The Neurobiology of Behavior and Its Applicability for Animal Welfare: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
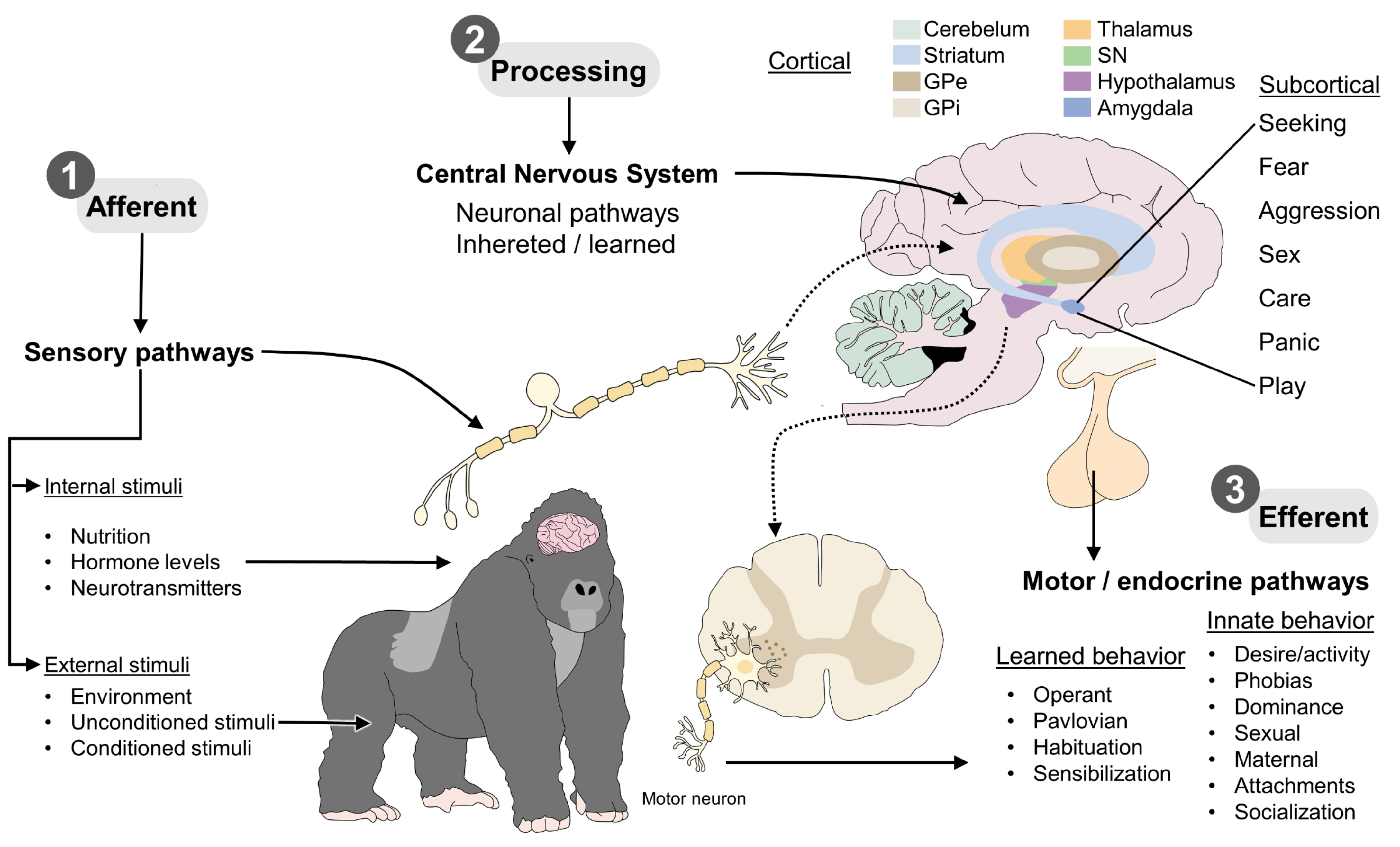
2. Levels of Information Processing
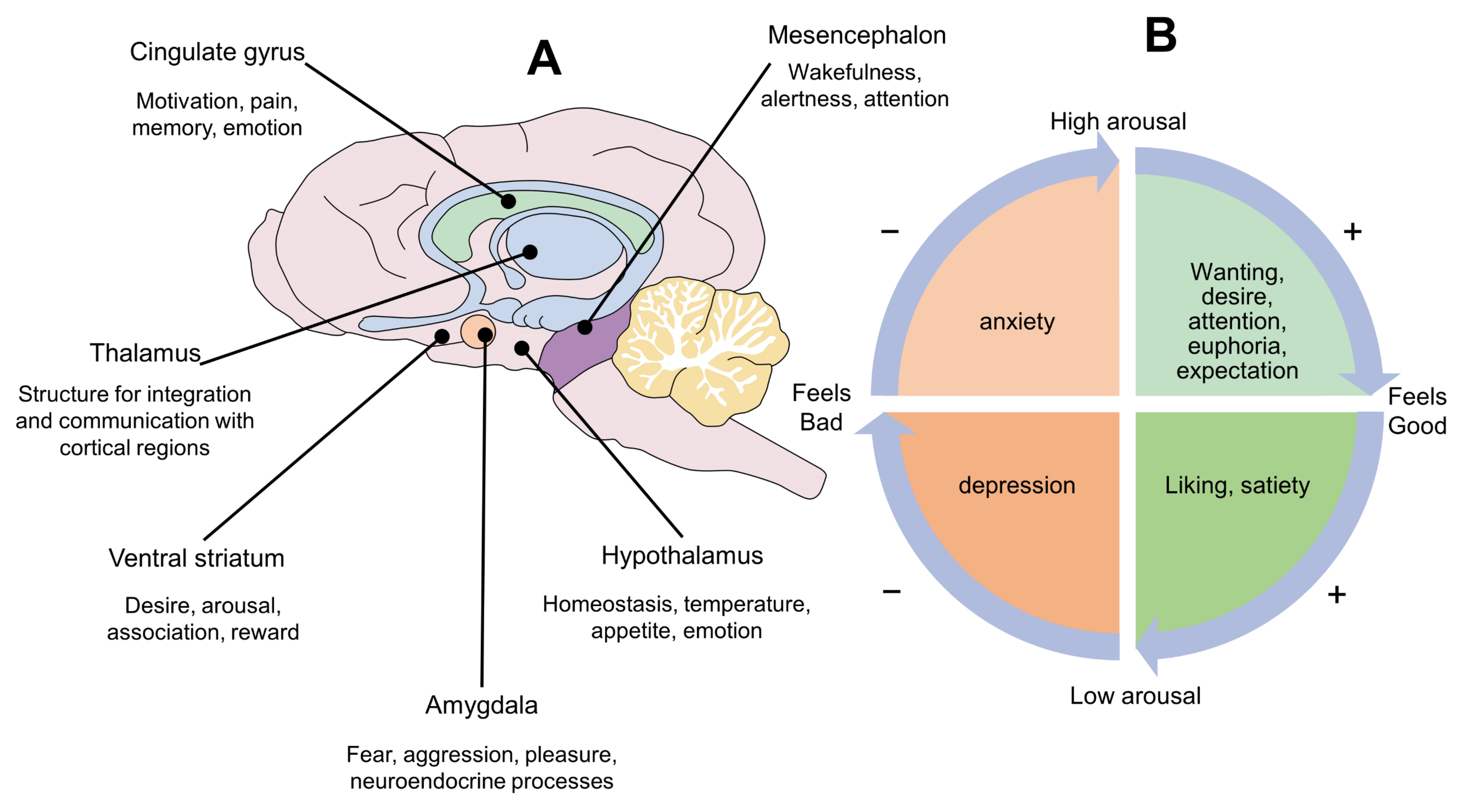
3. Panksepp’s Circumplex of Emotions
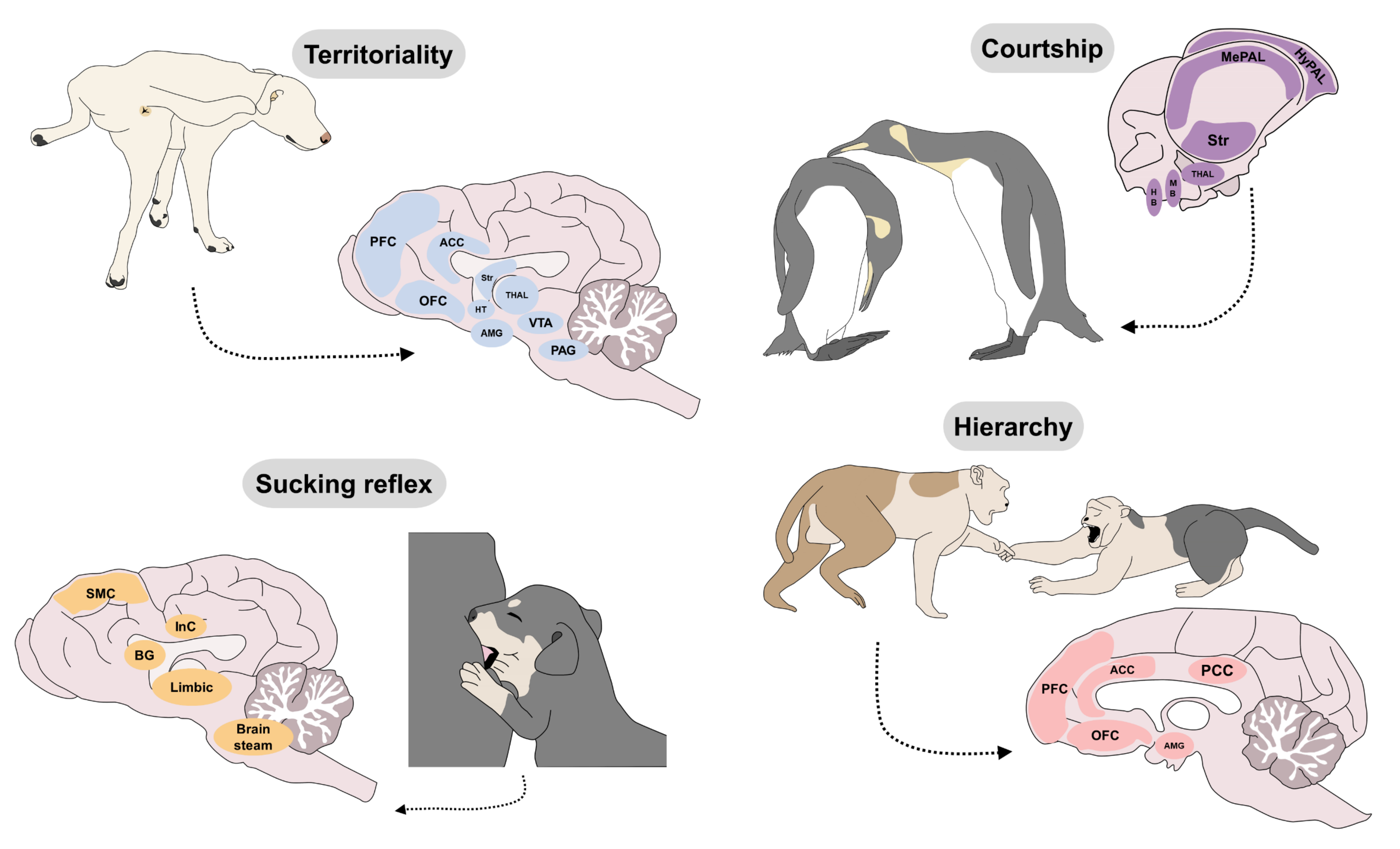
4. The Seven Basic Neurobiological Systems of Behavior
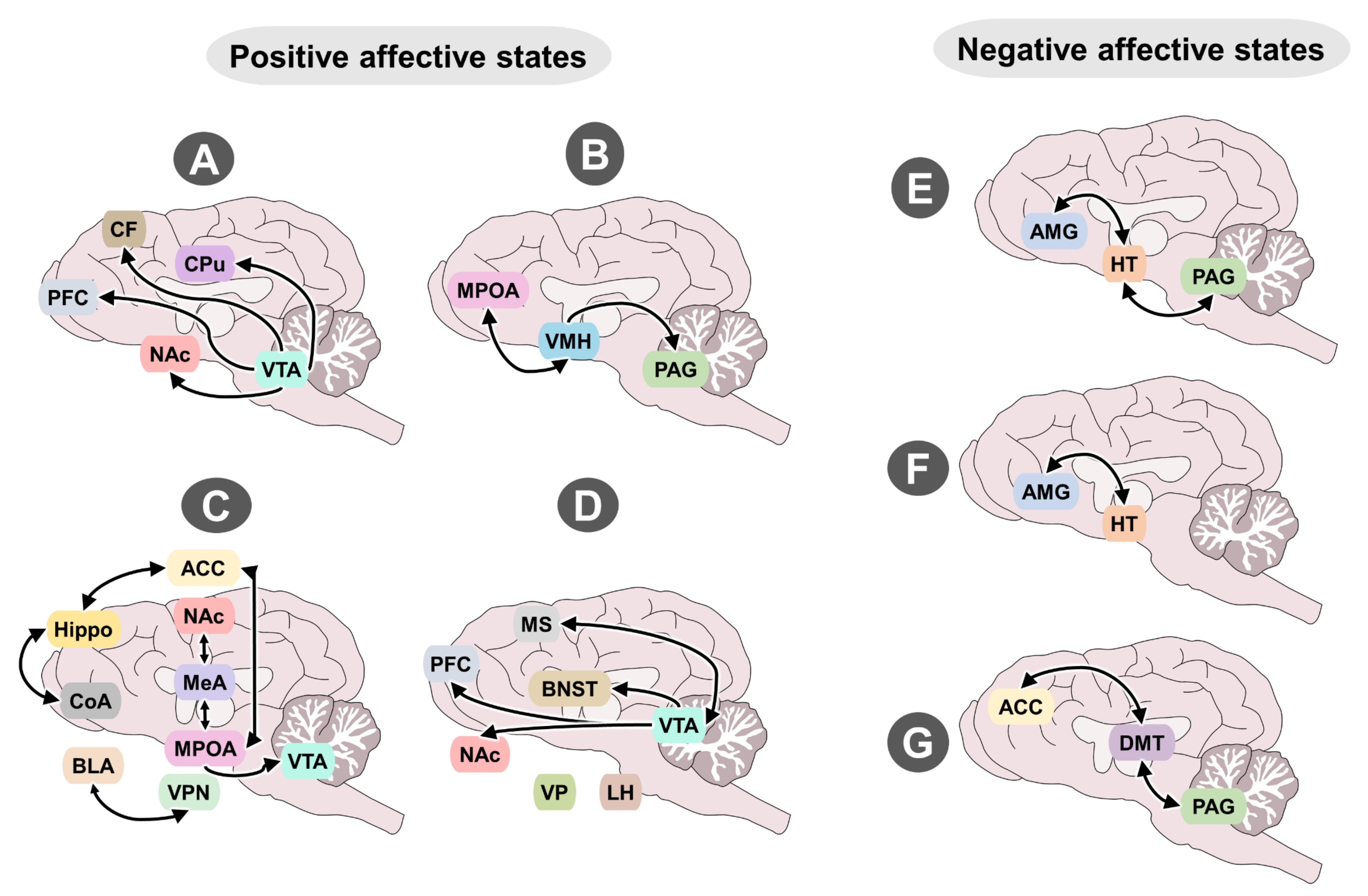
4.1. Neurobiological Systems That Evoke Positive Emotions
4.1.1. Seeking
4.1.2. Lust
4.1.3. Care
4.1.4. Play
4.2. Neurobiological Systems That Evoke Negative Emotions
4.2.1. Rage
4.2.2. Fear
4.2.3. Panic
5. Genetic Bases of Behavior and Its Relation to Welfare
6. Innate Responses That Impact Welfare
7. How Learning Influences Animal Welfare
7.1. Making Memories
7.2. Types of Memory
7.3. Classical Conditioning
7.4. Operant Conditioning
7.5. Habituation
7.6. Sensitization
7.7. Social Learning
8. Motivation and Satiety
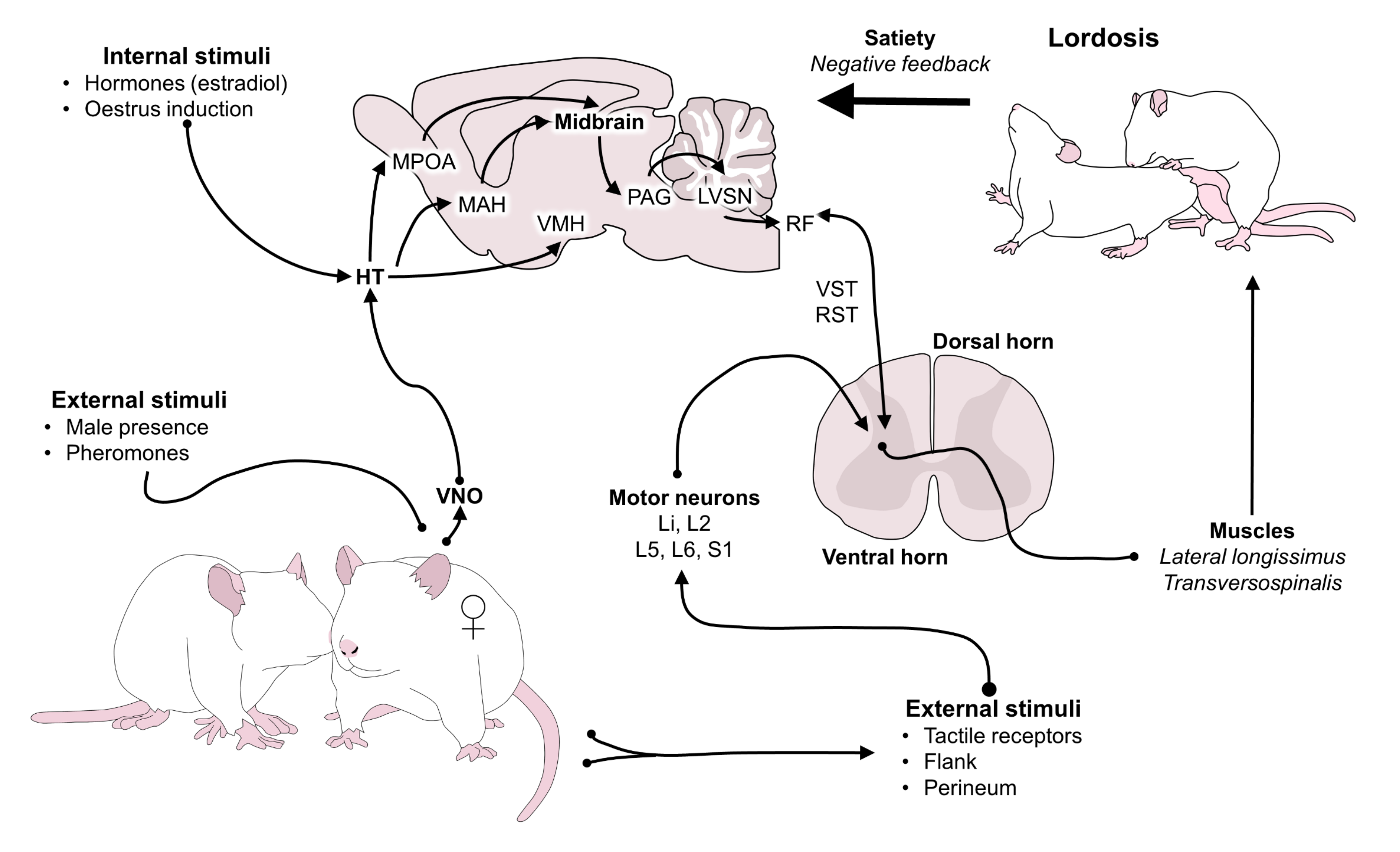
9. Brambell’s Five Freedoms and Neurobiology
10. Focusing on Emotional States
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duncan, I.J. The welfare of farm animals: An ethological approach. Sci. Prog. 1987, 71, 317–326. [Google Scholar] [PubMed]
- Coria-Avila, G.A.; Herrera-Covarrubias, D. The neuroscience of animal welfare: Theory 80–20. eNeurobiologia 2012, 3, 161012. [Google Scholar]
- Korte, S.M.; Olivier, B.; Koolhaas, J.M. A new animal welfare concept based on allostasis. Physiol. Behav. 2007, 92, 422–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Yeates, J.; Main, D. Assessment of positive welfare: A review. Vet. J. 2008, 175, 293–300. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998, 28, 309–369. [Google Scholar] [CrossRef]
- Pfaus, J. Frank A. Beach Award: Homologies of Animal and Human Sexual Behaviors. Horm. Behav. 1996, 30, 187–200. [Google Scholar] [CrossRef]
- Panksepp, J. Affective Neuroscience: The Foundations of Human and Animal Emotions; Oxford University Press: Oxford, MI, USA, 2004; 480p. [Google Scholar]
- Panksepp, J.; Clarici, A.; Vandekerckhove, M.; Yovell, Y. Neuro-Evolutionary Foundations of Infant Minds: From Psychoanalytic Visions of How Primal Emotions Guide Constructions of Human Minds toward Affective Neuroscientific Understanding of Emotions and Their Disorders. Psychoanal. Inq. 2019, 39, 36–51. [Google Scholar] [CrossRef]
- Davis, K.L.; Montag, C. Selected Principles of Pankseppian Affective Neuroscience. Front. Neurosci. 2019, 12, 1025. [Google Scholar] [CrossRef]
- Davis, K.L.; Montag, C. A Tribute to Jaak Panksepp (1943–2017). Pers. Neurosci. 2018, 1, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkarar-Gradwohl, F.G. Cross-Cultural Affective Neuroscience. Front. Psychol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ackermans, M.; Jonker, N.; Bennik, E.; de Jong, P. Hunger increases negative and decreases positive emotions in women with a healthy weight. Appetite 2021, 168, 105746. [Google Scholar] [CrossRef] [PubMed]
- Lefter, R.; Cojocariu, R.O.; Ciobica, A.; Balmus, I.-M.; Mavroudis, I.; Kis, A. Interactions between Sleep and Emotions in Humans and Animal Models. Medicina 2022, 58, 274. [Google Scholar] [CrossRef]
- Panksepp, J.; Knutson, B.; Burgdorf, J. The role of brain emotional systems in addictions: A neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction 2002, 97, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Ikemoto, S.; Panksepp, J. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav. Neurosci. 1996, 110, 331–345. [Google Scholar] [CrossRef]
- Nocjar, C.; Panksepp, J. Prior morphine experience induces long-term increases in social interest and in appetitive behavior for natural reward. Behav. Brain Res. 2007, 181, 191–199. [Google Scholar] [CrossRef]
- Alcaro, A.; Panksepp, J. The SEEKING mind: Primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci. Biobehav. Rev. 2011, 35, 1805–1820. [Google Scholar] [CrossRef]
- Wright, J.S.; Panksepp, J. An Evolutionary Framework to Understand Foraging, Wanting, and Desire: The Neuropsychology of the SEEKING System. Neuropsychoanalysis 2012, 14, 5–39. [Google Scholar] [CrossRef]
- Paredes-Ramos, P.; Manzo, J.; Coria-Avila, G.A. Home-made device improves the behavior of group housed dogs. eNeurobiologia 2014, 5, 1–7. [Google Scholar]
- Warwick, C.; Arena, P.; Lindley, S.; Jessop, M.; Steedman, C. Assessing reptile welfare using behavioural criteria. Practice 2013, 35, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Derdeyn, P.; Hui, M.; Macchia, D.; Beier, K.T. Uncovering the Connectivity Logic of the Ventral Tegmental Area. Front. Neural Circuits 2022, 15, 799688. [Google Scholar] [CrossRef] [PubMed]
- Garris, P.; Ciolkowski, E.; Pastore, P.; Wightman, R. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J. Neurosci. 1994, 14, 6084–6093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeets, W.J.; González, A. Catecholamine systems in the brain of vertebrates: New perspectives through a comparative approach. Brain Res. Rev. 2000, 33, 308–379. [Google Scholar] [CrossRef]
- Ferguson, L.M.; Ahrens, A.M.; Longyear, L.G.; Aldridge, J.W. Neurons of the Ventral Tegmental Area Encode Individual Differences in Motivational “Wanting” for Reward Cues. J. Neurosci. 2020, 40, 8951–8963. [Google Scholar] [CrossRef]
- Schultz, W. Predictive Reward Signal of Dopamine Neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef]
- Schultz, W. Book Review: Reward Signaling by Dopamine Neurons. Neurosci. 2001, 7, 293–302. [Google Scholar] [CrossRef]
- Wuensch, L.; Pool, E.R.; Sander, D. Individual differences in learning positive affective value. Curr. Opin. Behav. Sci. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Grandin, T. My Reflections on Understanding Animal Emotions for Improving the Life of Animals in Zoos. J. Appl. Anim. Welf. Sci. 2018, 21, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Podturkin, A.A.; Krebs, B.L.; Watters, J.V. A Quantitative Approach for Using Anticipatory Behavior as a Graded Welfare Assessment. J. Appl. Anim. Welf. Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Clayton, M.; Shrock, T. Making a Tiger’s Day: Free-Operant Assessment and Environmental Enrichment to Improve the Daily Lives of Captive Bengal Tigers (Panthera tigris tigris). Behav. Anal. Pract. 2020, 13, 883–893. [Google Scholar] [CrossRef]
- Glaeser, S.S.; Shepherdson, D.; Lewis, K.; Prado, N.; Brown, J.L.; Lee, B.; Wielebnowski, N. Supporting Zoo Asian Elephant (Elephas maximus) Welfare and Herd Dynamics with a More Complex and Expanded Habitat. Animals 2021, 11, 2566. [Google Scholar] [CrossRef] [PubMed]
- Coria-Avila, G.A.; Gavrila, A.M.; Boulard, B.; Charron, N.; Stanley, G.; Pfaus, J.G. Neurochemical basis of conditioned partner preference in the female rat: II. Disruption by flupenthixol. Behav. Neurosci. 2008, 122, 396–406. [Google Scholar] [CrossRef]
- Paredes-Ramos, P.; Diaz-Morales, J.V.; Espinosa-Palencia, M.; Coria-Avila, G.A.; Carrasco-Garcia, A.A. Clicker Training Accelerates Learning of Complex Behaviors but Reduces Discriminative Abilities of Yucatan Miniature Pigs. Animals 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Giacolini, T.; Conversi, D.; Alcaro, A. The Brain Emotional Systems in Addictions: From Attachment to Dominance/Submission Systems. Front. Hum. Neurosci. 2021, 14, 609467. [Google Scholar] [CrossRef]
- McCarthy, M.M. Molecular aspects of sexual differentiation of the rodent brain. Psychoneuroendocrinology 1994, 19, 415–427. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Bruschetta, D.; Milardi, D.; Quattrini, F.; Sciarrone, F.; La Rosa, G.; Bramanti, P.; Anastasi, G. Neuroanatomy and function of human sexual behavior: A neglected or unknown issue? Brain Behav. 2019, 9, e01389. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G. REVIEWS: Pathways of Sexual Desire. J. Sex. Med. 2009, 6, 1506–1533. [Google Scholar] [CrossRef]
- Larriva-Sahd, J.; Rondán, A.; Orozco-Estévez, H.; Sánchez-Robles, M.R. Evidence of a direct projection of the vomeronasal organ to the medial preoptic nucleus and hypothalamus. Neurosci. Lett. 1993, 163, 45–49. [Google Scholar] [CrossRef]
- Triemstra, J.L.; Nagatani, S.; I Wood, R. Chemosensory Cues are Essential for Mating-Induced Dopamine Release in MPOA of Male Syrian Hamsters. Neuropsychopharmacology 2005, 30, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Hull, E.; Lorrain, D.S.; Du, J.; Matuszewich, L.; Lumley, L.A.; Putnam, S.K.; Moses, J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav. Brain Res. 1999, 105, 105–116. [Google Scholar] [CrossRef]
- Mohankumar, S.M.J.; Balasubramanian, P.; Dharmaraj, M.; Mohankumar, P.S. Neuroendocrine regulation of adaptive mechanisms in livestock. In Environmental Stress and Amelioration in Livestock Production; Sejian, V., Naqvi, S.M.K., Ezeji, T., Lakritz, J., Lal, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 9783642292057, pp. 263–298. [Google Scholar]
- Panksepp, J.; Panksepp, J.B. Toward a cross-species understanding of empathy. Trends Neurosci. 2013, 36, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallin, C.M.; Bowen, S.E.; Brummelte, S. Opioid use during pregnancy can impair maternal behavior and the Maternal Brain Network: A literature review. Neurotoxicology Teratol. 2021, 86, 106976. [Google Scholar] [CrossRef] [PubMed]
- Coria-Avila, G.A.; Manzo, J.; Garcia, L.I.; Carrillo, P.; Miquel, M.; Pfaus, J. Neurobiology of social attachments. Neurosci. Biobehav. Rev. 2014, 43, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Shimmura, T.; Kamimura, E.; Azuma, T.; Kansaku, N.; Uetake, K.; Tanaka, T. Effect of broody hens on behaviour of chicks. Appl. Anim. Behav. Sci. 2010, 126, 125–133. [Google Scholar] [CrossRef]
- Baxter, M.; Bailie, C.L.; O’Connell, N.E. Play behaviour, fear responses and activity levels in commercial broiler chickens provided with preferred environmental enrichments. Animal 2019, 13, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Panksepp, J.; Moskal, J. Dopamine and SEEKING: Subcortical “Reward” Systems and Appetitive Urges. In Handbook of Approach and Avoidance Motivation; Elliot, A., Ed.; Psychology Press: East Sussex, UK, 2008; pp. 67–87. [Google Scholar]
- Panksepp, J.; Siviy, S.; Normansell, L. The psychobiology of play: Theoretical and methodological perspectives. Neurosci. Biobehav. Rev. 1984, 8, 465–492. [Google Scholar] [CrossRef]
- Panksepp, J.; Burgdorf, J. Laughing rats? Playful tickling arouses high-frequency ultrasonic chirping in young rodents. Am. J. Play. 2010, 2, 357–372. [Google Scholar]
- Burgdorf, J.; Knutson, B.; Panksepp, J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav. Neurosci. 2000, 114, 320–327. [Google Scholar] [CrossRef]
- Wöhr, M.; Schwarting, R.K.W. Affective communication in rodents: Ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013, 354, 81–97. [Google Scholar] [CrossRef]
- Hinchcliffe, J.K.; Mendl, M.; Robinson, E.S. Rat 50 kHz calls reflect graded tickling-induced positive emotion. Curr. Biol. 2020, 30, R1034–R1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Juniper, D.T.; Meagher, R.K. Effects of physical enrichment items and social housing on calves’ growth, behaviour and response to novelty. Appl. Anim. Behav. Sci. 2021, 237, 105295. [Google Scholar] [CrossRef]
- Windschnurer, I.; Häusler, A.; Waiblinger, S.; Coleman, G.J. Relationships between owner and household characteristics and enrichment and cat behaviour. Appl. Anim. Behav. Sci. 2022, 247, 105562. [Google Scholar] [CrossRef]
- Panksepp, J.; Zellner, M.R. Towards a neurobiologically based unified theory of aggression. Rev. Int. Psychol. Soc. 2004, 17, 37–61. [Google Scholar]
- Aleyasin, H.; E Flanigan, M.; Russo, S.J. Neurocircuitry of aggression and aggression seeking behavior: Nose poking into brain circuitry controlling aggression. Curr. Opin. Neurobiol. 2018, 49, 184–191. [Google Scholar] [CrossRef]
- Coria-Avila, G.; Paredes-Ramos, P.; Díaz-Estrada, V.X.; Tecamachaltzi-Silvarán, M.B. Agresión. In Neurofisiología de la Conducta; Coria-Avila, G., Ed.; Universidad Veracruzana: Xalapa, México, 2015; pp. 317–348. [Google Scholar]
- Yanowitch, R.; Coccaro, E.F. The Neurochemistry of Human Aggression. Adv. Genet. 2011, 75, 151–169. [Google Scholar] [CrossRef]
- Zapletal, D.; Macháček, M.; Suchý, P.; Straková, E.; Vitula, F. Male-to-female aggression in cage-housed common pheasants (Phasianus colchicus) during the breeding season was not related to male plasma testosterone level. Br. Poult. Sci. 2018, 59, 256–263. [Google Scholar] [CrossRef]
- Rayment, D.J.; Peters, R.A.; Marston, L.C.; de Groef, B. Relationships between serum serotonin, plasma cortisol, and behavioral factors in a mixed-breed, -sex, and -age group of pet dogs. J. Vet. Behav. 2020, 38, 96–102. [Google Scholar] [CrossRef]
- Alberghina, D.; Tropia, E.; Piccione, G.; Giannetto, C.; Panzera, M. Serum serotonin (5-HT) in dogs (Canis familiaris): Preanalytical factors and analytical procedure for use of reference values in behavioral medicine. J. Vet. Behav. 2019, 32, 72–75. [Google Scholar] [CrossRef]
- Odore, R.; Rendini, D.; Badino, P.; Gardini, G.; Cagnotti, G.; Meucci, V.; Intorre, L.; Bellino, C.; D’Angelo, A. Behavioral Therapy and Fluoxetine Treatment in Aggressive Dogs: A Case Study. Animals 2020, 10, 832. [Google Scholar] [CrossRef]
- Mezzomo, N.J.; Müller, T.E.; Franscescon, F.; Michelotti, P.; Souza, T.P.; Rosemberg, D.B.; Barcellos, L.J. Taurine-mediated aggression is abolished via 5-HT1A antagonism and serotonin depletion in zebrafish. Pharmacol. Biochem. Behav. 2020, 199, 173067. [Google Scholar] [CrossRef] [PubMed]
- Godyń, D.; Nowicki, J.; Herbut, P. Effects of Environmental Enrichment on Pig Welfare—A Review. Animals 2019, 9, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, H.-L.; Chong, Q.; Escribano, D.; Camerlink, I.; Manteca, X.; Llonch, P. Pre-weaning socialization and environmental enrichment affect life-long response to regrouping in commercially-reared pigs. Appl. Anim. Behav. Sci. 2020, 229, 105044. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Zhang, Z.; Zhang, X.; Chen, S. A Comparative Study on Two Territorial Fishes: The Influence of Physical Enrichment on Aggressive Behavior. Animals 2021, 11, 1868. [Google Scholar] [CrossRef]
- Woodward, M.A.; Winder, L.A.; Watt, P.J. Enrichment Increases Aggression in Zebrafish. Fishes 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Panksepp, J. The basic emotional circuits of mammalian brains: Do animals have affective lives? Neurosci. Biobehav. Rev. 2011, 35, 1791–1804. [Google Scholar] [CrossRef]
- Papes, F.; Logan, D.W.; Stowers, L. The Vomeronasal Organ Mediates Interspecies Defensive Behaviors through Detection of Protein Pheromone Homologs. Cell 2010, 141, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Adduci, L.B.; León, V.A.; Schlötelburg, A.; Busch, M.; Fraschina, J. Avoidance behaviour in laboratory house mice (Musmusculus) and Norway rats (Rattus norvegicus) towards predator odours. PLoS ONE 2021, 16, e0245441. [Google Scholar] [CrossRef]
- Panksepp, J. Fear and anxiety mechanisms of the brain. In Principles of Medical Biology. Biological Psychiatry; Bittar, E., Bittar, N., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 155–177. [Google Scholar]
- Panksepp, J. Toward a general psychobiological theory of emotions. Behav. Brain Sci. 1982, 5, 407–422. [Google Scholar] [CrossRef]
- Fernandez-Novo, A.; Pérez-Garnelo, S.; Villagrá, A.; Pérez-Villalobos, N.; Astiz, S. The Effect of Stress on Reproduction and Reproductive Technologies in Beef Cattle—A Review. Animals 2020, 10, 2096. [Google Scholar] [CrossRef]
- Destrez, A.; Haslin, E.; Boivin, X. What stockperson behavior during weighing reveals about the relationship between humans and suckling beef cattle: A preliminary study. Appl. Anim. Behav. Sci. 2018, 209, 8–13. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Schmidt, C.G.; Michel, V.; et al. Welfare of cattle at slaughter. EFSA J. 2020, 18, e06275. [Google Scholar] [CrossRef] [PubMed]
- Rørvang, M.V.; Christensen, J.W. Attenuation of fear through social transmission in groups of same and differently aged horses. Appl. Anim. Behav. Sci. 2018, 209, 41–46. [Google Scholar] [CrossRef]
- Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Costa, E.D. Thermography as a Non-Invasive Measure of Stress and Fear of Humans in Sheep. Animals 2018, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Mota-Rojas, D.; Broom, D.M.; Orihuela, A.; Velarde, A.; Napolitano, F.; Alonso-Spilsbury, M. Effects of human-animal relationship on animal productivity and welfare. J. Anim. Behav. Biometeorol. 2020, 8, 196–205. [Google Scholar] [CrossRef]
- Granati, G.; Cichella, F.; Lucidi, P. High-Tech Training for Birds of Prey. Animals 2021, 11, 530. [Google Scholar] [CrossRef]
- Campera, M.; Brown, E.; Imron, M.A.; Nekaris, K. Unmonitored releases of small animals? The importance of considering natural dispersal, health, and human habituation when releasing a territorial mammal threatened by wildlife trade. Biol. Conserv. 2020, 242, 108404. [Google Scholar] [CrossRef]
- Found, R.; Kloppers, E.L.; Hurd, T.E.; Clair, C.C.S. Intermediate frequency of aversive conditioning best restores wariness in habituated elk (Cervus canadensis). PLoS ONE 2018, 13, e0199216. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Cherry, M.J.; Gilbert, S.L.; Kohl, M.T.; Larson, C.L.; Newsome, T.M.; Prugh, L.R.; Suraci, J.P.; Young, J.K.; Smith, J.A. An applied ecology of fear framework: Linking theory to conservation practice. Anim. Conserv. 2021, 24, 308–321. [Google Scholar] [CrossRef]
- Panksepp, J.; Watt, D. Why Does Depression Hurt? Ancestral Primary-Process Separation-Distress (PANIC/GRIEF) and Diminished Brain Reward (SEEKING) Processes in the Genesis of Depressive Affect. Psychiatry 2011, 74, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Mota-Rojas, D.; Marcet-Rius, M.; Freitas-De-Melo, A.; Muns, R.; Mora-Medina, P.; Domínguez-Oliva, A.; Orihuela, A. Allonursing in Wild and Farm Animals: Biological and Physiological Foundations and Explanatory Hypotheses. Animals 2021, 11, 3092. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.; Nelson, E.; Bekkedal, M. Brain Systems for the Mediation of Social Separation-Distress and Social-Reward Evolutionary Antecedents and Neuropeptide Intermediaries. Ann. N. Y. Acad. Sci. 1997, 807, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. Feeling the Pain of Social Loss. Science 2003, 302, 237–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panksepp, J. Affective neuroscience of the emotional BrainMind: Evolutionary perspectives and implications for understanding depression. Dialog Clin. Neurosci. 2010, 12, 533–545. [Google Scholar] [CrossRef]
- Mellor, D.J. Animal emotions, behaviour and the promotion of positive welfare states. N. Zealand Vet. J. 2012, 60, 1–8. [Google Scholar] [CrossRef]
- McCrave, E.A. Diagnostic Criteria for Separation Anxiety in the Dog. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 247–255. [Google Scholar] [CrossRef]
- Schwartz, S. Separation anxiety syndrome in dogs and cats. J. Am. Vet. Med. Assoc. 2003, 222, 1526–1532. [Google Scholar] [CrossRef] [Green Version]
- Young, L.J.; Wang, Z. The neurobiology of pair bonding. Nat. Neurosci. 2004, 7, 1048–1054. [Google Scholar] [CrossRef]
- Bendesky, A.; Kwon, Y.-M.; Lassance, J.-M.; Lewarch, C.; Yao, S.; Peterson, B.K.; He, M.X.; Dulac, C.; Hoekstra, H.E. The genetic basis of parental care evolution in monogamous mice. Nature 2017, 544, 434–439. [Google Scholar] [CrossRef]
- Lim, M.M.; Wang, Z.; Olazábal, D.E.; Ren, X.; Terwilliger, E.F.; Young, L.J. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 2004, 429, 754–757. [Google Scholar] [CrossRef]
- Bagot, R.C.; Zhang, T.-Y.; Wen, X.; Nguyen, T.T.T.; Nguyen, H.-B.; Diorio, J.; Wong, T.P.; Meaney, M.J. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc. Natl. Acad. Sci. USA 2012, 109, 17200–17207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, C.A.; Vadlamudi, S.; Boccia, M.L.; Moy, S.S. Variations in Maternal Behavior in C57BL/6J Mice: Behavioral Comparisons between Adult Offspring of High and Low Pup-Licking Mothers. Front. Psychiatry 2011, 2, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodman, N.H.; Karlsson, E.; Moon-Fanelli, A.; Galdzicka, M.; Perloski, M.; Shuster, L.; Lindblad-Toh, K.; I Ginns, E. A canine chromosome 7 locus confers compulsive disorder susceptibility. Mol. Psychiatry 2010, 15, 8–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halperin, D.; Stavsky, A.; Kadir, R.; Drabkin, M.; Wormser, O.; Yogev, Y.; Dolgin, V.; Proskorovski-Ohayon, R.; Perez, Y.; Nudelman, H.; et al. CDH2 mutation affecting N-cadherin function causes attention-deficit hyperactivity disorder in humans and mice. Nat. Commun. 2021, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Irwin, D.; Liu, Y.-H.; Cheng, L.-G.; Wang, L.; Wang, G.-D.; Zhang, Y.-P. Balancing Selection on CDH2 May Be Related to the Behavioral Features of the Belgian Malinois. PLoS ONE 2014, 9, e110075. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, D.K.; Ruvinsky, A.O.; Trut, L.N. Inherited activation-inactivation of the star gene in foxes. J. Hered. 1981, 72, 267–274. [Google Scholar] [CrossRef]
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Can domestication shape Canidae brain morphology? The accessory olfactory bulb of the red fox as a case in point. Ann. Anat.-Anat. Anz. 2022, 240, 151881. [Google Scholar] [CrossRef]
- Stelow, E.A.; Bain, M.J.; Kass, P.H. The Relationship Between Coat Color and Aggressive Behaviors in the Domestic Cat. J. Appl. Anim. Welf. Sci. 2016, 19, 1–15. [Google Scholar] [CrossRef]
- Singh, N.; Albert, F.W.; Plyusnina, I.; Trut, L.; Pääbo, S.; Harvati, K. Facial shape differences between rats selected for tame and aggressive behaviors. PLoS ONE 2017, 12, e0175043. [Google Scholar] [CrossRef]
- Souza, J.C.D.S.M.D.; Alves, L.K.S.; Guimarães, E.B.B.; Madella, G.D.S.; Carnino, B.B.; de Moraes, E.I.C.; Dibo, P.V.; Braga, N.C.; De Souza, J.M.; Zhou, B.; et al. Flavored sisal ropes as environmental enrichment for nursery piglets. J. Anim. Behav. Biometeorol. 2020, 8, 308–312. [Google Scholar] [CrossRef]
- Krugmann, K.; Warnken, F.; Krieter, J.; Czycholl, I. Are Behavioral Tests Capable of Measuring Positive Affective States in Growing Pigs? Animals 2019, 9, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manell, E.; Hedenqvist, P.; Jensen-Waern, M. Training Pigs for Oral Glucose Tolerance Test—Six Years’ Experience of a Refined Model. Animals 2021, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Han, M.-R.; Lee, G.; Lee, S.S.; Kim, Y.-J.; Adams, M.E. Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling. PLoS Genet. 2015, 11, e1005513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorostiza, E.A. Does Cognition Have a Role in Plasticity of “Innate Behavior”? A Perspective from Drosophila. Front. Psychol. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Corradi, L.; Filosa, A. Neuromodulation and Behavioral Flexibility in Larval Zebrafish: From Neurotransmitters to Circuits. Front. Mol. Neurosci. 2021, 14, 718951. [Google Scholar] [CrossRef]
- Lea, S.E.G.; Chow, P.K.Y.; Leaver, L.A.; McLaren, I.P.L. Behavioral flexibility: A review, a model, and some exploratory tests. Anim. Learn. Behav. 2020, 48, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Vilhunen, S.; Hirvonen, H. Innate antipredator responses of Arctic charr (Salvelinus alpinus) depend on predator species and their diet. Behav. Ecol. Sociobiol. 2003, 55, 1–10. [Google Scholar] [CrossRef]
- Mooney, R. The neurobiology of innate and learned vocalizations in rodents and songbirds. Curr. Opin. Neurobiol. 2020, 64, 24–31. [Google Scholar] [CrossRef]
- Williams, H.; Lachlan, R.F. Evidence for cumulative cultural evolution in bird song. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200322. [Google Scholar] [CrossRef]
- Beny, Y.; Kimchi, T. Innate and learned aspects of pheromone-mediated social behaviours. Anim. Behav. 2014, 97, 301–311. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and Animal Behavior: Chemical Signals and Signature Mixes, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; 426p. [Google Scholar]
- Liberles, S.D. Mammalian Pheromones. Annu. Rev. Physiol. 2014, 76, 151–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, L.; Arnesen, C.; Zedrosser, A.; Rosell, F. Fears from the past? The innate ability of dogs to detect predator scents. Anim. Cogn. 2020, 23, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermiston, C.; Montrose, V.T.; Taylor, S. The effects of dog-appeasing pheromone spray upon canine vocalizations and stress-related behaviors in a rescue shelter. J. Vet. Behav. 2018, 26, 11–16. [Google Scholar] [CrossRef]
- Squire, L.R. Memory and Brain; Oxford University Press: New York, NY, USA, 1987; 336p. [Google Scholar]
- Liu, X.; Ramirez, S.; Pang, P.T.; Puryear, C.B.; Govindarajan, A.; Deisseroth, K.; Tonegawa, S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 2012, 484, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudy, J.W. The Neurobiology of Learning and Memory, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2013; 400p. [Google Scholar]
- Powell, R.; Symbaluk, D.G.; Macdonald, S.E. Introduction to Learning and Behavior, 2nd ed.; Wadsworth Publishing: Belmont, CA, USA, 2005; 547p. [Google Scholar]
- D’Ingeo, S.; Quaranta, A.; Siniscalchi, M.; Stomp, M.; Coste, C.; Bagnard, C.; Hausberger, M.; Cousillas, H. Horses associate individual human voices with the valence of past interactions: A behavioural and electrophysiological study. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Proops, L.; Grounds, K.; Smith, A.V.; McComb, K. Animals Remember Previous Facial Expressions that Specific Humans Have Exhibited. Curr. Biol. 2018, 28, 1428–1432.e4. [Google Scholar] [CrossRef] [Green Version]
- Quaranta, A.; D’Ingeo, S.; Siniscalchi, M. Odour-Evoked Memory in Dogs: Do Odours Help to Retrieve Memories of Food Location? Animals 2020, 10, 1249. [Google Scholar] [CrossRef]
- Mills, D.; Marchant-Forde, J.N.; McGreevy, P.; Morton, D.; Nicol, C.; Philips, C.; Sandoe, P.; Swaisgood, R. The Encyclopedia of Applied Animal Behaviour and Welfare; CAB International: Oxfordshire, UK, 2010; 704p. [Google Scholar]
- Elgier, A.M.; Jakovcevic, A.; Mustaca, A.E.; Bentosela, M. Pointing following in dogs: Are simple or complex cognitive mechanisms involved? Anim. Cogn. 2012, 15, 11568. [Google Scholar] [CrossRef]
- Overall, K. Manual of Clinical Behavioral Medicine for Dogs and Cat; Elsevier Mosby: St. Louis, MI, USA, 2013; 832p. [Google Scholar]
- Mota-Rojas, D.; Mariti, C.; Zdeinert, A.; Riggio, G.; Mora-Medina, P.; Reyes, A.d.M.; Gazzano, A.; Domínguez-Oliva, A.; Lezama-García, K.; José-Pérez, N.; et al. Anthropomorphism and Its Adverse Effects on the Distress and Welfare of Companion Animals. Animals 2021, 11, 3260. [Google Scholar] [CrossRef]
- Grandin, T. Livestock Handling and Transport, 4th ed.; CAB International: Oxford, UK, 2014; pp. 39–64. [Google Scholar]
- Heiblum, M. Advances in the diagnosis, and treatment of noise phobias in companion animals. Isr. J. Vet. Med. 2008, 63, 34–36. [Google Scholar]
- Dai, F.; Mazzola, S.; Cannas, S.; Heinzl, E.U.L.; Padalino, B.; Minero, M.; dalla Costa, E. Habituation to Transport Helps Reducing Stress-Related Behavior in Donkeys During Loading. Front. Vet. Sci. 2020, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Palo, P.; Maggiolino, A.; Albenzio, M.; Caroprese, M.; Centoducati, P.; Tateo, A. Evaluation of different habituation protocols for training dairy jennies to the milking parlor: Effect on milk yield, behavior, heart rate and salivary cortisol. Appl. Anim. Behav. Sci. 2018, 204, 72–80. [Google Scholar] [CrossRef]
- Napolitano, F.; Mota-Rojas, D.; Álvarez-Macías, A.; Braghieri, A.; Mora-Medina, P.; Bertoni, A.; Cruz-Monterrosa, R.; De Rosa, G. Factores productivos y su incidencia en el bienestar de la búfala lechera en sistemas de producción extensivos e intensivos. Soc. Rur. Prod. Med. Amb. 2020, 20, 155–173. [Google Scholar]
- Collins, C.; Corkery, I.; Haigh, A.; McKeown, S.; Quirke, T.; O'Riordan, R. The effects of environmental and visitor variables on the behavior of free-ranging ring-tailed lemurs (Lemur catta) in captivity. Zoo Biol. 2017, 36, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Saltz, D.; Berger-Tal, O.; Motro, U.; Shkedy, Y.; Raanan, N. Conservation implications of habituation in Nubian ibex in response to ecotourism. Anim. Conserv. 2019, 22, 220–227. [Google Scholar] [CrossRef]
- Fàbregas, M.C.; Fosgate, G.; Ganswindt, A.; Bertschinger, H.; Hofmeyr, M.; Meyer, L.C.R. Rehabilitation method affects behavior, welfare, and adaptation potential for subsequent release of orphaned white rhinoceros. Acta Ethologica 2020, 23, 105–114. [Google Scholar] [CrossRef]
- Glenk, L.M.; Foltin, S. Therapy Dog Welfare Revisited: A Review of the Literature. Vet. Sci. 2021, 8, 226. [Google Scholar] [CrossRef]
- Hennessy, M.; Willen, R.; Schiml, P. Psychological Stress, Its Reduction, and Long-Term Consequences: What Studies with Laboratory Animals Might Teach Us about Life in the Dog Shelter. Animals 2020, 10, 2061. [Google Scholar] [CrossRef]
- Troha, R.G.; Dong, D.; Markus, E.J. Observational learning of a shifting goal location in rats: Impact of distance, observed performance, familiarity, and delay. J. Neurosci. Methods 2020, 335, 108617. [Google Scholar] [CrossRef]
- Alem, S.; Perry, C.J.; Zhu, X.; Loukola, O.J.; Ingraham, T.; Søvik, E.; Chittka, L. Associative Mechanisms Allow for Social Learning and Cultural Transmission of String Pulling in an Insect. PLOS Biol. 2016, 14, e1002564. [Google Scholar] [CrossRef]
- Brown, C.; Laland, K.N. Social learning in fishes: A review. Fish Fish. 2003, 4, 280–288. [Google Scholar] [CrossRef]
- Slagsvold, T.; Wiebe, K.L. Social learning in birds and its role in shaping a foraging niche. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, M.C.O.; Messier, F.; Chivers, D.P. First Documentation of Cultural Transmission of Predator Recognition by Larval Amphibians. Ethology 2007, 113, 621–627. [Google Scholar] [CrossRef]
- Whiten, A.; van de Waal, E. Social learning, culture and the ‘socio-cultural brain’ of human and non-human primates. Neurosci. Biobehav. Rev. 2017, 82, 58–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabenhorst, F.; Báez-Mendoza, R.; Genest, W.; Deco, G.; Schultz, W. Primate Amygdala Neurons Simulate Decision Processes of Social Partners. Cell 2019, 177, 986–998.e15. [Google Scholar] [CrossRef]
- Van de Waal, E.; Borgeaud, C.; Whiten, A. Potent Social Learning and Conformity Shape a Wild Primate’s Foraging Decisions. Science 2013, 340, 483–485. [Google Scholar] [CrossRef]
- Thornton, A.; Clutton-Brock, T. Social learning and the development of individual and group behaviour in mammal societies. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 978–987. [Google Scholar] [CrossRef]
- Tallett, A.; Blundell, J.; Rodgers, R. Endogenous opioids and cannabinoids: System interactions in the regulation of appetite, grooming and scratching. Physiol. Behav. 2008, 94, 422–431. [Google Scholar] [CrossRef]
- Elmore, M.R.P.; Garner, J.P.; Johnson, A.K.; Kirkden, R.D.; Richert, B.T.; Pajor, E.A. Getting around social status: Motivation and enrichment use of dominant and subordinate sows in a group setting. Appl. Anim. Behav. Sci. 2011, 133, 154–163. [Google Scholar] [CrossRef]
- Orihuela, A.; Mota-Rojas, D.; Strappini, A.; Serrapica, F.; Braghieri, A.; Mora-Medina, P.; Napolitano, F. Neurophysiological Mechanisms of Cow–Calf Bonding in Buffalo and Other Farm Animals. Animals 2021, 11, 1968. [Google Scholar] [CrossRef]
- Mora-Medina, P.; Napolitano, F.; Mota-Rojas, D.; Berdugo-Gutiérrez, J.; Ruiz, J.; Guerrero-Legarreta, I. Imprinting, Sucking and Allosucking Behaviors in Buffalo Calves. J. Buffalo Sci. 2018, 7, 49–57. [Google Scholar] [CrossRef]
- Hartcher, K.M.; Jones, B. The welfare of layer hens in cage and cage-free housing systems. World's Poult. Sci. J. 2017, 73, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Vasdal, G.; Vas, J.; Newberry, R.; Moe, R.O. Effects of environmental enrichment on activity and lameness in commercial broiler production. J. Appl. Anim. Welf. Sci. 2019, 22, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Bailie, C.L.; O’Connell, N.E. Evaluation of a dustbathing substrate and straw bales as environmental enrichments in commercial broiler housing. Appl. Anim. Behav. Sci. 2018, 200, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, R.; Wang, L.; Li, J.; Su, Y.; Li, X.; Bao, J. Effect of social order, perch, and dust-bath allocation on behavior in laying hens. Anim. Biosci. 2022, 35, 299–307. [Google Scholar] [CrossRef]
- Sandilands, V.; Baker, L.; Donbavand, J.; Brocklehurst, S. Effects of Different Scratch Mat Designs on Hen Behaviour and Eggs Laid in Enriched Cages. Animals 2021, 11, 1544. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Jones, S.L.; Flanagan-Cato, L.M.; Blaustein, J.D. Female sexual behavior. In Knobil and Neill’s Physiology of Reproduction. Two-Volume Set; Elsevier: London, UK, 2015; pp. 2287–2370. [Google Scholar]
- Veissier, I.; Boissy, A. Stress and welfare: Two complementary concepts that are intrinsically related to the animal's point of view. Physiol. Behav. 2007, 92, 429–433. [Google Scholar] [CrossRef]
- Riggio, G.; Pirrone, F.; Lunghini, E.; Gazzano, A.; Mariti, C. Zookeepers’ Perception of Zoo Canid Welfare and its Effect on Job Satisfaction, Worldwide. Animals 2020, 10, 916. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Warwick, C.; Steedman, C.; Jessop, M.; Arena, P.; Pilny, A.; Nicholas, E. Exotic pet suitability: Understanding some problems and using a labeling system to aid animal welfare, environment, and consumer protection. J. Vet. Behav. 2018, 26, 17–26. [Google Scholar] [CrossRef]
- Lawrence, A.; Vigors, B. Farm animal welfare: Origins and interplay with economics and policy. In The Economics of Farm Animal Welfare; Ahmadi, B., Moran, D., D’Eath, R., Eds.; CAB International: Oxfordshire, UK, 2020; pp. 1–30. [Google Scholar]
- Panksepp, J. A critical role for "affective neuroscience" in resolving what is basic about basic emotions. Psychol. Rev. 1992, 99, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.; Yovell, Y. Preclinical Modeling of Primal Emotional Affects (SEEKING, PANIC and PLAY): Gateways to the Development of New Treatments for Depression. Psychopathology 2014, 47, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Dreschel, N.A.; Granger, D.A. Physiological and behavioral reactivity to stress in thunderstorm-phobic dogs and their caregivers. Appl. Anim. Behav. Sci. 2005, 95, 153–168. [Google Scholar] [CrossRef]
- Prato-Previde, E.; Cannas, S.; Palestrini, C.; Ingraffia, S.; Battini, M.; Ludovico, L.; Ntalampiras, S.; Presti, G.; Mattiello, S. What’s in a Meow? A Study on Human Classification and Interpretation of Domestic Cat Vocalizations. Animals 2020, 10, 2390. [Google Scholar] [CrossRef]
- Green, A.C.; Lidfors, L.M.; Lomax, S.; Favaro, L.; Clark, C.E. Vocal production in postpartum dairy cows: Temporal organization and association with maternal and stress behaviors. J. Dairy Sci. 2021, 104, 826–838. [Google Scholar] [CrossRef]
- Weary, D.M.; Ross, S.; Fraser, D. Vocalizations by isolated piglets: A reliable indicator of piglet need directed towards the sow. Appl. Anim. Behav. Sci. 1997, 53, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, I.; Humeau, Y.; Grenier, F.; Ciocchi, S.; Herry, C.; Lüthi, A. Amygdala Inhibitory Circuits and the Control of Fear Memory. Neuron 2009, 62, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Hanson, M.; Jojola, S.M.; Rawson, N.E.; Crowe, M.; Laska, M. Facial expressions and other behavioral responses to pleasant and unpleasant tastes in cats (Felis silvestris catus). Appl. Anim. Behav. Sci. 2016, 181, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Lezama-García, K.; Orihuela, A.; Olmos-Hernández, A.; Reyes-Long, S.; Mota-Rojas, D. Facial expressions and emotions in domestic animals. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Hernández, E.; Martínez-Burnes, J.; Whittaker, A.L. The Utility of Grimace Scales for Practical Pain Assessment in Laboratory Animals. Animals 2020, 10, 1838. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Marcet-Rius, M.; Ogi, A.; Hernández-Ávalos, I.; Mariti, C.; Martínez-Burnes, J.; Mora-Medina, P.; Casas, A.; Domínguez, A.; Reyes, B.; et al. Current Advances in Assessment of Dog’s Emotions, Facial Expressions, and Their Use for Clinical Recognition of Pain. Animals 2021, 11, 3334. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.; Keeling, L.J. Routine activities and emotion in the life of dairy cows: Integrating body language into an affective state framework. PLoS ONE 2018, 13, e0195674. [Google Scholar] [CrossRef] [Green Version]
- Siniscalchi, M.; D’Ingeo, S.; Quaranta, A. Lateralized emotional functioning in domestic animals. Appl. Anim. Behav. Sci. 2021, 237, 105282. [Google Scholar] [CrossRef]
- Orihuela, A.; Mota-Rojas, D.; Velarde, A.; Strappini, A.; Thielo de la Vega, L.; Borderas, T.F.; Alonso, S.M.L. Environmental enrichment to improve behaviour in farm animals. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2018, 13, 1–25. [Google Scholar] [CrossRef]
- Rius, M.M.; Cozzi, A.; Bienboire-Frosini, C.; Teruel, E.; Chabaud, C.; Monneret, P.; Leclercq, J.; Lafont-Lecuelle, C.; Pageat, P. Selection of putative indicators of positive emotions triggered by object and social play in mini-pigs. Appl. Anim. Behav. Sci. 2018, 202, 13–19. [Google Scholar] [CrossRef]
- Tamioso, P.R.; Molento, C.F.M.; Boivin, X.; Chandèze, H.; Andanson, S.; Delval, É.; Hazard, D.; da Silva, G.P.; Taconeli, C.A.; Boissy, A. Inducing positive emotions: Behavioural and cardiac responses to human and brushing in ewes selected for high vs low social reactivity. Appl. Anim. Behav. Sci. 2018, 208, 56–65. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coria-Avila, G.A.; Pfaus, J.G.; Orihuela, A.; Domínguez-Oliva, A.; José-Pérez, N.; Hernández, L.A.; Mota-Rojas, D. The Neurobiology of Behavior and Its Applicability for Animal Welfare: A Review. Animals 2022, 12, 928. https://doi.org/10.3390/ani12070928
Coria-Avila GA, Pfaus JG, Orihuela A, Domínguez-Oliva A, José-Pérez N, Hernández LA, Mota-Rojas D. The Neurobiology of Behavior and Its Applicability for Animal Welfare: A Review. Animals. 2022; 12(7):928. https://doi.org/10.3390/ani12070928
Chicago/Turabian StyleCoria-Avila, Genaro A., James G. Pfaus, Agustín Orihuela, Adriana Domínguez-Oliva, Nancy José-Pérez, Laura Astrid Hernández, and Daniel Mota-Rojas. 2022. "The Neurobiology of Behavior and Its Applicability for Animal Welfare: A Review" Animals 12, no. 7: 928. https://doi.org/10.3390/ani12070928
APA StyleCoria-Avila, G. A., Pfaus, J. G., Orihuela, A., Domínguez-Oliva, A., José-Pérez, N., Hernández, L. A., & Mota-Rojas, D. (2022). The Neurobiology of Behavior and Its Applicability for Animal Welfare: A Review. Animals, 12(7), 928. https://doi.org/10.3390/ani12070928








