force Tests
2.6. Statistical Analyses
3. Results
3.1. Experiment I
3.1.1. Frequency Distribution of Cattle in Harvesting Lots by Supplementation and Implant Treatments
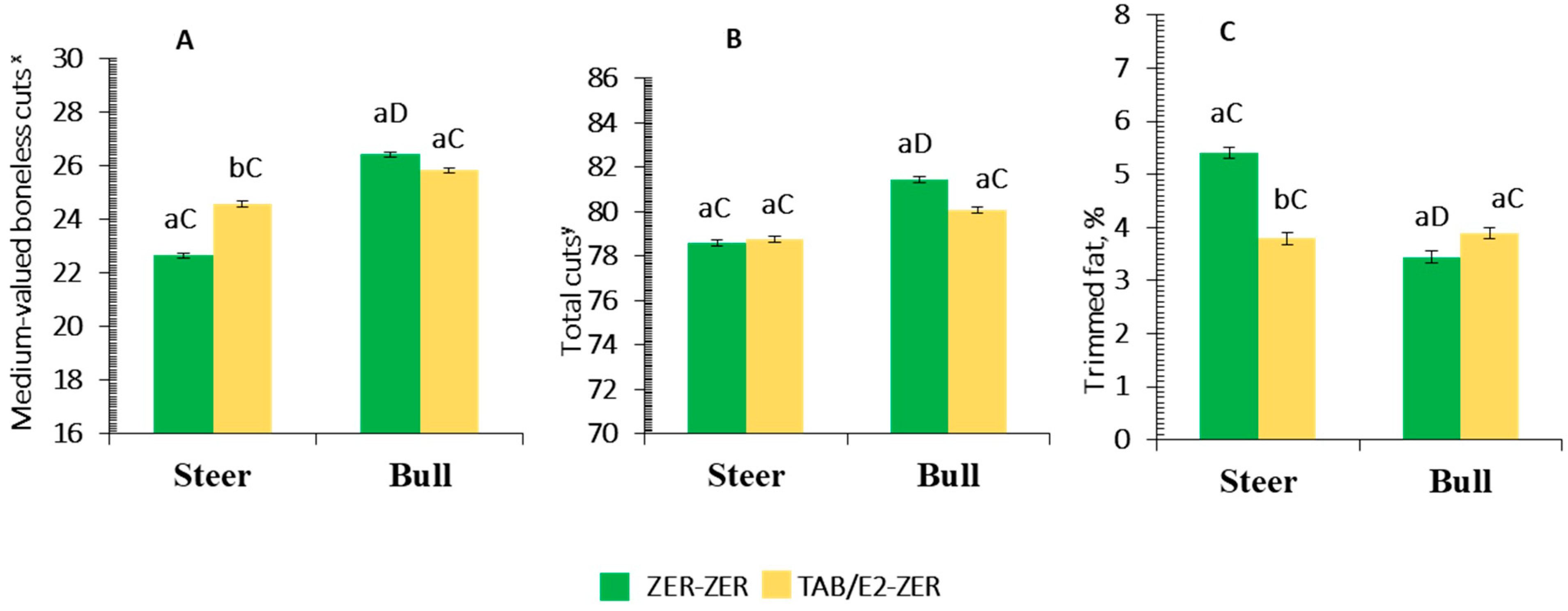
3.1.2. Growth Traits, Carcass Dressing, and Yield of Hindquarter’s Subprimal and Coproducts
Pasture Supplementation and Implant Protocol Main Effects
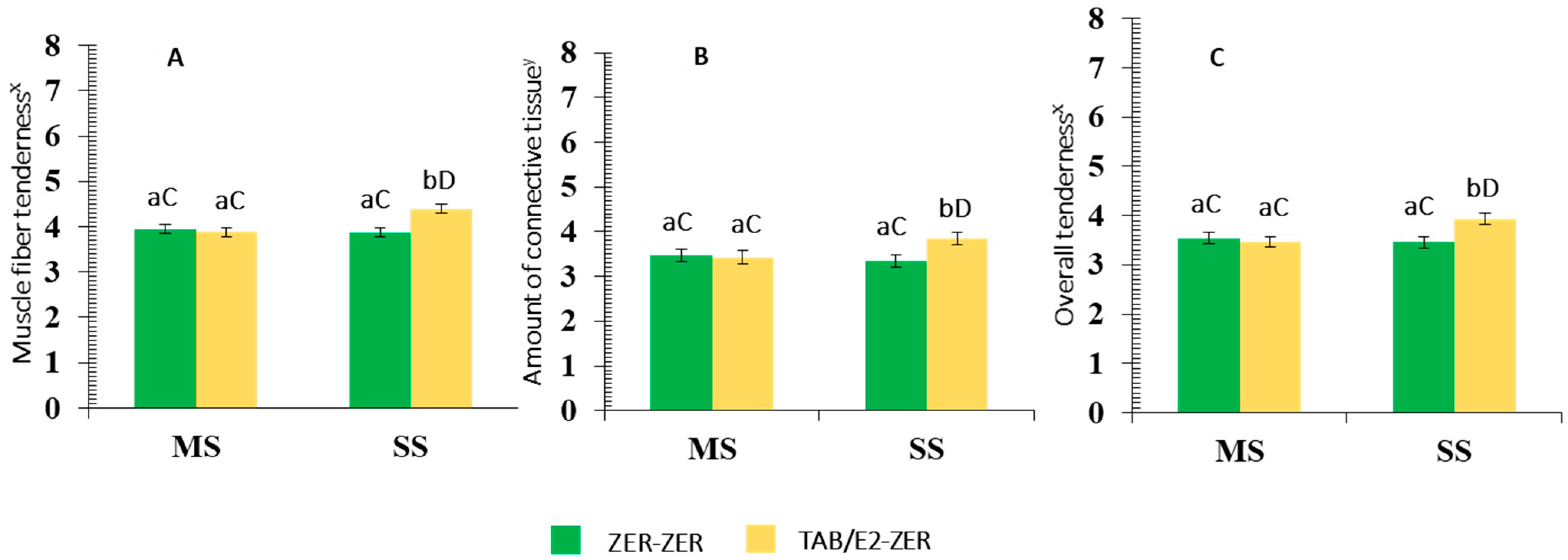
3.1.3. Cookery and Quality Traits
Interaction Effects
Pasture Supplementation and Implant Protocol Main Effects
3.2. Experiment II
3.2.1. Frequency Distribution of Cattle in Harvesting Lots by Male Class and Implant Protocol
3.2.2. Growth Performance Traits
3.2.3. Carcass Traits
3.2.4. Frequency Distribution of Carcass Categories/Grades According to Male Class and Implant Protocol
3.2.5. Carcass Yield in Subprimal Cuts and Coproducts
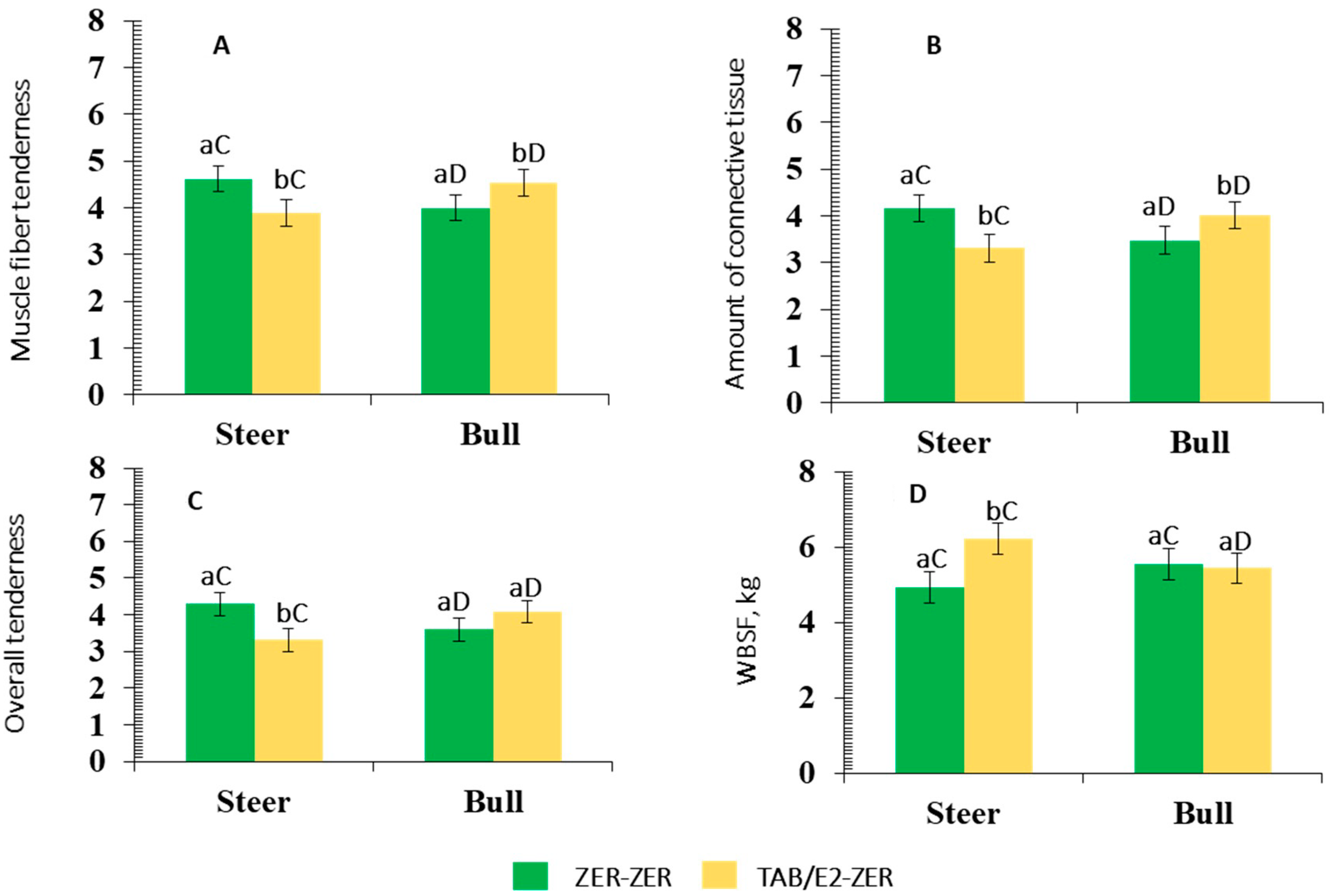
3.2.6. Cookery and Meat Quality Traits
Male Class and Implant Protocol-Independent Effects
4. Discussion
4.1. General
4.2. Grass-Fed Finishing Performance
4.3. Carcass Performance
4.4. Eating Quality and Cookery Traits
4.4.1. Effects of Pasture Supplementation
4.4.2. Effects of Implants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riera-Sigala, T.J.; Rodas-González, A.; Rodríguez-Matos, C.; Avellaneda, J.F.; Huerta-Leidenz, N. Growth traits and carcass weights of purebred Brahman and F1 Brahman x Bos taurus bulls raised and fattened semi-intensively on improved savannah. Arch. Latinoam. Prod. Anim. 2004, 12, 66–72. [Google Scholar]
- Huerta-Leidenz, N.; Jerez-Timaure, N.; Rodas-González, A. Carcass performance of cows, heifers, and bulls fattened to pasture in the savanna ecosystem. NACAMEH 2020, 4, 41–60. Available online: http://nacameh.cbsuami.org/volumenes/v14n1/Nacameh_v14n1p41_HuertaLeidenz_etal.pdf (accessed on 20 April 2021). [CrossRef]
- Congreso de la República de Venezuela. Decreto Presidencial No. 1896. Gaceta Oficial de la República de Venezuela No. 36.242; Congreso de la República de Venezuela: Caracas, Venezuela, 1997. [Google Scholar]
- Plasse, D.; Fossi, H.; Hoogesteijn, E.; Verde, O.; Rodríguez, R.; Rodríguez, C.M.; Bastidas, P. Growth of F1 Bos taurus x Bos indicus versus Bos indicus beef cattle in Venezuela. I. Weights at birth, weaning and 18 months. J. Anim. Breed. Genet. 1995, 112, 117–132. [Google Scholar] [CrossRef]
- Plasse, D.; Fossi, H.; Hoogesteijn, R.; Verde, O.; Rodríguez, C.M.; Rodríguez, R.; Bastidas, P. Growth of F1 Bos taurus × Bos indicus versus Bos indicus beef cattle in Venezuela. II. Initial, final, and carcass weight of bulls, and breeding weight of heifers. J. Anim. Breed. Genet. 1995, 112, 1331–1345. [Google Scholar] [CrossRef]
- Torres, G.R. The agroecosystem modules de Apure as an instrument to confront drought. Rev. Fac. Agron. 1994, 11, 175–189. [Google Scholar]
- Smith, J.K.; Chacón-Moreno, E.J.; Jongman, R.H.G.; Wenting, P.P.; Loedeman, J.H. Effect of Dike Construction on Water Dynamics in the Flooding Savannahs of Venezuela. Earth Surf. Proc. Land. 2006, 31, 81–96. [Google Scholar] [CrossRef]
- Mwangi, F.W.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Kinobe, R.T.; Malau-Aduli, A.E.O. Diet and genetics influence beef cattle performance, and meat quality. Foods 2019, 8, 648. [Google Scholar] [CrossRef]
- Poppi, D.P.; McLennan, S.R. Nutritional research to meet future challenges. Anim. Prod. Sci. 2010, 50, 329–338. [Google Scholar] [CrossRef]
- Poppi, D.P.; Quigley, S.P.; Corrêa Carvalho da Silva, T.A.; McLennan, S.R. Challenges of beef cattle production from tropical pastures. Rev. Bras. Zootec. 2018, 47, e20160419. [Google Scholar] [CrossRef]
- Seideman, S.C.; Cross, H.R.; Oltjen, R.R.; Schanbacher, B.D. Utilization of the intact male for red meat production: A review. J. Anim. Sci. 1982, 55, 826–840. [Google Scholar] [CrossRef]
- Ismail, H.A. Effect of Castration on Feedlot Performance, Carcass Characteristics and Meat Quality of Western Sudan Baggara Bulls. Master’s Thesis, University of Khartoun, Khartoun State, Sudan, 2006. [Google Scholar]
- Venkata Reddy, B.; Sivakumar, A.S.; Jeong, D.W.; Woo, Y.-B.; Park, S.J.; Lee, S.Y.; Byun, J.-Y.; Kim, C.H.; Cho, S.H.; Hwang, I. Beef quality traits of heifer in comparison with steer, bull, and cow at various feeding environments. Anim. Sci. J. 2014, 86, 11–16. [Google Scholar] [CrossRef]
- Morón-Fuenmayor, O.; Araujo Febres, O.; Brillembourg, D. Efecto de la condición sexual y del implante con ATB+l7β-estradiol sobre el crecimiento de animales mestizos Santa Gertrudis. Rev. Fac. Agron. 1994, 11, 81–87. Available online: https://www.researchgate.net/profile/Effect-of-sex-conditions.pdf (accessed on 15 January 2022).
- Aricett, J.A.; Rotta, P.P.; do Prato, R.M.; Perotto, D.; Moletta, J.L.; Matsushita, M.; do Prado, I.N. Carcass characteristics, chemical composition, and fatty acid profile of Longissimus muscle of bulls and steers finished in a pasture system bulls and steers finished in pasture systems. Asian-Australas J. Anim. Sci. 2008, 21, 14411–14448. [Google Scholar] [CrossRef]
- Rodríguez, J.; Unruh, J.; Villarreal, M.; Murillo, O.; Rojas, S.; Camacho, J.; Reinhardt, C. Carcass and meat quality characteristics of Brahman cross bulls and steers finished on tropical pastures in Costa Rica. Meat Sci. 2014, 96, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Moron-Fuenmayor, O.; Rumbos-Gomez, J.L. Dual implantation use and breed type effect on bulls under savanna conditions. Arch. Latinoam. Prod. Anim. 1997, 5, 180–181. [Google Scholar]
- Moron-Fuenmayor, O.; Rumbos-Gomez, J.L. Uses of anabolic agents on the growth of crossbred commercial bulls as a management strategy in Venezuelan savannas. Arch. Latinoam. Prod. Anim. 1997, 5, 183–185. [Google Scholar]
- Chacón, E.; Marchena, H. Tecnologías alimentarias apropiadas para la producción con bovinos a pastoreo. In Desarrollo Sostenible en la Ganadería de Doble Propósito; González Stagnaro, C., Madrid Bury, N., Soto Belloso, E., Eds.; Fundación GIRARZ, Ediciones Astro Data: Maracaibo, Venezuela, 2008; pp. 435–453. [Google Scholar]
- Depablos, L.; Ordoñez, J.; Godoy, S.; Chicco, C.F. Suplementación mineral proteica de novillas a pastoreo en los Llanos Centrales de Venezuela. Zootec. Trop. 2009, 27, 249–262. [Google Scholar]
- Smith, Z.K.; Johnson, B.J. Mechanisms of steroidal implants to improve beef cattle growth: A review. J. Appl. Anim. Res. 2020, 48, 133–141. [Google Scholar] [CrossRef]
- Araujo-Febres, O.; Pietrosemoli, E. Hormonal and non-hormonal implants on commercial steers on pasture with supplementation. Rev. Fac. Agron. 1991, 8, 2092–3017. Available online: https://www.revfacagronluz.org.ve/v08_3/0803z050.html (accessed on 15 January 2022).
- Morón- Fuenmayor, O.; Araujo-Febres, O.; Rincón- Urdaneta, E. Efecto del implante, de la castración y mestizaje en toretes mestizos comerciales a pastoreo con suplementación. Rev. Fac. Agron. 1992, 9, 49–62. [Google Scholar]
- Moron-Fuenmayor, O.; Pietrosemoli, S.; Aranguren, J.A.; Fossi, A. Use of Anabolic agents alone or in combination on growth in grazing steers. Rev. Cient. FCV-LUZ 1999, 9, 299–304. [Google Scholar]
- Prado Ortega, M.J.; Nouel Borges, G.E.; Prado Ortega, J.G. Uso de lisado de órganos y zeranol en el engorde de novillos mestizos comerciales a pastoreo bajo condiciones de bosque seco tropical. Rev. Cient. FCV-LUZ 2002, 2, 555–558. [Google Scholar]
- Arias, R.; Santa-Cruz, C.; Velásquez, A. Effect of High Potency Growth Implants on Average Daily Gain of Grass-Fattened Steers. Animals 2019, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Arias, R.; Manrique, S.; Velasquez, A. Effect of an anabolic growth-stimulating implant on the productive and economic response of steers of three breeds. Agro. Sur. 2021, 49, 21–29. [Google Scholar] [CrossRef]
- Huerta-Leidenz, N.; Jerez-Timaure, N.; Sarturi, J.O.; Brashears, M.M.; Miller, M.F.; Moya, A.; Godoy, S. Effects of Sex Class, a Combined Androgen and Estrogen Implant, and Pasture Supplementation on Growth and Carcass Performance and Meat Quality of Zebu-Type Grass-Fed Cattle. Animals 2021, 11, 3441. [Google Scholar] [CrossRef]
- Mackenzie, J.R. Effects of zeranol implant on behavior, growth rate, and carcass characteristics of Friesian bulls. N. Z. J. Exp. Agric. 1983, 11, 225–229. [Google Scholar] [CrossRef]
- Rubio, O.; Montiel, N.S. Effect comparative on the gain of weight the two anabolic implants in hybrid Bos-indicus bulls and castrate bulls pasturing. Rev. Cient. FCV-LUZ 1994, 4, 131–138. Available online: https://www.produccioncientificaluz.org/index.php/cientifica/article/view/14139/14118 (accessed on 15 January 2022).
- Foutz, C.P.; Dolezal, H.G.; Gardner, T.L.; Gill, J.L.; Hensley, J.L.; Morgan, J.B. Anabolic implants effects on steers performance, carcass traits, subprimal yields, and Longissimus muscle properties. J. Anim. Sci. 1997, 75, 1256–1265. [Google Scholar] [CrossRef]
- Watson, R.; Polkinghorne, R.; Gee, A.; Porter, M.; Thompson, J.M.; Ferguson, D.; Pethick, D.; McIntyre, B. Effect of hormonal growth promotants on palatability and carcass traits of various muscles from steer and heifer carcasses from a Bos indicus-Bos taurus composite cross. Aust. J. Exp. Agric. 2008, 48, 1415–1424. [Google Scholar] [CrossRef][Green Version]
- Packer, D.T.; Geesink, G.H.; Thompson, J.M.; Polkinghorne, R.J.; Ball, A.B.; McGilchrist, P. The impact of different hormonal growth promotants (HGP) on desmin degradation and collagen content of various muscles from pasture and feedlot finished steer carcasses. Meat Sci. 2021, 182, 108515. [Google Scholar] [CrossRef]
- Lean, I.J.; Golder, H.M.; Lees, N.M.; Mc Gilchrist, P.; Santos, J.E. Effects of hormonal growth promotants on beef quality: A meta-analysis. J. Anim. Sci. 2018, 96, 675–2697. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Leidenz, N.; Jerez-Timaure, N.; Godoy, S.; Rodríguez-Matos, C.; Araujo-Febres, O. Fattening performance and carcass traits of implanted and supplemented grass-fed bulls. Rev. Cient. FCV-LUZ 2021, 31, 53–60. [Google Scholar] [CrossRef]
- Fondo Nacional de Ciencia, Tecnología e Innovación (MCT-FONACIT). Código de Bioética y Bioseguridad, 2nd ed.; Ministerio del Poder Popular para Ciencia, Tecnología e Industrias Intermedias y el Fondo Nacional de Ciencia, Tecnología e Innovación: Caracas, Venezuela, 2002; pp. 1–35. Available online: https://cupdf.com/download/bioetica-fonacit (accessed on 30 September 2021).
- González-Ronquillo, M.; Aparicio, R.; Torres, R.; Domínguez Vera, I.A. Producción de biomasa, composición química y producción de gas in vitro de la vegetación de una sábana estacional modulada. Zootec. Trop. 2009, 27, 47–417. [Google Scholar]
- Torin, C.; Rodríguez, L.; Piñate, P.; Verdecia, I. Agroclimatic characteristics in the municipality San Fernando de Apure State, Venezuela. Rev. Cient. UDO Agric. 2012, 12, 178–186. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM). Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016; p. 494. [Google Scholar]
- Venezuela, Decreto Presidencial No. 181. Gaceta Oficial de la República de Venezuela No. 4737; Congreso de la República de Venezuela: Caracas, Venezuela, 1994. [Google Scholar]
- Comisión Venezolana de Normas Industriales (COVENIN). Norma Venezolana 792–82: Carne de Bovino. Definición e Identificación de las Piezas de Una Canal; FONDONORMA: Caracas, Venezuela, 1982; pp. 1–10. [Google Scholar]
- Comisión Venezolana de Normas Industriales. Norma Venezolana 2072–83: Ganado Bovino. Inspección Postmortem; FONDONORMA: Caracas, Venezuela, 1983; pp. 1–10. Available online: http://www.sencamer.gob.ve/sencamer/normas/2072–83.pdf (accessed on 25 February 2021).
- Rodas González, A.; Huerta-Leidenz, N.; Jerez-Timaure, N. Benchmarking Venezuelan quality grades for grass-fed cattle carcasses. Meat Muscle Biol. 2017, 1, 718. [Google Scholar] [CrossRef][Green Version]
- United States Department of Agriculture (USDA). Official United States Standards for Grades of Carcass Beef; Agricultural Marketing Service: Washington, DC, USA, 2017.
- American Meat Science Association. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat, 2nd ed.; Version 1.02; American Meat Science Association: Champaign, IL, USA, 2016. [Google Scholar]
- Rodas-González, A.; Huerta-Leidenz, N.; Jerez- Timaure, N.; Miller, M.F. Establishing tenderness thresholds of Venezuelan beef steaks using consumer and trained sensory panels. Meat Sci. 2009, 83, 218–223. [Google Scholar] [CrossRef]
- Shapiro, S.; Wilk, M. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org (accessed on 13 May 2021).
- Steel, D.; Torrie, H. Bioestadística: Principios y Procedimientos, 2nd ed.; McGraw Hill: New York, NY, USA, 1985; Available online: http://www.repositorio.cenpat-conicet.gob.ar/bitstream/handle/123456789/1206/bioestad-steel.pdf?sequence=1 (accessed on 15 May 2021).
- Montiel, N. Comparison of two types of anabolic on the gain of weight in cattle Bos indicus to pasture with two supplementary levels. Rev. Cient. FCV-LUZ 1994, 4, 113–118. Available online: https://www.produccioncientificaluz.org/index.php/cientifica/article/view/14135/14115 (accessed on 15 January 2022).
- Connell, J.; Huerta-Leidenz, N.; Rodas-Gonzalez, A. Fattening on pasture of beef calves differing in muscle thickness, frame size, and apparent Brahman genotype subjected to implant and supplementation regimes. Arch. Latinoam. Prod. Anim. 2002, 10, 156–163. [Google Scholar]
- Saddy, J.; Combellas, J.; Tesorero, M.; Gabaldón, L. Comparación de dos sistemas de alimentación con cama de pollos sobre la ganancia de peso en bovinos. Zootec. Trop. 2002, 20, 111–119. Available online: https://hdl.handle.net/1807/163 (accessed on 20 November 2021).
- Zamora-Bonilla, L.N. Evaluación de tres dietas con base en una mezcla tamo de arroz-pollinaza en toretes Cebú comercial en el trópico bajo del Valle del Alto Magdalena, Colombia. Rev. Colomb. Cienc. Anim. 2008, 1, 2227. [Google Scholar]
- Mekasha, Y.; Urge, M.; Kurtu, M.Y.; Bayissa, M. Effect of strategic supplementation with different proportion of agro-industrial by-products and grass hay on body weight change and carcass characteristics of tropical Ogaden bulls (Bos indicus) grazing native pasture. Afr. J. Agric. Res. 2011, 6, 825–833. [Google Scholar] [CrossRef]
- Cunha Rocha, T.; de Alencar Fontes, C.A.; Tavares Soares da Solva, R.; Fonseca Processi, E.; Amaral Ferreira do Valle, F.R.; Teixeira Lombardi, C.; Lopes Oliveira, R.; Rocha Bezerra, L. Performance, nitrogen balance and microbial efficiency of beef cattle under concentrate supplementation strategies in intensive management of a tropical pasture. Trop. Anim. Health Prod. 2016, 48, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Lozano, M.S.; Ngapo, T.M.; Huerta-Leidenz, N. Tropical Beef: Is There an Axiomatic Basis to Define the Concept? Foods 2021, 10, 1025. [Google Scholar] [CrossRef]
- Huerta-Leidenz, N.; Hernández, O.; Rodas-González, A.; Ordóñez, V.J.; Pargas, H.L.; Rincón, E.; del Villar, A.; Bracho, B. Body weight and carcass dressing as affected by sex class, breed type, muscle thickness, age, and provenance of Venezuelan cattle. NACAMEH 2013, 7, 75–96. [Google Scholar] [CrossRef]
- Flórez, H.; Martínez, G.; Ballesteros, H.; León, L.M.; Castañeda, S.; Moreno, E.; Arias, L.E.; Torres, J.C.; Rodríguez, C.A.; Peña, F.; et al. Beef yield of creole and European bovines and their crosses with zebu under conditions of the Colombian Orinoquia. AICA 2014, 4, 12–15. [Google Scholar]
- Jerez-Timaure, N.; Huerta-Leidenz, N. Effects of breed type and supplementation during grazing on carcass traits and meat quality of bulls fattened on improved savannah. Livest. Sci. 2009, 121, 219–226. [Google Scholar] [CrossRef]
- Reed, K.F.; Bonfá, H.C.; Dijstra, J.; Casper, D.P.; Kebreab, E. Estimating the energetic cost of feeding excess dietary nitrogen to dairy cows. J. Dairy Sci. 2017, 100, 7116–7126. [Google Scholar] [CrossRef]
- Broderick, G.A. Effects of varying dietary protein and energy levels on the production of lactating dairy cows. J. Dairy Sci. 2003, 86, 1370–1381. [Google Scholar] [CrossRef]
- Berg, R.T.; Butterfield, R.M. Methods of measuring and predicting carcass composition. In New Concepts of Cattle Growth; Chapter 8; The Internet-First University Press: Sydney, Australia, 1976; pp. 202–203. [Google Scholar]
- Chase, C.C., Jr.; Chenoweth, P.J.; Larsen, R.E.; Hammond, A.C.; Olson, T.A.; West, R.L.; Johnson, D.D. Growth, puberty, and carcass characteristics of Brahman, Senepol, and Tuli-sired F1 Angus bulls. J. Anim. Sci. 2001, 89, 2006–2015. [Google Scholar] [CrossRef]
- Vazquez-Mendoza, O.V.; Aranda-Osorio, G.; Huerta-Bravo, M.; Kholif, A.E.; Elghandour, M.M.Y.; Salem, A.Z.M.; Maldonado-Simán, E. Carcass and meat properties of six genotypes of young bulls finished under feedlot tropical conditions of Mexico. Anim. Prod. Sci. 2017, 57, 1186–1192. [Google Scholar] [CrossRef]
- Acosta Castellanos, N.T. Efecto del sistema productivo sobre la dureza de cuatro cortes comerciales de ganado doble propósito del trópico alto en Cundinamarca, Colombia. Tesis de Maestría, Facultad de Cs. Agrarias, Universidad Nacional de Colombia, Bogotá, Colombia, 2018; 74p. Available online: https://repositorio.unal.edu.co/bitstream/handle/unal/77463/Efecto%20del%20sistema%20productivo%20sobre%20la%20dureza.pdf?sequence=1&isAllowed=y (accessed on 9 December 2021).
- Schmutz, M.; Weindl, P.; Carrasco, S.; Bellof, G.; Schmidt, E. Effect of breed, grazing system and concentrate supplementation on fattening performance, carcass value, and meat quality of steers. Arch. Anim. Breed. 2013, 56, 943–957. [Google Scholar] [CrossRef]
- Duynisveld, J.L.; Charmley, E.; Mir, P. Meat quality and fatty acid composition of pasture finished beef steers fed barley and soybeans. Can. J. Anim. Sci. 2006, 86, 535–545. [Google Scholar] [CrossRef]
- Platter, W.J.; Tatum, J.D.; Belk, K.E.; Scanga, J.A.; Smith, G.C. Effects of repetitive use of hormonal implants on beef carcass quality, tenderness, and consumer ratings of beef palatability. J. Anim. Sci. 2003, 81, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Duckett, S.K.; Pratt, S.L. Anabolic implants, and meat quality: Meat Science and Muscle Biology Symposium. J. Anim. Sci. 2014, 92, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.W.; Henricks, D.M.; Skelley, G.C.; Grimes, L.W. Use of trenbolone acetate and estradiol in intact and castrate male cattle: Effects on growth, serum hormones, and carcass characteristics. J. Anim. Sci. 1991, 69, 2452–2462. [Google Scholar] [CrossRef]
| Breed Type | Steer | Bull | Total (n) | ||
|---|---|---|---|---|---|
| ZER-ZER a | TBA/E2-ZER b | ZER-ZER a | TBA/E2-ZER | ||
| F1-Angus | 1 | 2 | 3 | 3 | 9 |
| F1-Romosinuano | 3 | 2 | 3 | 3 | 11 |
| F1-Senepol | 2 | 2 | 4 | 2 | 10 |
| F1-Simmental | 1 | 2 | 4 | 3 | 10 |
| Commercial Brahman | 2 | 2 | 2 | 4 | 10 |
| Total | 9 | 10 | 16 | 15 | 50 |
| Time until Harvest (Days) | SUPPL | Implant Protocol | Total (n) | ||
|---|---|---|---|---|---|
| MS a n (%) | SS b n (%) | ZER-ZER c n (%) | TBA/E2-ZER d n (%) | ||
| 181 | 1 (7.7) | 12 (92.33) | 5 (38.5) | 8 61.5) | 13 |
| 195 | 0 (0) | 13 (100) | 7 (46.2) | 6 (46.2) | 13 |
| 209 | 5 (29.4) | 12 (70.0) | 10 (41.2) | 7 (41.2) | 17 |
| 223 | 13 (72.2) | 5 (27.8) | 7 (61.1) | 11 (61.1) | 18 |
| 237 | 21 (100) | 0 (0) | 12 (42.9) | 9 (42.9) | 21 |
| 258 | 17 (100) | 0 (0) | 9 (47.1) | 8 (47.1) | 17 |
| χ2 = 65.98; p = 0.0006 | χ2 = 2.66; p = 0.75 | N = 99 | |||
| Variable | Supplementation (SUPPL) | Implant Protocol (IMPL) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| MS a (n = 57) | SS b (n = 42) | ZER-ZER c (n = 50) | TBA/E2-ZER d (n = 49) | SEM | SUPPL | IMPL | SUPPL × IMPL | |
| Initial BW, kg | 347.16 | 346.90 | 344.44 | 349.7 | 2.80 | 0.96 | 0.45 | 0.88 |
| Adjusted final BW, kg e | 490.31 | 487.54 | 484.95 | 493.40 | 12.5 | 0.46 | 0.54 | 0.32 |
| Adjusted ADG f (0–d of shipment), g | 606.22 | 711.98 | 640.22 | 662.18 | 8.3 | <0.01 | 0.18 | 0.57 |
| Cold carcass weight, kg | 288.27 | 294.21 | 289.02 | 292.60 | 1.82 | 0.71 | 0.84 | 0.10 |
| Cold carcass dressing,% | 56.46 | 57.95 | 57.23 | 56.96 | 0.17 | <0.01 | 0.65 | 0.13 |
| High-valued boneless cuts, % g | 33.04 | 35.06 | 34.62 | 33.16 | 0.31 | <0.01 | 0.25 | 0.49 |
| Total clean bone,% | 7.92 | 7.71 | 7.71 | 7.76 | 0.12 | 0.50 | 0.78 | 0.72 |
| Trimmed fat,% | 3.48 | 3.80 | 3.53 | 4.02 | 0.16 | 0.83 | 0.78 | 0.91 |
| Variable | Supplementation (SUPPL) | Implant Protocol (IMPL) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| MS a (n = 57) | SS b (n = 42) | ZER-ZER c (n = 50) | TBA/E2-ZER d (n = 49) | SEM | SUPPL | IMPL | SUPPL × IMPL | |
| Cooking loss,% | 32.44 | 34.18 | 32.86 | 33.48 | 0.29 | <0.01 | 0.16 | 0.06 |
| Cooking time, min | 79.19 | 78.37 | 77.81 | 79.90 | 0.42 | 0.07 | 0.01 | 0.11 |
| WBSF, kg e | 5.93 | 5.53 | 5.99 | 5.53 | 0.07 | <0.01 | <0.01 | 0.19 |
| Muscle fiber tenderness f | 3.91 | 4.13 | 3.91 | 4.10 | 0.06 | 0.02 | 0.04 | 0.02 |
| Amount of connective tissue g | 3.45 | 3.59 | 3.42 | 3.60 | 0.06 | 0.10 | 0.15 | <0.01 |
| Overall tenderness f | 3.50 | 3.70 | 3.51 | 3.66 | 0.06 | <0.01 | 0.32 | 0.02 |
| Juiciness h | 4.70 | 4.95 | 4.17 | 4.89 | 0.03 | 0.01 | 0.01 | 0.37 |
| Flavor intensity i | 5.77 | 5.74 | 5.75 | 5.78 | 0.02 | 0.56 | 0.66 | 0.75 |
| Time until Harvest (Days) | Male class | Implant Protocol | Total (n) | ||
|---|---|---|---|---|---|
| Steer n (%) | Bull n (%) | ZER-ZER a n (%) | TBA/E2-ZER b n (%) | ||
| 181 | 8 (46.7) | 8 (53.3) | 7 (46.7) | 8 (53.3) | 15 |
| 195 | 7 (41.2) | 10 (58.8) | 11 (64.7) | 6 (35.3) | 17 |
| 209 | 3 (30.0) | 7 (70.0) | 4 (40.0) | 6 (60.0) | 10 |
| 223 | 2 (28.6) | 5 (71.4) | 3 (42.9) | 4 (57.1) | 7 |
| χ2 = 1.06; p = 0.78 | χ2 = 2.06; p = 0.56 | N = 49 | |||
| Variable | Male Class | Implant protocol | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Steer (n = 19) | Bull (n = 31) | ZER-ZER a (n = 25) | TBA/E2-ZER b (n = 25) | SEM | CLASS | IMPL | CLASS × IMPL | |
| Initial BW, kg | 333.78 | 348.90 | 339.16 | 346.40 | 3.75 | 0.03 | 0.36 | 0.22 |
| Muscle thickness score c | 2.15 | 2.16 | 2.16 | 2.16 | 0.08 | 0.12 | 0.16 | 0.07 |
| Frame size score d | 2.11 | 1.96 | 2.08 | 1.95 | 0.09 | 0.57 | 0.41 | 0.11 |
| Hip height, cm | 135.01 | 134.67 | 133.8 | 135.8 | 3.61 | 0.16 | 0.97 | 0.14 |
| Chronological age, mo. | 28.80 | 29.66 | 29.21 | 29.46 | 0.78 | 0.04 | 0.54 | 0.12 |
| BW at end of supplementation test, kg | 475.26 | 500.64 | 484.56 | 497.44 | 13.69 | 0.03 | 0.42 | 0.65 |
| Final BW at shipment d e, kg | 484.21 | 511.22 | 494.88 | 507.04 | 13.80 | 0.02 | 0.32 | 0.85 |
| ADG1 (0–180 d), g | 800.29 | 843.01 | 814.43 | 839.11 | 16.77 | 0.22 | 0.41 | 0.71 |
| ADG2 (0–d of shipment), g | 777.63 | 817.57 | 790.71 | 814.07 | 15.75 | 0.04 | 0.12 | 0.27 |
| Adjusted BW at shipment kg f | 464.84 | 490.77 | 475.08 | 486.75 | 14.67 | <0.01 | 0.44 | 0.73 |
| Adjusted ADG2 g, g | 677.74 | 714.42 | 689.89 | 711.07 | 4.75 | 0.18 | 0.25 | 0.45 |
| Fattening days | 195.00 | 199.52 | 197.24 | 198.36 | 5.47 | 0.07 | 0.18 | 0.15 |
| Variables | Male Class | Implant Protocol | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Steer (n = 19) | Bull (n = 31) | ZER-ZER a (n = 25) | TBA/E2-ZER b (n = 25) | SEM | CLASS | IMPL | CLASS × IMPL | |
| Hot carcass weight, kg | 285.31 | 302.74 | 293.08 | 299.16 | 3.08 | 0.02 | 0.48 | 0.86 |
| Hot carcass dressing yield,% | 58.92 | 59.23 | 59.21 | 59.01 | 0.21 | 0.64 | 0.68 | 0.94 |
| Cold carcass weight, kg | 279.61 | 296.68 | 287.21 | 293.17 | 3.02 | <0.01 | 0.21 | 0.71 |
| Cold carcass dressing yield,% | 57.74 | 58.04 | 58.03 | 57.93 | 0.20 | 0.48 | 0.63 | 0.95 |
| Conformation score c | 3.16 | 3.74 | 3.48 | 3.56 | 0.13 | <0.01 | 0.10 | 0.15 |
| Ribeye area, cm2 | 81.70 | 85.58 | 86.11 | 82.09 | 2.66 | 0.07 | 0.56 | 0.16 |
| Finish score d | 3.10 | 2.87 | 3.00 | 2.92 | 0.08 | 0.43 | 0.87 | 0.64 |
| Back-fat thickness, mm | 3.37 | 1.77 | 2.48 | 2.28 | 0.25 | <0.01 | 0.38 | 0.52 |
| Marbling score e | 4.95 | 5.48 | 5.20 | 5.36 | 0.15 | 0.03 | 0.66 | 0.80 |
| Skeletal maturity f | 177.36 | 187.42 | 178.4 | 188.8 | 5.34 | 0.21 | 0.11 | 0.73 |
| Lean maturity f | 188.42 | 214.83 | 196.0 | 213.6 | 8.89 | 0.04 | 0.07 | 0.269 |
| Overall maturity f | 183.68 | 201.29 | 186.8.0 | 202.40 | 6.81 | 0.09 | 0.08 | 0.35 |
| Adipose maturity g | 3.00 | 2.90 | 2.92 | 2.96 | 0.08 | 0.27 | 0.84 | 0.73 |
| Carcass length, cm | 130.32 | 131.84 | 130.58 | 131.84 | 3.51 | 0.06 | 0.11 | 0.46 |
| Thigh width, cm | 60.13 | 62.27 | 61.36 | 61.56 | 1.65 | 0.04 | 0.84 | 0.85 |
| Length of pelvic limb, cm | 54.97 | 57.12 | 57.21 | 55.41 | 1.58 | 0.62 | 0.16 | 0.57 |
| Leg perimeter, cm | 119.15 | 120.80 | 120.24 | 120.12 | 3.22 | 0.49 | 0.90 | 0.83 |
| Thoracic depth, cm | 36.58 | 38.30 | 37.32 | 37.99 | 1.09 | 0.64 | 0.88 | 0.49 |
| Carcass Category/Grade | Male Class | Implant Protocol a | ||
|---|---|---|---|---|
| Steer n (%) | Bull n (%) | ZER-ZER n (%) | TBA/E2-ZER n (%) | |
| Venezuelan carcass category b | ||||
| AA | 8 (42.1) | 0 (0.0) | 5 (20) | 3 (12) |
| A | 4 (21.1) | 3 (9.7) | 4 (16) | 3 (12) |
| B | 5 (26.3) | 21 (67.7) | 13 (52) | 13 (52) |
| C | 2 (10.5) | 7 (22.6) | 3 (12) | 6 (24) |
| χ2 = 18.98; p = 0.002 | χ2 = 1.64; p = 0.65 | |||
| USDA Carcass Quality Grades c | ||||
| Select | 4 (21.1) | 0 (0) | 3 (12) | 1 (4) |
| High Standard | 10 (52.6) | 4 (12.9) | 7 (28) | 7 (28) |
| Low Standard | 5 (26.3) | 15 (48.4) | 9 (36) | 11 (44) |
| Bull | 0 (0) | 12 (38.7) | 6 (24) | 6 (24) |
| χ2 = 21.96; p = 0.001 | χ2 = 1.20; p = 0.75 | |||
| USDA Carcass Yield Grades d | ||||
| 1 | 6 (31.6) | 15 (48.4) | 10 (40) | 11 (44) |
| 2 | 2 (10.5) | 0 (0) | 1 (4) | 1 (4) |
| 3 | 11 (57.9) | 16 (51.6) | 14 (56) | 13 (52) |
| χ2 = 4.14; p = 0.12 | χ2 = 0.080; p = 0.96 | |||
| Total | 19 | 31 | 25 | 25 |
| Variable | Male Class | Implant Protocol | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Steer (n = 19) | Bull (n = 31) | ZER-ZER a (n = 25) | TBA/E2-ZER b (n = 25) | SEM | CLASS | IMPL | CLASS × IMPL | |
| High-valued boneless cuts,% c | 34.01 | 33.26 | 33.81 | 33.28 | 0.20 | 0.31 | 0.95 | 0.35 |
| Medium-valued boneless cuts,% d | 23.66 | 25.16 | 25.07 | 25.31 | 0.32 | <0.01 | 0.01 | 0.01 |
| Low-valued cuts,% e | 21.53 | 21.33 | 21.53 | 21.29 | 0.21 | 0.29 | 0.27 | 0.28 |
| Total clean bone,% | 7.49 | 7.56 | 7.55 | 7.51 | 0.09 | 0.39 | 0.21 | 0.19 |
| Trimmed fat,% | 4.56 | 3.66 | 4.15 | 3.85 | 0.18 | <0.01 | 0.02 | <0.01 |
| Total cuts,% f | 79.21 | 80.80 | 80.40 | 79.94 | 0.31 | <0.01 | 0.17 | 0.05 |
| Variables | Male Class | Implant Protocol | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Steers (n = 19) | Bulls (n = 31) | ZER-ZER a (n = 25) | TBA/E2-ZER b (n = 25) | SEM | CLASS | IMPL | CLASS × IMPL | |
| Cooking loss,% | 31.60 | 34.23 | 31.81 | 34.66 | 0.11 | 0.04 | <0.01 | 0.24 |
| Cooking time, min | 77.75 | 78.87 | 77.30 | 79.62 | 0.64 | 0.58 | 0.82 | 0.16 |
| WBSF, kg c | 5.52 | 5.50 | 5.29 | 5.74 | 0.29 | 0.11 | 0.04 | <0.01 |
| Muscle fiber tenderness d | 4.29 | 4.27 | 4.26 | 4.30 | 0.07 | 0.07 | 0.81 | <0.01 |
| ACT e | 3.78 | 3.75 | 3.76 | 3.75 | 0.08 | 0.87 | 0.96 | <0.01 |
| Overall tenderness d | 3.85 | 3.84 | 3.89 | 3.79 | 0.08 | 0.08 | 0.55 | <0.01 |
| Juiciness f | 4.98 | 4.97 | 4.89 | 5.07 | 0.06 | 0.05 | 0.06 | 0.14 |
| Flavor intensity g | 5.97 | 5.74 | 5.76 | 5.93 | 0.05 | 0.02 | 0.08 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta-Leidenz, N.; Jerez-Timaure, N.; Rodas-González, A.; Sarturi, J.O.; Brashears, M.M.; Miller, M.F.; Brashears, M.T. The Effects of Castration, Implant Protocol, and Supplementation of Bos indicus-Influenced Beef Cattle under Tropical Savanna Conditions on Growth Performance, Carcass Characteristics, and Meat Quality. Animals 2022, 12, 366. https://doi.org/10.3390/ani12030366
Huerta-Leidenz N, Jerez-Timaure N, Rodas-González A, Sarturi JO, Brashears MM, Miller MF, Brashears MT. The Effects of Castration, Implant Protocol, and Supplementation of Bos indicus-Influenced Beef Cattle under Tropical Savanna Conditions on Growth Performance, Carcass Characteristics, and Meat Quality. Animals. 2022; 12(3):366. https://doi.org/10.3390/ani12030366
Chicago/Turabian StyleHuerta-Leidenz, Nelson, Nancy Jerez-Timaure, Argenis Rodas-González, Jhones Onorino Sarturi, Mindy M. Brashears, Markus F. Miller, and Michel Todd Brashears. 2022. "The Effects of Castration, Implant Protocol, and Supplementation of Bos indicus-Influenced Beef Cattle under Tropical Savanna Conditions on Growth Performance, Carcass Characteristics, and Meat Quality" Animals 12, no. 3: 366. https://doi.org/10.3390/ani12030366
APA StyleHuerta-Leidenz, N., Jerez-Timaure, N., Rodas-González, A., Sarturi, J. O., Brashears, M. M., Miller, M. F., & Brashears, M. T. (2022). The Effects of Castration, Implant Protocol, and Supplementation of Bos indicus-Influenced Beef Cattle under Tropical Savanna Conditions on Growth Performance, Carcass Characteristics, and Meat Quality. Animals, 12(3), 366. https://doi.org/10.3390/ani12030366






