From In Vitro Data to In Vivo Interspecies Danger Communication: A Study of Chemosensing via the Mouse Grueneberg Ganglion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Cell Culture
2.1.2. HEK 293 Cell Transfection
2.1.3. Calcium Imaging Experiments on Transfected HEK 293 Cells
2.2. Ex Vivo Experiments
2.2.1. Animals
2.2.2. GG Tissue Slice Preparation
2.2.3. Calcium Imaging Experiments
2.3. In Vivo Experiments
2.3.1. Animals
2.3.2. Behavioural Test
2.4. Statistical Analyses
3. Results
3.1. TAS2Rs-Expressing HEK Cells Respond to Predator Secretions
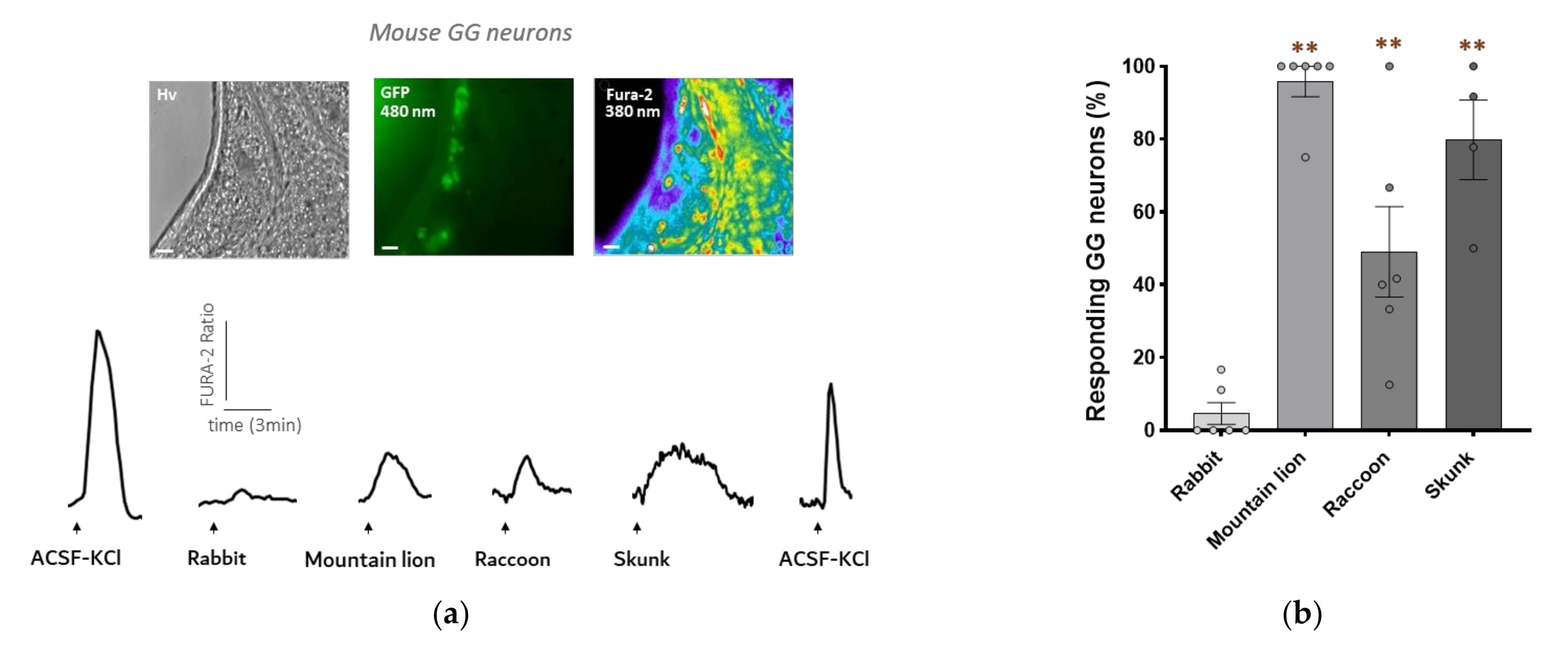
3.2. Mouse GG Neurons Respond to Raccoon Urine and Skunk Anal Secretions
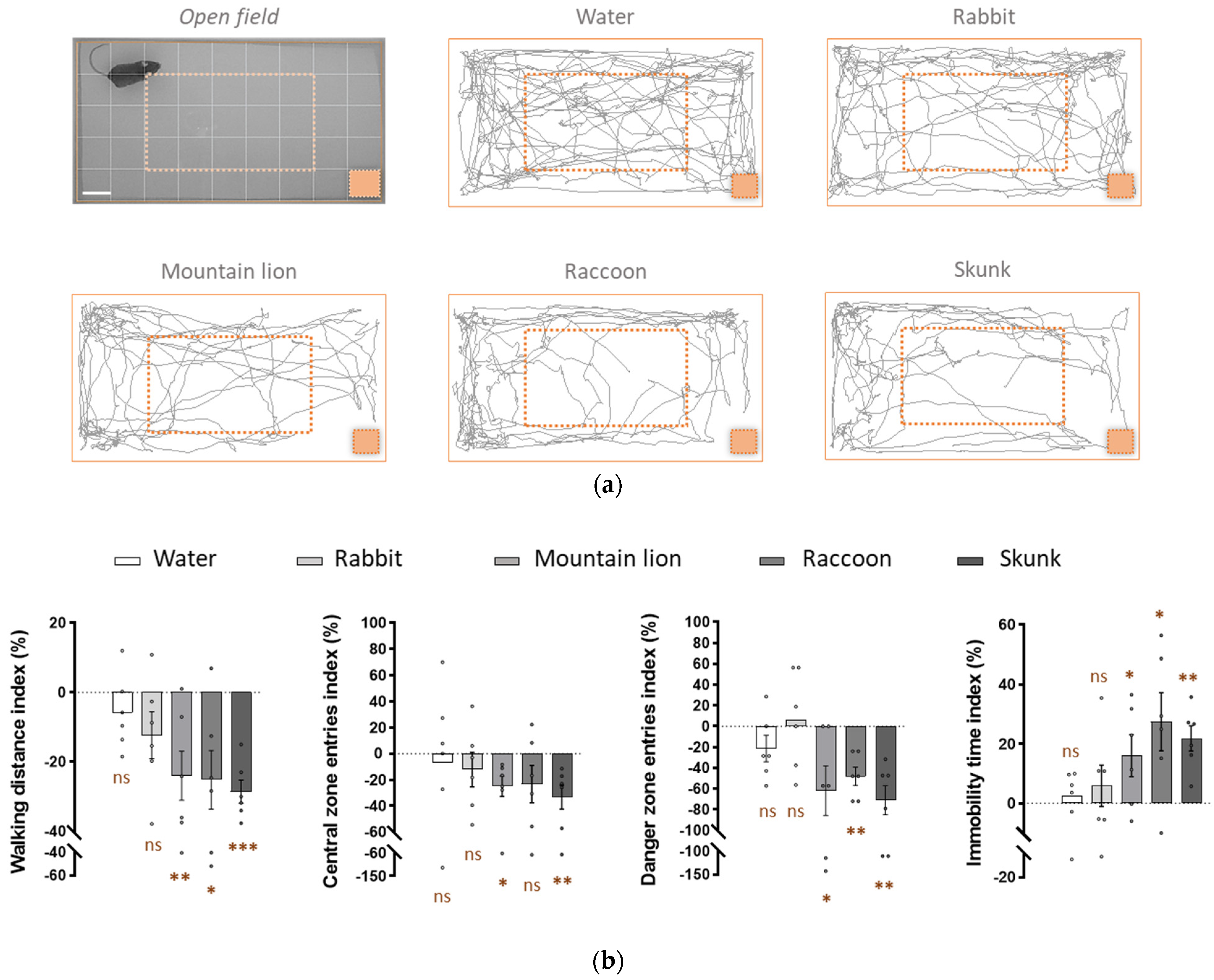
3.3. Raccoon Urine and Skunk Secretions Induce Fear-Related Behaviours in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tirindelli, R.; Dibattista, M.; Pifferi, S.; Menini, A. From Pheromones to Behavior. Physiol. Rev. 2009, 89, 921–956. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liberles, S.D. Aversion and attraction through olfaction. Curr. Biol. 2015, 25, R120–R129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enjin, A.; Suh, G.S.B. Neural mechanisms of alarm pheromone signaling. Mol. Cells 2013, 35, 177–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bind, R.H.; Minney, S.M.; Rosenfeld, S.; Hallock, R.M. The role of pheromonal responses in rodent behavior: Future directions for the development of laboratory protocols. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2013, 52, 124–129. [Google Scholar] [PubMed]
- Mohrhardt, J.; Nagel, M.; Fleck, D.; Ben-Shaul, Y.; Spehr, M. Signal detection and coding in the accessory olfactory system. Chem. Senses 2018, 43, 667–695. [Google Scholar] [CrossRef] [Green Version]
- Brown, W.L.; Eisner, T.; Whittaker, R.H. Allomones and Kairomones: Transspecific Chemical Messengers. BioScience 1970, 20, 21–22. [Google Scholar] [CrossRef]
- Fortes-Marco, L.; Lanuza, E.; Martinez-Garcia, F. Of pheromones and kairomones: What receptors mediate innate emotional responses? Anat. Rec. 2013, 296, 1346–1363. [Google Scholar] [CrossRef] [Green Version]
- Stowers, L.; Logan, D.W. Olfactory mechanisms of stereotyped behavior: On the scent of specialized circuits. Curr. Opin. Neurobiol. 2010, 20, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Mucignat-Caretta, C.; Redaelli, M.; Caretta, A. One nose, one brain: Contribution of the main and accessory olfactory system to chemosensation. Front. Neuroanat. 2012, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Hattori, T.; Asaba, A.; Inoue, N.; Kanomata, N.; Kikusui, T.; Kobayakawa, R.; Kobayakawa, K. Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, E311–E320. [Google Scholar] [CrossRef] [Green Version]
- Lopez, F.; Delgado, R.; Lopez, R.; Bacigalupo, J.; Restrepo, D. Transduction for Pheromones in the Main Olfactory Epithelium Is Mediated by the Ca2+-Activated Channel TRPM5. J. Neurosci. 2014, 34, 3268–3278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischer, J. The Grueneberg ganglion: Signal transduction and coding in an olfactory and thermosensory organ involved in the detection of alarm pheromones and predator-secreted kairomones. Cell Tissue Res. 2021, 383, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Brechbühl, J.; Moine, F.; Klaey, M.; Nenniger-Tosato, M.; Hurni, N.; Sporkert, F.; Giroud, C.; Broillet, M.-C. Mouse alarm pheromone shares structural similarity with predator scents. Proc. Natl. Acad. Sci. USA 2013, 110, 4762–4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwayanagi, M.; Miyazono, S.; Osada, K. Pyrazine analogues from wolf urine induced unlearned fear in rats. Heliyon 2017, 3, e00391. [Google Scholar] [CrossRef] [Green Version]
- Brechbühl, J.; Moine, F.; Tosato, M.N.; Sporkert, F.; Broillet, M.C. Identification of pyridine analogs as new predator-derived kairomones. Front. Neurosci. 2015, 9, 253. [Google Scholar] [CrossRef]
- Mueller, K.L.; Hoon, M.A.; Erlenbach, I.; Chandrashekar, J.; Zuker, C.S.; Ryba, N.J.P. The receptors and coding logic for bitter taste. Nature 2005, 434, 225–229. [Google Scholar] [CrossRef]
- Avau, B.; Depoortere, I. The bitter truth about bitter taste receptors: Beyond sensing bitter in the oral cavity. Acta Physiol. 2016, 216, 407–420. [Google Scholar] [CrossRef]
- Moine, F.; Brechbühl, J.; Nenniger Tosato, M.; Beaumann, M.; Broillet, M.C. Alarm pheromone and kairomone detection via bitter taste receptors in the mouse Grueneberg ganglion. BMC Biol. 2018, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ooi, A.; Wong, A.; Esau, L.; Lemtiri-Chlieh, F.; Gehring, C. A guide to transient expression of membrane proteins in HEK-293 cells for functional characterization. Front. Physiol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- García, J.T.; García, F.J.; Alda, F.; González, J.L.; Aramburu, M.J.; Cortés, Y.; Prieto, B.; Pliego, B.; Pérez, M.; Herrera, J.; et al. Recent invasion and status of the raccoon (Procyon lotor) in Spain. Biol. Invasions 2012, 14, 1305–1310. [Google Scholar] [CrossRef] [Green Version]
- Kent, L.; Tang-Martínez, Z. Evidence of individual odors and individual discrimination in the raccoon, Procyon lotor. J. Mammal. 2014, 95, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Wood, W.F. The History of Skunk Defensive Secretion Research. Chem. Educ. 1999, 4, 44–50. [Google Scholar] [CrossRef]
- Kingston, R.E.; Chen, C.A.; Okayama, H. Calcium Phosphate Transfection. Curr. Protoc. Immunol. 1999, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brechbühl, J.; Luyet, G.; Moine, F.; Rodriguez, I.; Broillet, M.C. Imaging pheromone sensing in a mouse vomeronasal acute tissue slice preparation. J. Vis. Exp. 2011, 58, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, S.M.; Zheng, C.; Koos, D.S.; Feinstein, P.; Fraser, S.E.; Mombaerts, P. Structure and emergence of specific olfactory glomeruli in the mouse. J. Neurosci. 2001, 21, 9713–9723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs then and now: The need for clarity in definition and purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120–132. [Google Scholar] [PubMed]
- Oe, S.; Sashika, M.; Fujimoto, A.; Shimozuru, M.; Tsubota, T. Predation impacts of invasive raccoons on rare native species. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A.M.; Bowman, J.; Sadowski, C.; Nituch, L.A.; Bruce, L.; Halonen, T.; Puukka, K.; Rouvinen-Watt, K.; Aho, J.; Nieminen, P. Physiological adaptations to prolonged fasting in the overwintering striped skunk (Mephitis mephitis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 555–563. [Google Scholar] [CrossRef]
- Brechbühl, J.; Klaey, M.; Moine, F.; Bovay, E.; Hurni, N.; Nenniger-Tosato, M.; Broillet, M.-C. Morphological and physiological species-dependent characteristics of the rodent Grueneberg ganglion. Front. Neuroanat. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Brechbühl, J.; Moine, F.; Broillet, M.-C. Mouse Grueneberg ganglion neurons share molecular and functional features with C. elegans amphid neurons. Front. Behav. Neurosci. 2013, 7, 193. [Google Scholar] [CrossRef]
- Pérez-Gómez, A.; Bleymehl, K.; Stein, B.; Pyrski, M.; Birnbaumer, L.; Munger, S.D.; Leinders-Zufall, T.; Zufall, F.; Chamero, P. Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr. Biol. 2015, 25, 1340–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brechbühl, J. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science 2009, 326, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, Y.; Fleischer, J.; Yang, R. Guanylyl cyclase-G is an alarm pheromone receptor in mice. EMBO J. 2018, 37, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Brechbühl, J.; de Vallière, A.; Wood, D.; Nenniger Tosato, M.; Broillet, M.C. The Grueneberg ganglion controls odor-driven food choices in mice under threat. Commun. Biol. 2020, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Yoo, S.J.; Bleymehl, K.; Omura, M.; Zapiec, B.; Pyrski, M.; Blum, T.; Khan, M.; Bai, Z.; Leinders-Zufall, T.; et al. Danger perception and stress response through an olfactory sensor for the bacterial metabolite hydrogen sulfide. Neuron 2021, 109, 2469–2484.e7. [Google Scholar] [CrossRef]
- Wennig, R.; Schneider, S.; Meys, F. GC/MS based identification of skunk spray maliciously deployed as “biological weapon” to harm civilians. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Liberles, S.D. Trace amine-associated receptors: Ligands, neural circuits, and behaviors. Curr. Opin. Neurobiol. 2015, 34, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Stenseth, N.C.; Leirs, H.; Skonhoft, A.; Davis, S.A.; Pech, R.P.; Andreassen, H.P.; Singleton, G.R.; Lima, M.; Machang’u, R.S.; Makundi, R.H.; et al. Mice, rats, and people: The bio-economics of agricultural rodent pests. Front. Ecol. Environ. 2003, 1, 367–375. [Google Scholar] [CrossRef]
- Adduci, L.B.; León, V.A.; Busch, M.; Fraschina, J. Effects of different odours on the reproductive success of Mus musculus as an alternative method of control. Pest Manag. Sci. 2019, 75, 1887–1893. [Google Scholar] [CrossRef]
- Mackenzie, L.; Nalivaiko, E.; Beig, M.I.; Day, T.A.; Walker, F.R. Ability of predator odour exposure to elicit conditioned versus sensitised post-traumatic stress disorder-like behaviours, and forebrain δFosB expression, in rats. Neuroscience 2010, 169, 733–742. [Google Scholar] [CrossRef]
- Albrechet-Souza, L.; Gilpin, N.W. The predator odor avoidance model of post-traumatic stress disorder in rats. Behav. Pharmacol. 2019, 30, 105–114. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.C.; Brechbühl, J.; Ferreira, F.; Amez-Droz, M.; Broillet, M.-C. From In Vitro Data to In Vivo Interspecies Danger Communication: A Study of Chemosensing via the Mouse Grueneberg Ganglion. Animals 2022, 12, 356. https://doi.org/10.3390/ani12030356
Lopes AC, Brechbühl J, Ferreira F, Amez-Droz M, Broillet M-C. From In Vitro Data to In Vivo Interspecies Danger Communication: A Study of Chemosensing via the Mouse Grueneberg Ganglion. Animals. 2022; 12(3):356. https://doi.org/10.3390/ani12030356
Chicago/Turabian StyleLopes, Ana Catarina, Julien Brechbühl, Flavio Ferreira, Marjorie Amez-Droz, and Marie-Christine Broillet. 2022. "From In Vitro Data to In Vivo Interspecies Danger Communication: A Study of Chemosensing via the Mouse Grueneberg Ganglion" Animals 12, no. 3: 356. https://doi.org/10.3390/ani12030356
APA StyleLopes, A. C., Brechbühl, J., Ferreira, F., Amez-Droz, M., & Broillet, M.-C. (2022). From In Vitro Data to In Vivo Interspecies Danger Communication: A Study of Chemosensing via the Mouse Grueneberg Ganglion. Animals, 12(3), 356. https://doi.org/10.3390/ani12030356





