Biochemical Properties of Black and Green Teas and Their Insoluble Residues as Natural Dietary Additives to Optimize In Vitro Rumen Degradability and Fermentation but Reduce Methane in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Tea Leaves, Dietary Ingredients, and Diet Preparation
2.2. Rumen Liquor Collection and Buffered Inoculum Preparation
2.3. In Vitro Method and Sampling
2.4. VFA, NH3, CH4, and CO2 Analyses
2.5. Proximate, Detergent Fibre, and Secondary Metabolite Analyses
2.6. Mineral, Alkaloid and Polyphenol Analyses
2.7. Calculation and Statistical Analysis
3. Results
3.1. Chemical Properties
3.2. In Vitro Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramdani, D.; Chaudhry, A.S.; Seal, C.J. Chemical composition, plant secondary metabolites, and minerals of green and black teas and the effect of different tea-to-water ratios during their extraction on the composition of their spent leaves as potential additives for ruminants. J. Agric. Food Chem. 2013, 61, 4961–4967. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Velioglu, Y.S. Determination of alkaloids and phenolic compounds in black tea processed by two different methods in different plucking seasons. J. Sci. Food Agric. 2007, 87, 1408–1416. [Google Scholar] [CrossRef]
- Ramdani, D.; Chaudhry, A.S.; Seal, C.J. Alkaloid and polyphenol analyses by HPLC in green and black teas powders and their potential as additives in ruminant diets. In Proceedings of the 1st International Conference and Exhibition on Powder Technology Indonesia, Jatinangor, Sumedang, Indonesia, 8–9 August 2017; Volume 1927, p. 030008. [Google Scholar]
- Mueller-Harvey, I. Review unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Cieślak, A. Potential of phytofactors to mitigate rumen ammonia and methane production. J. Anim. Feed Sci. Technol. 2010, 19, 319–337. [Google Scholar] [CrossRef]
- Ramdani, D.; Budinuryanto, D.C.; Mayasari, N. The effect of paddy straw and concentrate containing green tea dust on performance and nutrient digestibility in feedlot lambs. Turk. J. Vet. Anim. Sci. 2020, 44, 668–674. [Google Scholar] [CrossRef]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Liu, J.X.; Zhu, W.Y.; Denman, S.E.; McSweeney, C.S. Effect of tea saponin on methanogenesis, microbial community structure and expression of mcrA gene, in cultures of rumen micro-organisms. Lett. Appl. Microbiol. 2008, 47, 421–426. [Google Scholar] [CrossRef]
- Mao, H.L.; Wang, J.K.; Zhou, Y.Y.; Liu, J.X. Effects of addition of tea saponins and soybean oil on methane production, fermentation and microbial population in the rumen of growing lambs. Livest. Sci. 2010, 129, 56–62. [Google Scholar] [CrossRef]
- Jayanegara, A.; Wina, E.; Takahashi, J. Meta-analysis on methane mitigating properties of saponin-rich sources in the rumen: Influence of addition levels and plant sources. Asian-Australas. J. Anim. Sci. 2014, 27, 1426–1435. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Chaudhry, A.S.; Beneke, R.G.; Suhubdy Young, B.A. Modification of methane emission in sheep by cysteine and a microbial preparation. Sci. Total Environ. 1997, 204, 117–123. [Google Scholar] [CrossRef]
- Chaudhry, A.S.; Khan, M.M.H. Impacts of different spices on in vitro ruminal degradability, fermentation and methane of wheat flour or ryegrass hay based substrates. Livest. Sci. 2012, 146, 84–90. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef] [Green Version]
- Boadi, D.; Benchaar, C.; Chiquette, J.; Massé, D. Mitigation strategies to reduce enteric methane emissions from dairy cows: Update review. Can. J. Anim. Sci. 2004, 84, 319–335. [Google Scholar] [CrossRef]
- Attwood, G.; McSweeney, C. Methanogen genomics to discover targets for methane mitigation technologies and options for alternative H2 utilization in the rumen. Aust. J. Exp. Agric. 2008, 48, 28–37. [Google Scholar] [CrossRef]
- Shrubsole, M.J.; Lu, W.; Chen, Z.; Shu, X.O.; Zheng, Y.; Dai, Q.; Cai, Q.; Gu, K.; Ruan, Z.X.; Gao, Y.T.; et al. Drinking green tea modestly reduces breast cancer risk. J. Nutr. 2009, 139, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Andlauer, W.; Héritier, J. Rapid electrochemical screening of antioxidant capacity (RESAC) of selected tea samples. Food Chem. 2011, 125, 1517–1520. [Google Scholar] [CrossRef]
- Stewart, A.J.; Mullen, W.; Crozier, A. On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol. Nutr. Food Res. 2005, 49, 52–60. [Google Scholar] [CrossRef]
- Gardner, E.J.; Ruxton, C.H.S.; Leeds, A.R. Black tea—helpful or harmful? A review of the evidence. Eur. J. Clin. Nutr. 2007, 61, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Prasanthi, J.R.P.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Kondo, M.; Hirano, K.; Kita, K.; Jayanegara, A.; Yokota, H. Fermentation characteristics, tannin contents and in vitro ruminal degradation of green tea and black tea by-products ensiled at different temperatures. Asian-Australas. J. Anim. Sci. 2014, 27, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Hirano, Y.; Kita, K.; Jayanegara, A.; Yokota, H. Nutritive evaluation of spent green and black tea leaf silages by in vitro gas production characteristics, ruminal degradability and post-ruminal digestibility assessed with inhibitory activity of their tannins. Anim. Sci. J. 2018, 89, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Kita, K.; Yokota, H. Ensiled or oven-dried green tea by-product as protein feedstuffs: Effects of tannin on nutritive value in goats. Asian-Australas. J. Anim. Sci. 2007, 20, 880–886. [Google Scholar] [CrossRef]
- Xu, C.; Cai, Y.; Moriya, N.; Ogawa, M. Nutritive value for ruminants of green tea grounds as a replacement of brewers’ grains in totally mixed ration silage. Anim. Feed Sci. Technol. 2007, 138, 228–238. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on ruminant saliva: The composition and output of sheep's saliva. Biochem. J. 1948, 49, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Ramdani, D.; Chaudhry, A.S.; Hernaman, I.; Seal, C.J. Comparing Tea Leaf Products and Other Forages for In-Vitro Degradability, Fermentation, and Methane for Their Potential Use as Natural Additives for Ruminants. KnE Life Sci. 2017, 2, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.H.; Chaudhry, A.S. Chemical composition of selected forages and spices and the effect of these spices on in vitro rumen degradability of some forages. Asian-Australas. J. Anim. Sci. 2010, 23, 889–900. [Google Scholar]
- AOAC. Animal Feed (Chapter 4). In Official Methods of Analysis of AOAC International; AOAC: Gaithersburg, MI, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral-detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Kluwer Academic: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Hegarty, R.S. Reducing rumen methane emissions through elimination of rumen protozoa. Aust. J. Agric. Res. 1999, 50, 1321–1328. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 43, 351–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, J.L.; Dijkstra, J.; Kebreab, E.; Bannink, A.; Odongo, N.E.; McBride, B.W.; France, J. Aspect of rumen microbiology central to mechanistic modelling of methane production in cattle. J. Agric. Sci. 2008, 146, 213–233. [Google Scholar] [CrossRef] [Green Version]
- McAllister, T.A.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Le Van, T.D.; Robinson, J.A.; Ralph, J.; Greening, R.C.; Smolenski, W.J.; Leedle, J.A.Z.; Schaefer, D.M. Assessment of reductive acetogenesis with indigenous ruminal bacterium populations and acetitomaculum ruminis. Appl. Environ. Microbiol. 1998, 64, 3429–3436. [Google Scholar] [CrossRef] [Green Version]
- López, S.; McInntosh, F.M.; Wallace, R.J.; Newbold, C.J. Effect of adding acetogenic bacteria on methane production by mixed rumen microorganisms. Anim. Feed Sci. Technol. 1999, 78, 1–9. [Google Scholar] [CrossRef]
- Leadbetter, J.R.; Schmidt, T.M.; Graber, J.R.; Breznak, J.A. Acetogenesis from H2 plus CO2 by spirochetes from termite guts. Science 1999, 283, 686–689. [Google Scholar] [CrossRef] [Green Version]
- Popják, G.; French, T.H.; Hunter, G.D.; Martin, A.J.P. Mode of formation of milk fatty acids from acetate in the goats. Biochem. J. 1951, 48, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Tea Leaf Products | RH or RS | CON |
|---|---|---|
| BTL or GTL | ||
| 0 | 300 | 700 |
| 50 | 250 | 700 |
| 100 | 200 | 700 |
| STL | ||
| 0 | 300 | 700 |
| 100 | 200 | 700 |
| 200 | 100 | 700 |
| Ingredients | DM | OM | Ash | CP | EE | ME | NDFom | ADFom | ADLom | TP | TT | TS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BTL | 942 a | 939 c | 61.4 d | 242 b | 12.6 de | 6.40 c | 323 f | 309 ef | 27.4 d | 151 b | 133 b | 86.1 b |
| GTL | 937 a | 938 c | 61.8 d | 240 b | 20.8 b | 7.08 bc | 254 g | 211 g | 37.6 d | 231 a | 204 a | 276 a |
| SBTL | 126 f | 961 a | 38.7 f | 234 b | 13.5 cd | 6.59 c | 474 d | 410 d | 44.5 d | 90.9 c | 79.1 c | 34.0 c |
| SGTL | 134 f | 957 b | 43.3 e | 246 a | 23.1 b | 7.39 b | 405 e | 294 f | 40.3 d | 110 b | 99.9 b | 55.6 b |
| CSBTL | 205 d | 959 a | 41.3 f | 253 a | 12.6 de | 6.87 bc | 576 c | 449 c | 48.8 d | 34.4 de | 31.7 d | 12.4 d |
| CSGTL | 170 e | 955 b | 44.9 e | 261 a | 17.8 bc | 7.49 b | 560 c | 334 e | 42.7 d | 44.7 d | 39.8 d | 26.8 cd |
| RH | 840 c | 908 e | 92.4 b | 200 c | 20.2 b | 6.79 bc | 649 b | 507 b | 435 b | 9.89 ef | 2.19 e | 16.9 cd |
| RS | 944 a | 818 e | 182 a | 60.4 e | 9.9 e | 4.01 d | 787 a | 684 a | 598 a | 6.12 f | 1.08 e | 24.3 cd |
| CON | 864 b | 921 d | 78.9 c | 176 d | 56.6 a | 10.1 a | 271 g | 144 h | 134 c | 3.95 f | 1.61 e | 32.2 cd |
| SEM | 2.37 *** | 0.56 *** | 0.56 *** | 1.77 *** | 0.80 *** | 0.15 *** | 5.35 *** | 5.32 *** | 4.99 *** | 5.74 *** | 4.28 *** | 5.06 *** |
| Compounds(mg/kg DM) | BTL | GTL | SBTL | SGTL | CSBTL | CSGTL | SEM |
|---|---|---|---|---|---|---|---|
| Ca | 6441 c | 6699 c | 8281 b | 8881 b | 10,374 a | 10,753 a | 171 *** |
| K | 7808 a | 8095 a | 2521 b | 2542 b | 632 c | 906 c | 186 *** |
| P | 2413 ab | 2521 a | 1904 d | 2213 bc | 2013 cd | 2183 bcd | 61.8 *** |
| Mg | 1726 bc | 1993 a | 1641 bc | 1848 ab | 1726 c | 1864 bc | 44.7 *** |
| Mn | 527 c | 663 b | 536 c | 747 a | 536 c | 804 a | 13.6 *** |
| Fe | 116 d | 119 d | 169 bc | 143 c | 182 d | 346 ba | 6.68 *** |
| Na | 150 c | 78.2 d | 180.1 c | 94.6 d | 1789 a | 1303 b | 8.11 *** |
| Cu | 23.8 a | 16.9 b | 24.0 a | 16.6 b | 26.9 a | 23.8 a | 1.16 *** |
| Zn | 21.7 ab | 21.2 ab | 22.4 ab | 19.5 b | 23.7 a | 20.4 b | 0.63 ** |
| Ni | 1.69 a | 1.58 a | 1.17 b | 0.49 c | 0.69 c | 0.40 c | 0.08 *** |
| Cr | 1.22 b | 1.32 b | 1.45 b | 1.08 b | 1.24 b | 2.37 a | 0.14 *** |
| Pb | 0.59 b | 0.51 b | 0.73 b | 0.39 b | 0.65 b | 1.48 a | 0.14 ** |
| Cd | 0.04 | 0.04 | 0.04 | 0.04 | 0.07 | 0.09 | 0.01 NS |
| Compounds (g/kg DM) | BTL | GTL | SBTL | SGTL | CSBTL | CSGTL | SEM |
|---|---|---|---|---|---|---|---|
| Theobromine | 1.37 b | 2.58 a | 0.40 d | 0.76 c | 0.03 e | 0.11 e | 0.02 *** |
| Caffeine | 27.4 b | 28.9 a | 9.47 c | 9.89 c | 0.93 d | 0.91 d | 0.16 *** |
| Total alkaloids | 28.8 b | 31.5 a | 9.87 d | 10.7 c | 0.96 e | 1.02 e | 0.16 *** |
| Gallocatechin | n.d. | 4.93 a | n.d. | 1.61 b | n.d. | 0.81 c | 0.07 *** |
| Epigallocatechin | 3.51 c | 22.4 a | 0.80 d | 9.02 b | 0.07 e | 3.22 c | 0.14 *** |
| Catechin | 0.40 b | 1.30 a | 0.14 c | 0.40 b | 0.03 c | 0.14 d | 0.01 *** |
| Epicatechin | 0.28 c | 2.13 a | 0.03 d | 1.37 b | 0.08 d | 0.25 c | 0.02 *** |
| Epigallocatechin gallate | 4.45 d | 94.6 a | 2.30 e | 51.8 b | 3.69 de | 10.7 c | 0.41 *** |
| Gallocatechin gallate | 0.60 c | 1.15 a | 0.16 d | 0.85 b | 0.16 d | 0.75 b | 0.03 *** |
| Epicatechin gallate | 5.41 c | 25.5 a | 2.30 e | 14.4 b | 1.90 e | 4.23 d | 0.15 *** |
| Catechin gallate | 1.33 c | 3.10 a | 0.51 e | 1.97 b | 0.39 e | 0.68 d | 0.03 *** |
| Total catechins | 16.0 d | 155 a | 6.24 e | 73.3 b | 6.32 e | 20.8 c | 0.68 *** |
| Theaflavin | 2.33 a | 0.28 c | 1.44 b | 0.18 d | 0.33 c | 0.07 e | 0.01 *** |
| Theaflavin-3-gallate | 4.57 a | 0.22 d | 3.27 b | 0.13 de | 0.77 c | 0.03 e | 0.03 *** |
| Theaflavin-3′-gallate | 2.8 a | 0.35 d | 2.08 b | 0.22 e | 0.49 c | 0.09 f | 0.02 *** |
| Theaflavin-3,3′-digallate | 6.98 a | 0.38 d | 5.78 b | 0.24 de | 1.19 c | 0.08 e | 0.05 *** |
| Total theaflavins | 16.7 a | 1.23 d | 12.6 b | 0.77 d | 2.77 c | 0.28 e | 0.11 *** |
| Rutin | 2.03 b | 2.11 a | 0.80 d | 1.13 c | n.d. | n.d. | 0.02 *** |
| Measurement | Levels (g/kg DM) n = 16 | Tea Types n = 24 | Diet Types n = 24 | SEM and Significances | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | Black | Green | RH | RS | L | T | D | L*T | L*D | T*D | L*D*T | |
| IVOMD (g/kg OM) | 726 | 715 | 708 | 706 | 727 | 753 | 681 | 7.39 NS | 5.74 * | 5.89 *** | 10.5 NS | 11.2 NS | 8.33 NS | 15.8 NS |
| NH3 (mg/L) | 152 a | 141 b | 126 c | 144 | 135 | 134 | 145 | 1.51 *** | 1.24 *** | 1.24 *** | 2.09 *** | 2.25 NS | 1.80 NS | 3.41 NS |
| pH | 6.69 a | 6.67 b | 6.67 b | 6.68 | 6.67 | 6.65 | 6.70 | 0.002 *** | 0.002 *** | 0.002 *** | 0.003 ** | 0.003 NS | 0.003 NS | 0.005 NS |
| tVFA (mmol/L) | 48.6 | 48.5 | 50.2 | 48.4 | 49.8 | 48.6 | 49.6 | 1.47 NS | 1.18 NS | 1.18 NS | 2.16 NS | 2.16 NS | 1.73 NS | 3.26 NS |
| A:P ratio | 2.70 b | 2.78 ab | 2.83 b | 2.74 | 2.81 | 2.77 | 2.78 | 0.03 ** | 0.02* | 0.02 NS | 0.04 NS | 0.04 NS | 0.03 NS | 0.05 NS |
| Acetate | 28.2 | 28.3 | 29.8 | 28.2 | 29.4 | 28.4 | 29.2 | 1.04 NS | 0.84 NS | 0.84 NS | 1.52 NS | 1.52 NS | 1.21 NS | 2.30 NS |
| Propionate | 10.4 | 10.2 | 10.5 | 10.3 | 10.4 | 10.3 | 10.5 | 0.31 NS | 0.25 NS | 0.25 NS | 0.45 NS | 0.45 NS | 0.36 NS | 0.68 NS |
| iso-Butyrate | 0.84 | 0.83 | 0.82 | 0.83 | 0.83 | 0.82 | 0.84 | 0.01 NS | 0.01 NS | 0.01 NS | 0.02 NS | 0.02 NS | 0.01 NS | 0.03 NS |
| n-Butyrate | 6.70 | 6.71 | 6.77 | 6.69 | 6.76 | 6.74 | 6.71 | 0.11 NS | 0.09 NS | 0.09 NS | 0.16 NS | 0.16 NS | 0.13 NS | 0.24 NS |
| iso-Valerate | 1.42 | 1.39 | 1.35 | 1.38 | 1.39 | 1.38 | 1.39 | 0.02 NS | 0.01 NS | 0.01 NS | 0.03 NS | 0.03 NS | 0.02 NS | 0.04 NS |
| Valerate | 1.04 | 1.02 | 0.97 | 1.00 | 1.02 | 1.06 | 0.97 | 0.01 NS | 0.01 NS | 0.01 NS | 0.02 NS | 0.02 NS | 0.01 NS | 0.03 NS |
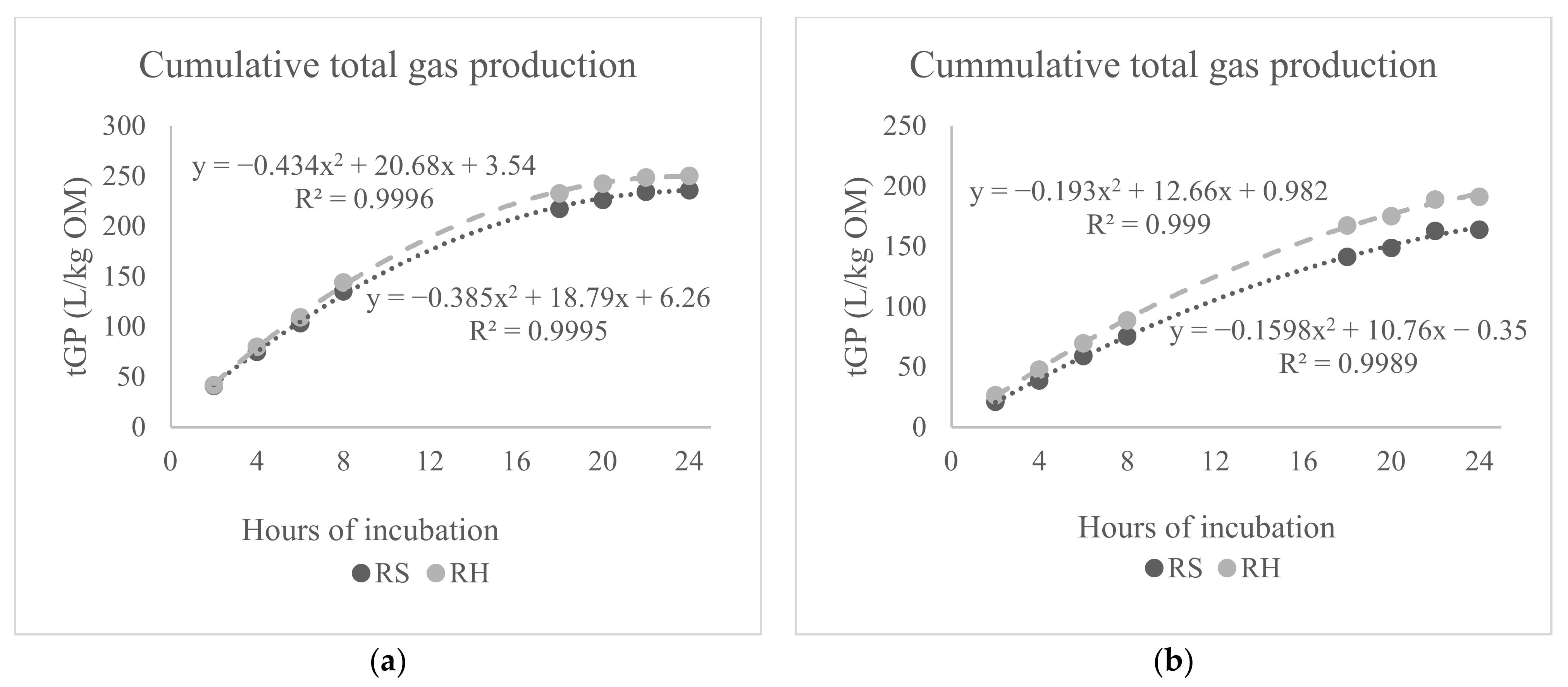
| tGP (L/kg OM) | 243 | 244 | 243 | 241 | 246 | 251 | 236 | 1.40 NS | 1.17 ** | 1.17 *** | 2.14 NS | 2.14 NS | 1.70 NS | 3.23 NS |
| CH4 (% total gas) | 14.1 a | 13.3 b | 13.1 b | 13.5 | 13.4 | 13.3 | 13.6 | 0.18 ** | 0.15 NS | 0.15 NS | 0.26 NS | 0.26 NS | 0.21 NS | 0.40 NS |
| CH4 (L/kg OM) | 34.3 a | 32.3 b | 31.7 b | 32.6 | 33.0 | 33.4 | 32.1 | 0.45 ** | 0.37 NS | 0.37* | 0.67 NS | 0.67 NS | 0.54 NS | 1.02 NS |
| CO2 (% total gas) | 67.5 | 66.6 | 67.4 | 67.0 | 67.4 | 67.6 | 66.7 | 1.87 NS | 1.49 NS | 1.52 NS | 2.64 NS | 2.82 * | 2.21 NS | 3.99 NS |
| CO2 (L/kg OM) | 165 | 162 | 164 | 162 | 166 | 170 | 158 | 4.80 NS | 3.82 NS | 3.92* | 6.79 NS | 6.79 NS | 5.67 NS | 10.3 NS |
| Measurement | Levels (g/kg DM) (n = 32) | STL Types (n = 48) | Diet Types (n = 48) | SEM and Significance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | Black | Green | RH | RS | L | S | D | L*S | L*D | S*D | L*S*D | |
| IVOMD (g/kg OM) | 797 | 799 | 798 | 794 | 803 | 821 | 775 | 6.40 NS | 4.18 NS | 4.26 *** | 9.04 NS | 6.13 NS | 6.03 NS | 13.7 NS |
| NH3 (mg/L) | 154 | 154 | 153 | 154 | 153 | 152 | 155 | 1.05 NS | 0.72 NS | 0.72 *** | 1.49 NS | 1.49* | 1.04 NS | 2.11 NS |
| pH | 6.72 | 6.70 | 6.69 | 6.71 | 6.70 | 6.67 | 6.74 | 0.01 NS | 0.01 NS | 0.01 *** | 0.01 NS | 0.01 NS | 0.01 NS | 0.02 NS |
| tVFA (mmol/L) | 40.7 | 40.0 | 38.8 | 40.4 | 39.9 | 42.7 | 37.6 | 0.95 NS | 0.63 NS | 0.63 *** | 1.34 NS | 1.34 NS | 0.89 NS | 1.89 NS |
| A:P ratio | 1.75 c | 1.83 a | 1.81 b | 1.79 | 1.81 | 1.84 | 1.76 | 0.005 *** | 0.005 ** | 0.005 *** | 0.010 * | 0.010 *** | 0.006 NS | 0.014 NS |
| Acetate | 22.2 a | 21.7 ab | 21.3 b | 22.1 | 22.0 | 23.6 | 20.5 | 0.51 * | 0.34 NS | 0.34 *** | 0.73 NS | 0.73 NS | 0.49 NS | 1.03 NS |
| Propionate | 12.7 a | 12.4 ab | 11.8 b | 12.4 | 12.2 | 12.9 | 11.7 | 0.29 * | 0.19 NS | 0.19 *** | 0.40 NS | 0.40 NS | 0.27 NS | 0.57 NS |
| iso-Butyrate | 0.56 | 0.55 | 0.53 | 0.58 | 0.51 | 0.58 | 0.51 | 0.02 NS | 0.01 NS | 0.01 *** | 0.02 NS | 0.02 NS | 0.02 NS | 0.03 NS |
| n-Butyrate | 3.44 | 3.53 | 3.35 | 3.48 | 3.40 | 3.66 | 3.23 | 0.09 NS | 0.06 NS | 0.06 *** | 0.12 NS | 0.12 NS | 0.08 NS | 0.18 NS |
| iso-Valerate | 0.69 | 0.71 | 0.68 | 0.71 | 0.68 | 0.73 | 0.65 | 0.02 NS | 0.02 NS | 0.02 ** | 0.03 NS | 0.03 NS | 0.02 NS | 0.05 NS |
| Valerate | 1.15 | 1.14 | 1.09 | 1.15 | 1.10 | 1.28 | 0.98 | 0.03 NS | 0.02 NS | 0.02 ** | 0.04 NS | 0.04 NS | 0.03 NS | 0.06 NS |
| tGP (L/kg OM) | 175 | 179 | 177 | 175 | 179 | 193 | 161 | 1.55 NS | 1.04 ** | 1.05 *** | 2.19 NS | 2.19 *** | 1.49 NS | 3.10 NS |
| CH4 (% total gas) | 13.8 a | 13.3 b | 13.4 b | 13.6 | 13.5 | 13.3 | 13.7 | 0.12 ** | 0.08 NS | 0.08 ** | 0.16 NS | 0.16 NS | 0.11 NS | 0.23 NS |
| CH4 (L/kg OM) | 24.2 | 23.8 | 23.6 | 23.7 | 24.1 | 25.7 | 22.0 | 0.29 NS | 0.20 NS | 0.20 ** | 0.41 NS | 0.41 ** | 0.28 NS | 0.58 NS |
| CO2 (% total gas) | 68.7 | 67.1 | 65.8 | 66.7 | 67.7 | 70.1 | 64.3 | 1.22 NS | 0.83 NS | 0.83 *** | 1.72 NS | 1.72 NS | 1.17 NS | 2.44 NS |
| CO2 (L/kg OM) | 121 | 121 | 117 | 117 | 122 | 135 | 104 | 2.46 NS | 1.68 * | 1.68 *** | 3.48 NS | 3.48 ** | 2.40 NS | 4.92NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramdani, D.; Jayanegara, A.; Chaudhry, A.S. Biochemical Properties of Black and Green Teas and Their Insoluble Residues as Natural Dietary Additives to Optimize In Vitro Rumen Degradability and Fermentation but Reduce Methane in Sheep. Animals 2022, 12, 305. https://doi.org/10.3390/ani12030305
Ramdani D, Jayanegara A, Chaudhry AS. Biochemical Properties of Black and Green Teas and Their Insoluble Residues as Natural Dietary Additives to Optimize In Vitro Rumen Degradability and Fermentation but Reduce Methane in Sheep. Animals. 2022; 12(3):305. https://doi.org/10.3390/ani12030305
Chicago/Turabian StyleRamdani, Diky, Anuraga Jayanegara, and Abdul Shakoor Chaudhry. 2022. "Biochemical Properties of Black and Green Teas and Their Insoluble Residues as Natural Dietary Additives to Optimize In Vitro Rumen Degradability and Fermentation but Reduce Methane in Sheep" Animals 12, no. 3: 305. https://doi.org/10.3390/ani12030305
APA StyleRamdani, D., Jayanegara, A., & Chaudhry, A. S. (2022). Biochemical Properties of Black and Green Teas and Their Insoluble Residues as Natural Dietary Additives to Optimize In Vitro Rumen Degradability and Fermentation but Reduce Methane in Sheep. Animals, 12(3), 305. https://doi.org/10.3390/ani12030305







