Analysis of Indirect Biomarkers of Effect after Exposure to Low Doses of Bisphenol A in a Study of Successive Generations of Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Breeding, and Housing Conditions
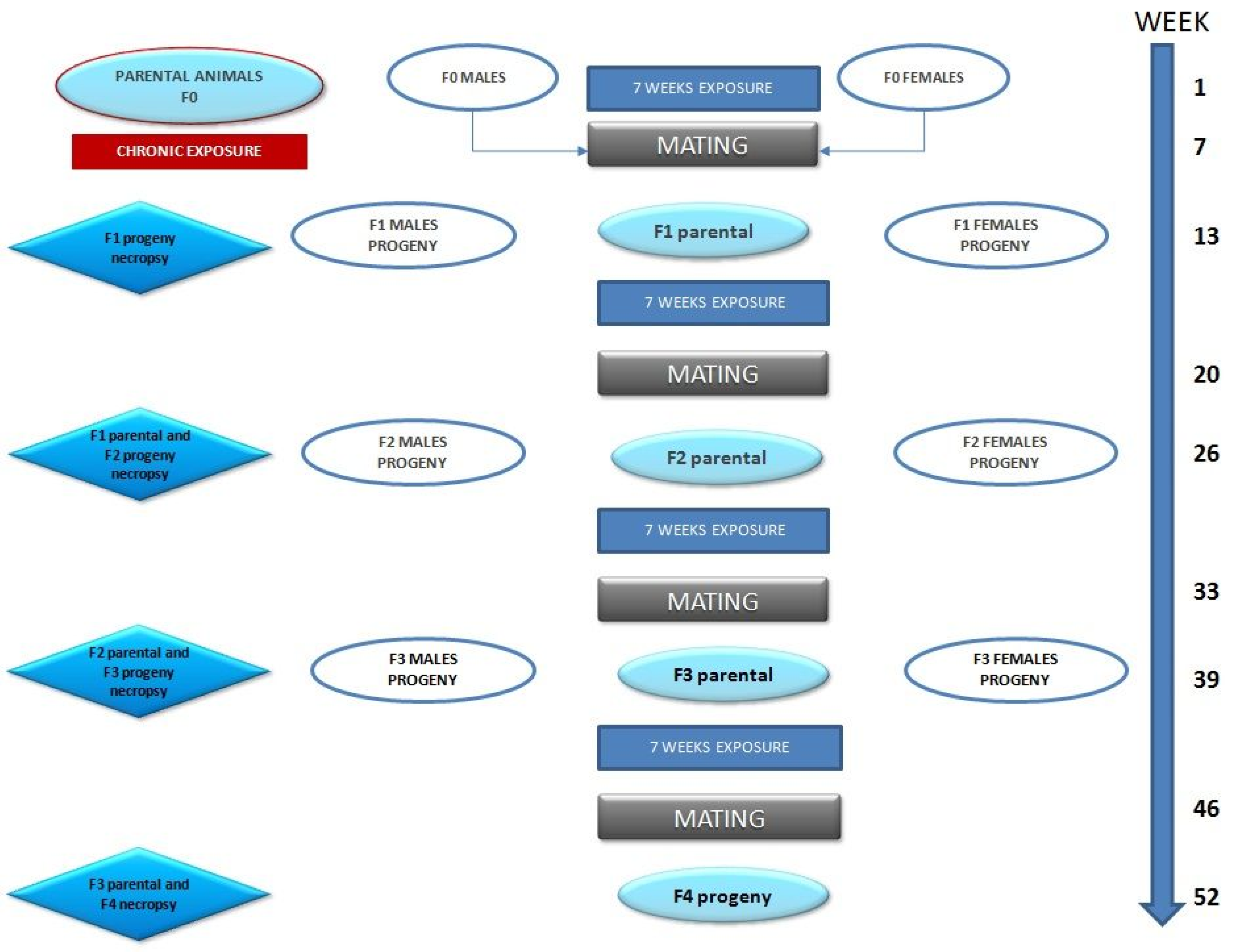
2.2. Study Design
2.3. Parent Animals; Experimental Evaluations
2.3.1. Clinical Data of the Parent Animals (F0, F1, F2, and F3)
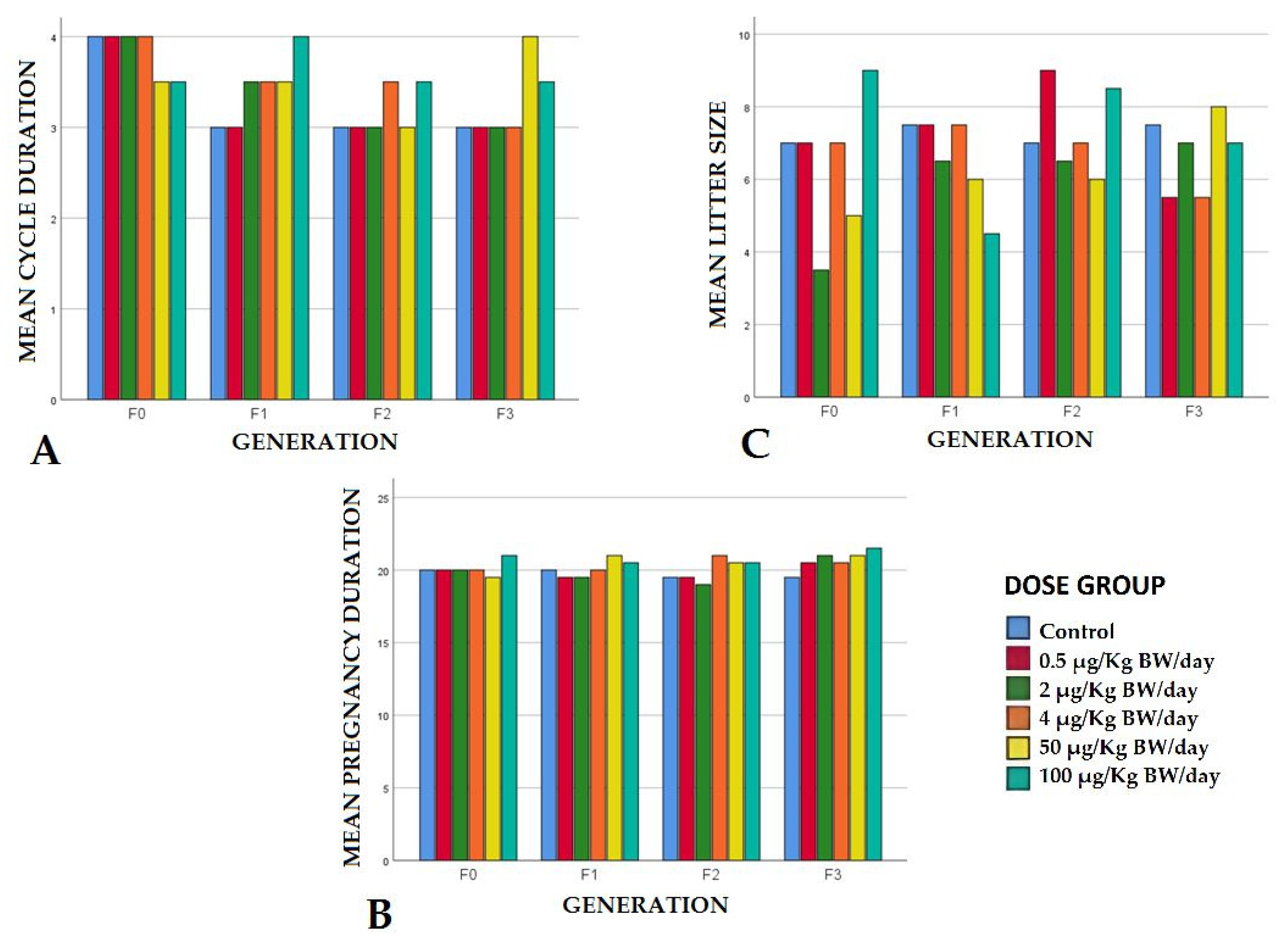
2.3.2. Evaluation and Duration of the Estrous Cycle
2.3.3. Parental Reproductive Parameters
2.3.4. Biochemical Analysis
2.4. Progeny (F1, F2, F3, and F4); Experimental Evaluations
Biochemical Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Parent Animals
3.1.1. Clinical Observations
3.1.2. Increase in Weight, Feed, and Water Intake
3.1.3. Parental Reproductive Parameters
Estrous Cycle
Pregnancy Duration
Litter Size and Stillbirths
Percentage of Pups Alive at Weaning
Sex Ratio
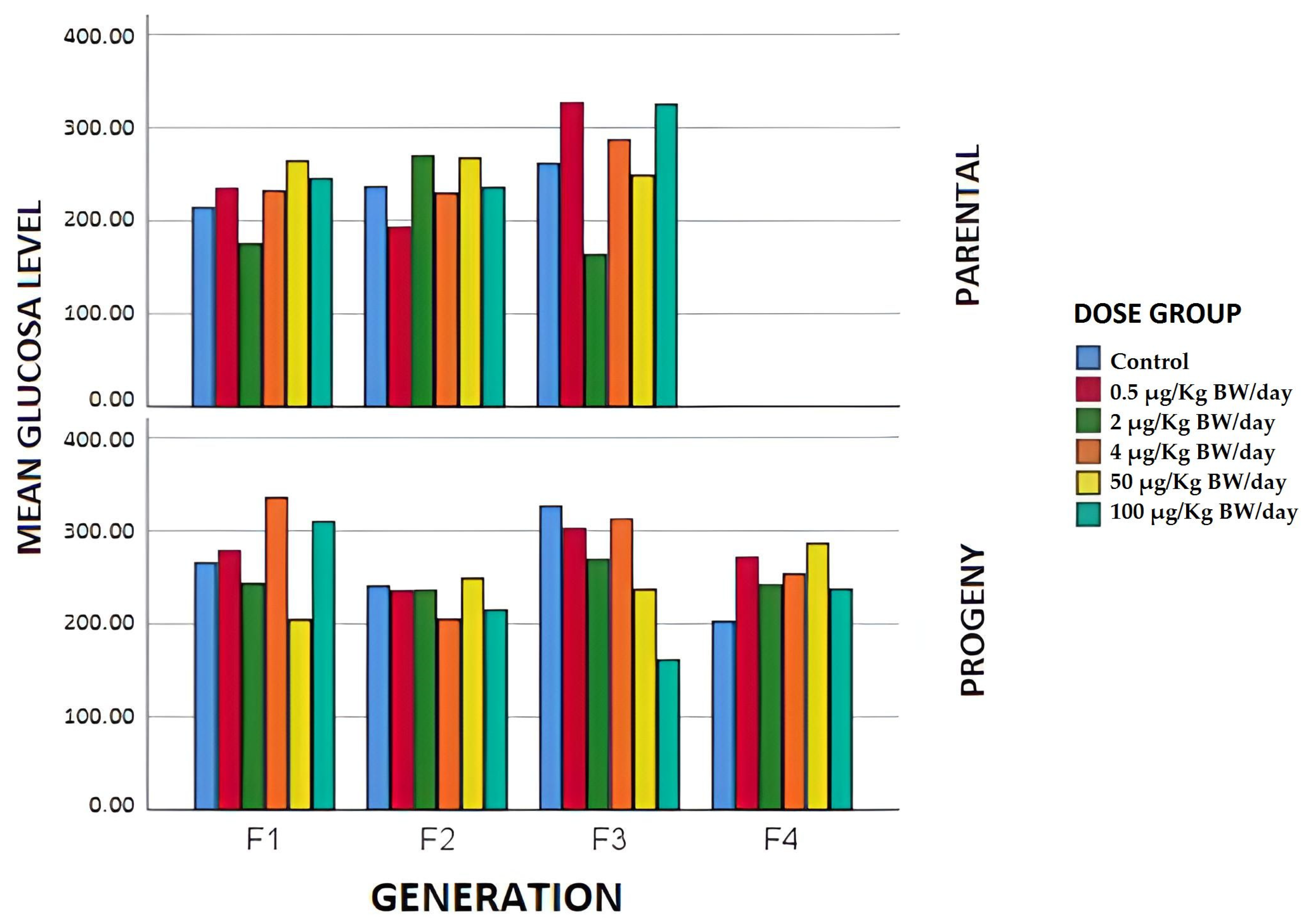
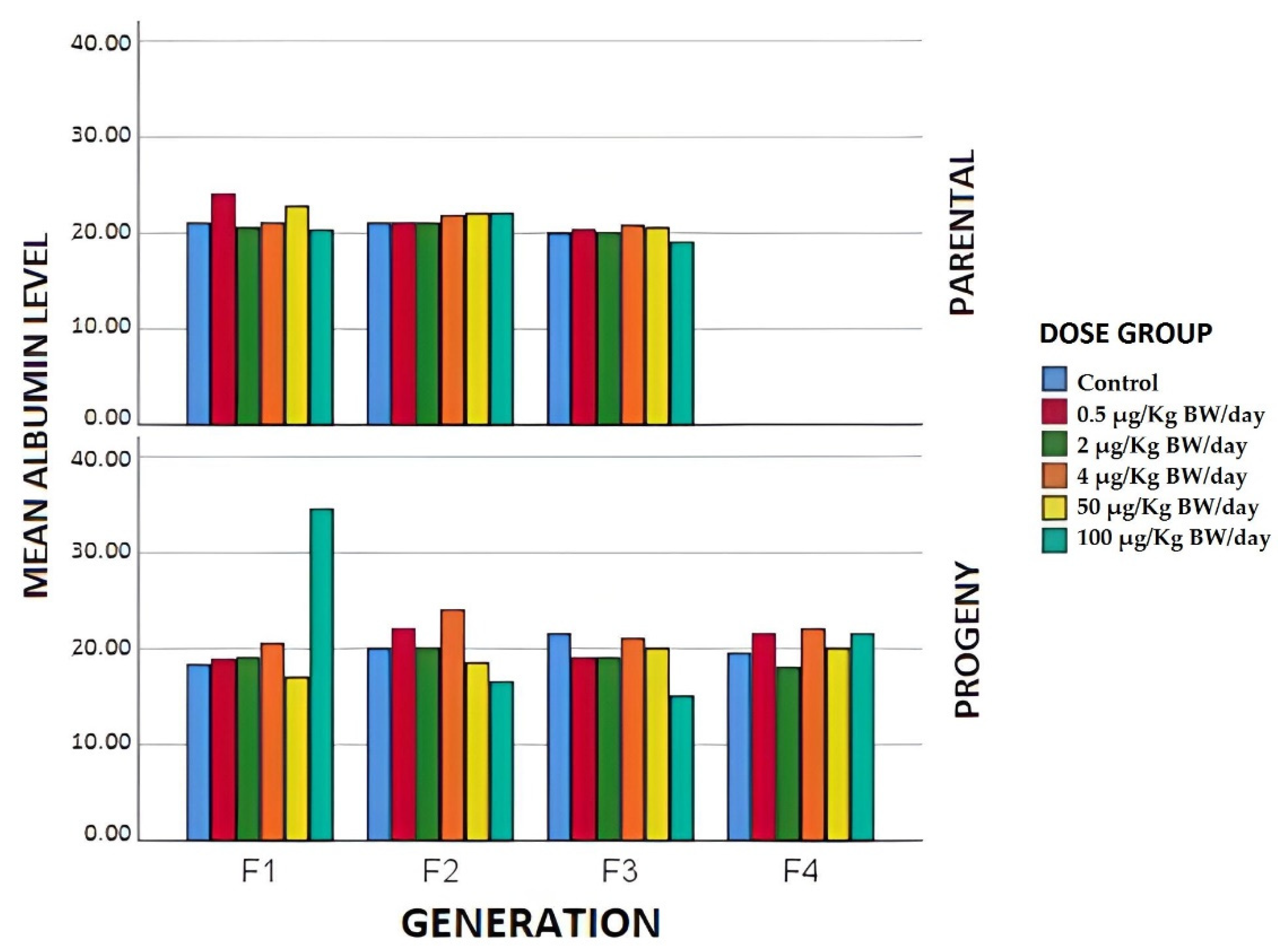
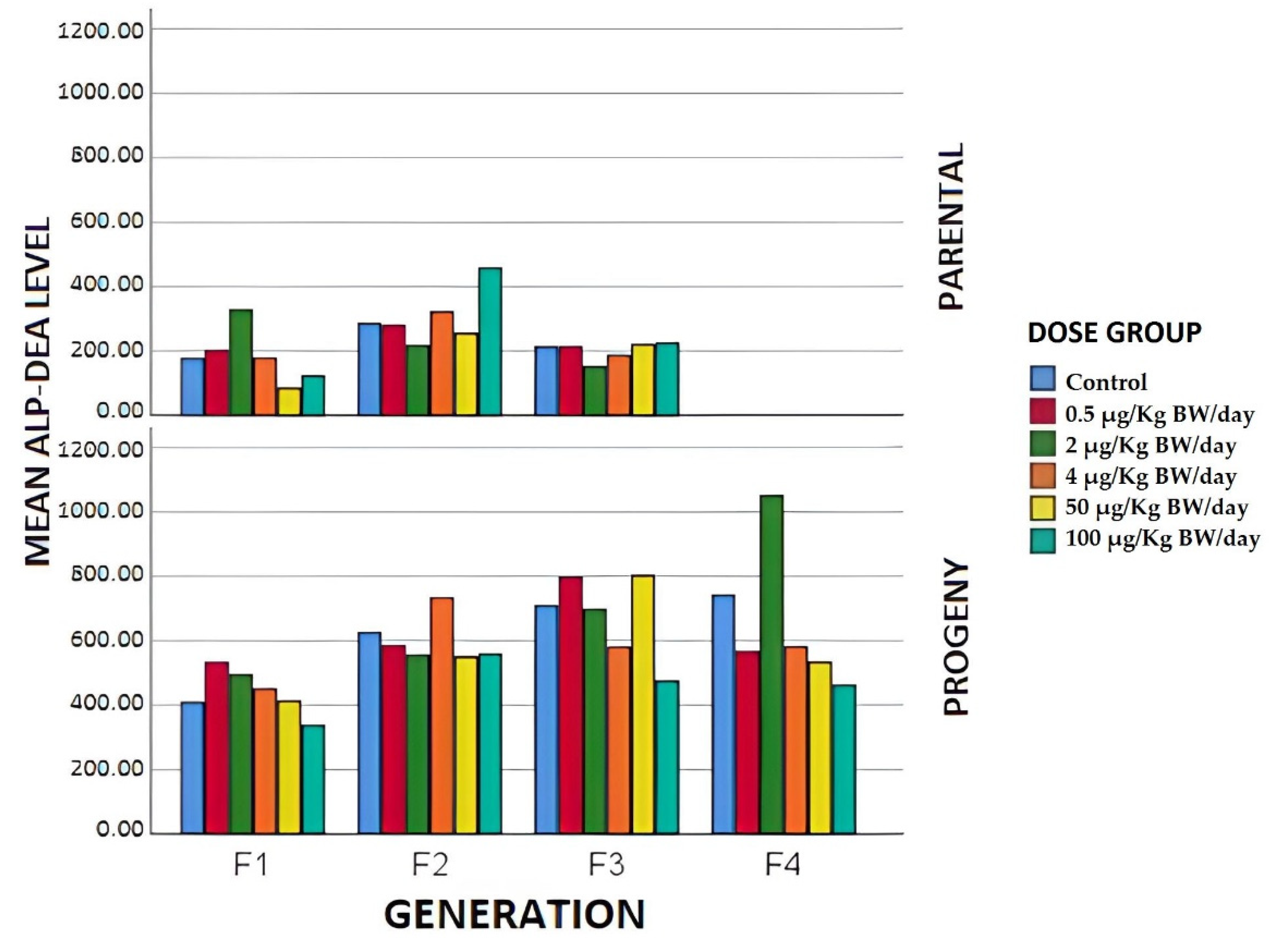
3.1.4. Biochemical Parameters
3.2. Progeny
3.2.1. Litter Weights
Eye and Ear Opening Age
3.2.2. Biochemical Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Noureddine El Moussawi, S.; Ouaini, R.; Matta, J.; Chébib, H.; Cladière, M.; Camel, V. Simultaneous migration of bisphenol compounds and trace metals in canned vegetable food. Food Chem. 2019, 288, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Errico, S.; Bianco, M.; Mita, L.; Migliaccio, M.; Rossi, S.; Nicolucci, C.; Menale, C.; Portaccio, M.; Gallo, P.; Mita, D.G.; et al. Migration of bisphenol A into canned tomatoes produced in Italy: Dependence on temperature and storage conditions. Food Chem. 2014, 160, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Björnsdotter, M.K.; de Boer, J.; Ballesteros-Gómez, A. Bisphenol A and replacements in thermal paper: A review. Chemosphere 2017, 182, 691–706. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA) Home Page. Available online: https://echa.europa.eu/hot-topics/bisphenol-a (accessed on 28 October 2021).
- Vandenberg, L.N.; Hunt, P.A.; Myers, J.P.; Vom Saal, F.S. Human exposures to bisphenol A: Mismatches between data and assumptions. Rev. Environ. Health 2013, 28, 37–58. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. CienSaude Colet. 2012, 17, 407–434, Erratum in CienSaude Colet. 2012, 17, 809. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, E. Environmental Effects of BPA: Focus on Aquatic Species. Dose Response 2015, 13, 1559325815598304. [Google Scholar] [CrossRef] [Green Version]
- Babić, S.; Barišić, J.; Bielen, A.; Bošnjak, I.; SauerbornKlobučar, R.; Ujević, I.; Strunjak-Perović, I.; TopićPopović, N.; Čož-Rakovac, R. Multilevel ecotoxicity assessment of environmentally relevant bisphenol A concentrations using the soil invertebrate Eisenia fetida. J. Hazard Mater. 2016, 318, 477–486. [Google Scholar] [CrossRef]
- Salgueiro-González, N.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Trends in analytical methodologies for the determination of alkylphenols and bisphenol A in water samples. Anal. Chim. Acta 2017, 962, 1–14. [Google Scholar] [CrossRef]
- Vermeirssen, E.L.M.; Dietschweiler, C.; Werner, I.; Burkhardt, M. Corrosion protection products as a source of bisphenol A and toxicity to the aquatic environment. Water Res. 2017, 123, 586–593. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manage. 2012, 104, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Manfo, F.P.; Jubendradass, R.; Nantia, E.A.; Moundipa, P.F.; Mathur, P.P. Adverse effects of bisphenol A on male reproductive function. Rev. Environ. Contam. Toxicol. 2014, 228, 57–82. [Google Scholar] [CrossRef] [PubMed]
- Söffker, M.; Tyler, C.R. Endocrine disrupting chemicals and sexual behaviors in fish--a critical review on effects and possible consequences. Crit. Rev. Toxicol. 2012, 42, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Blum, M.J. Exposure to an environmental estrogen breaks down sexual isolation between native and invasive species. Evol. Appl. 2012, 5, 901–912. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Deem, S.L.; Holliday, D.K.; Jandegian, C.M.; Kassotis, C.D.; Nagel, S.C.; Tillitt, D.E.; Vom Saal, F.S.; Rosenfeld, C.S. Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen. Comp. Endocrinol. 2015, 214, 195–219. [Google Scholar] [CrossRef]
- Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; Olson, G.R.; Davis, K.J.; Patton, R.E.; Twaddle, N.C.; Doerge, D.R.; Churchwell, M.I.; Bryant, M.S.; et al. A two-year toxicology study of bisphenol A (BPA) in Sprague-Dawley rats: CLARITY-BPA core study results. Food Chem. Toxicol. 2019, 132, 110728. [Google Scholar] [CrossRef]
- Lind, T.; Lejonklou, M.H.; Dunder, L.; Kushnir, M.M.; Öhman-Mägi, C.; Larsson, S.; Melhus, H.; Lind, P.M. Developmental low-dose exposure to bisphenol A induces chronic inflammation, bone marrow fibrosis and reduces bone stiffness in female rat offspring only. Environ. Res. 2019, 177. [Google Scholar] [CrossRef]
- Sprague, B.L.; Trentham-Dietz, A.; Hedman, C.J.; Wang, J.; Hemming, J.D.; Hampton, J.M.; Buist, D.S.; Aiello Bowles, E.J.; Sisney, G.S.; Burnside, E.S. Circulating serum xenoestrogens and mammographic breast density. Breast Cancer 2013, 15, R45. [Google Scholar] [CrossRef] [Green Version]
- Alavian-Ghavanini, A.; Lin, P.I.; Lind, P.M.; RisénRimfors, S.; HalinLejonklou, M.; Dunder, L.; Tang, M.; Lindh, C.; Bornehag, C.G.; Rüegg, J. Prenatal Bisphenol A Exposure is Linked to Epigenetic Changes in Glutamate Receptor Subunit Gene Grin2b in Female Rats and Humans. Sci. Rep. 2018, 8, 11315. [Google Scholar] [CrossRef] [Green Version]
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodríguez-Carrillo, A.; Fini, J.B.; Hofer, T.; Steffensen, I.L.; Dirven, H.; Barouki, R.; Olea, N.; et al. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020, 144, 105811. [Google Scholar] [CrossRef] [PubMed]
- Amraoui, W.; Adjabi, N.; Bououza, F.; Boumendjel, M.; Taibi, F.; Boumendjel, A.; Abdennour, C.; Messarah, M. Modulatory Role of Selenium and Vitamin E, Natural Antioxidants, against Bisphenol A-Induced Oxidative Stress in Wistar Albinos Rats. Toxicol. Res. 2018, 34, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Jiang, Y.; Li, Y.; Wan, Y.; Liu, J.; Ma, Y.; Mao, Z.; Chang, H.; Li, G.; Xu, B.; et al. Early-life exposure to bisphenol a induces liver injury in rats involvement of mitochondria-mediated apoptosis. PLoS ONE 2014, 9, e90443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, N.; Rubin, B.S.; Schaeberle, C.M.; Soto, A.M. Perinatal BPA exposure and reproductive axis function in CD-1 mice. Reprod. Toxicol. 2018, 79, 39–46. [Google Scholar] [CrossRef]
- Wu, D.; Wu, F.; Lin, R.; Meng, Y.; Wei, W.; Sun, Q.; Jia, L. Impairment of learning and memory induced by perinatal exposure to BPA is associated with ERα-mediated alterations of synaptic plasticity and PKC/ERK/CREB signaling pathway in offspring rats. Brain Res. Bull. 2020, 161, 43–54. [Google Scholar] [CrossRef]
- Goldman, J.M.; Murr, A.S.; Cooper, R.L. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B. Dev. Reprod. Toxicol. 2007, 80, 84–97. [Google Scholar] [CrossRef]
- Molina, A.M.; Lora, A.J.; Blanco, A.; Monterde, J.G.; Ayala, N.; Moyano, R. Endocrine-active compound evaluation: Qualitative and quantitative histomorphological assessment of zebrafish gonads after bisphenol-A exposure. Ecotoxicol. Environ. Saf. 2013, 88, 155–162. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Cheng, C.; Li, L.; Xiao, M.; Zhang, G.; Lu, X. Combined effects of di (2-ethylhexyl) phthalate and bisphenol A on thyroid hormone homeostasis in adolescent female rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 40882–40892. [Google Scholar] [CrossRef]
- Ropero, A.B.; Alonso-Magdalena, P.; García-García, E.; Ripoll, C.; Fuentes, E.; Nadal, A. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int. J. Androl. 2008, 31, 194–220. [Google Scholar] [CrossRef]
- Yurino, H.; Ishikawa, S.; Sato, T.; Akadegawa, K.; Ito, T.; Ueha, S.; Inadera, H.; Matsushima, K. Endocrine disruptors (environmental estrogens) enhance autoantibody production by B1 cells. Toxicol. Sci. 2004, 81, 139–147. [Google Scholar] [CrossRef]
- Elango, A.; Shepherd, B.; Chen, T.T. Effects of endocrine disrupters on the expression of growth hormone and prolactin mRNA in the rainbow trout pituitary. Gen. Comp. Endocrinol. 2006, 145, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Abril, N.; Morales-Prieto, N.; Monterde, J.; Ayala, N.; Lora, A.; Moyano, R. Hypothalamic-pituitary-ovarian axis perturbation in the basis of bisphenol A (BPA) reproductive toxicity in female zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 156, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Naville, D.; Gaillard, G.; Julien, B.; Vega, N.; Pinteur, C.; Chanon, S.; Vidal, H.; Le Magueresse-Battistoni, B. Chronic exposure to a pollutant mixture at low doses led to tissue-specific metabolic alterations in male mice fed standard and high-fat high-sucrose diet. Chemosphere 2019, 220, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, Y.; Tsutsumi, O.; Ikezuki, Y.; Kamei, Y.; Osuga, Y.; Yano, T.; Taketan, Y. Preimplantation exposure to bisphenol A advances postnatal development. Reprod. Toxicol. 2001, 15, 71–74. [Google Scholar] [CrossRef]
- Nah, W.H.; Park, M.J.; Gye, M.C. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin. Exp. Reprod. Med. 2011, 38, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Lejonklou, M.H.; Dunder, L.; Bladin, E.; Pettersson, V.; Rönn, M.; Lind, L.; Waldén, T.B.; Lind, P.M. Effects of Low-Dose Developmental Bisphenol A Exposure on Metabolic Parameters and Gene Expression in Male and Female Fischer 344 Rat Offspring. Environ. Health Perspect. 2017, 125, 067018. [Google Scholar] [CrossRef]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Sloan, C.S.; Castillo, N.P.; Veselica, M.M.; Seely, J.C.; Dimond, S.S.; Van Miller, J.P.; Shiotsuka, R.N.; et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 2008, 104, 362–384. [Google Scholar] [CrossRef]
- Moore-Ambriz, T.R.; Acuña-Hernández, D.G.; Ramos-Robles, B.; Sánchez-Gutiérrez, M.; Santacruz-Márquez, R.; Sierra-Santoyo, A.; Piña-Guzmán, B.; Shibayama, M.; Hernández-Ochoa, I. Exposure to bisphenol A in young adult mice does not alter ovulation but does alter the fertilization ability of oocytes. Toxicol. Appl. Pharmacol. 2015, 289, 507–514. [Google Scholar] [CrossRef]
- Mustieles, V.; Zhang, Y.; Yland, J.; Braun, J.M.; Williams, P.L.; Wylie, B.J.; Attaman, J.A.; Ford, J.B.; Azevedo, A.; Calafat, A.M.; et al. Maternal and paternal preconception exposure to phenols and preterm birth. Environ. Int. 2020, 137, 105523. [Google Scholar] [CrossRef]
- Honma, S.; Suzuki, A.; Buchanan, D.L.; Katsu, Y.; Watanabe, H.; Iguchi, T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod. Toxicol. 2002, 16, 117–222. [Google Scholar] [CrossRef]
- Dagher, J.B.; Hahn-Townsend, C.K.; Kaimal, A.; Mansi, M.A.; Henriquez, J.E.; Tran, D.G.; Laurent, C.R.; Bacak, C.J.; Buechter, H.E.; Cambric, C.; et al. Independent and combined effects of Bisphenol A and Diethylhexyl Phthalate on gestational outcomes and offspring development in Sprague-Dawley rats. Chemosphere 2021, 263, 128307. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, M.M.; Gajowik, A.; Jankowska-Steifer, E.A.; Radzikowska, J.; Tyrkiel, E.J. Reproductive and developmental F1 toxicity following exposure of pubescent F0 male mice to bisphenol A alone and in a combination with X-rays irradiation. Toxicology 2018, 410, 142–151. [Google Scholar] [CrossRef]
- Mouneimne, Y.; Nasrallah, M.; Khoueiry-Zgheib, N.; Nasreddine, L.; Nakhoul, N.; Ismail, H.; Abiad, M.; Koleilat, L.; Tamim, H. Bisphenol A urinary level, its correlates, and association with cardiometabolic risks in Lebanese urban adults. Environ. Monit. Assess. 2017, 189, 517. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S.; Paranjpe, M.; DaFonte, T.; Schaeberle, C.; Soto, A.M.; Obin, M.; Greenberg, A.S. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: The addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod. Toxicol. 2017, 68, 130–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassenaar, P.N.H.; Trasande, L.; Legler, J. Systematic Review and Meta-Analysis of Early-Life Exposure to Bisphenol A and Obesity-Related Outcomes in Rodents. Environ. Health Perspect. 2017, 125, 106001. [Google Scholar] [CrossRef] [Green Version]
- Le Magueresse-Battistoni, B.; Multigner, L.; Beausoleil, C.; Rousselle, C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018, 475, 74–91. [Google Scholar] [CrossRef]
- Liu, J.; Yu, P.; Qian, W.; Li, Y.; Zhao, J.; Huan, F.; Wang, J.; Xiao, H. Perinatal bisphenol A exposure and adult glucose homeostasis: Identifying critical windows of exposure. PLoS ONE 2013, 8, e64143. [Google Scholar] [CrossRef]
- Song, S.; Zhang, L.; Zhang, H.; Wei, W.; Jia, L. Perinatal BPA exposure induces hyperglycemia, oxidative stress and decreased adiponectin production in later life of male rat offspring. Int. J. Environ. Res. Public Health 2014, 11, 3728–3742. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, H.S.; Samarghandian, S.; Farkhondeh, T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol. Mech. Methods 2015, 25, 507–513. [Google Scholar] [CrossRef]
- Ozaydın, T.; Oznurlu, Y.; Sur, E.; Celik, I.; Uluısık, D.; Dayan, M.O. Effects of bisphenol A on antioxidant system and lipid profile in rats. Biotech. Histochem. 2018, 93, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Vieira, E.; Soriano, S.; Menes, L.; Burks, D.; Quesada, I.; Nadal, A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect. 2010, 118, 1243–1250. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Park, H. Association between urinary bisphenol A concentrations and type 2 diabetes in Korean adults: A population-based cross-sectional study. Int. J. Hyg. Environ. Health 2013, 216, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine metabolic disruptor. Ann. Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Marmugi, A.; Lasserre, F.; Beuzelin, D.; Ducheix, S.; Huc, L.; Polizzi, A.; Chetivaux, M.; Pineau, T.; Martin, P.; Guillou, H.; et al. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology 2014, 325, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Chitra, K.C.; Latchoumycandane, C.; Mathur, P.P. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 2003, 185. [Google Scholar] [CrossRef]
- Moon, M.K.; Kim, M.J.; Jung, I.K.; Koo, Y.D.; Ann, H.Y.; Lee, K.J.; Kim, S.H.; Yoon, Y.C.; Cho, B.J.; Park, K.S.; et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J. Korean Med. Sci. 2012, 27, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Tian, S.; Yan, J.; Jia, M.; Yan, S.; Li, R.; Zhang, R.; Zhu, W.; Zhou, Z. Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ. Pollut. 2019, 247, 935–943. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.N.; Zhang, L.; Peng, F.Y.; Jia, Y.; Wei, W.; Jia, L.H. Effect of Perinatal Bisphenol A Exposure on Serum Lipids and Lipid Enzymes in Offspring Rats of Different Sex. Biomed. Environ. Sci. 2016, 29, 686–689. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxid. Med. Cell. Longev. 2018, 3082438. [Google Scholar] [CrossRef]
- Cagen, S.Z.; Waechter, J.M., Jr.; Dimond, S.S.; Breslin, W.J.; Butala, J.H.; Jekat, F.W.; Joiner, R.L.; Shiotsuka, R.N.; Veenstra, G.E.; Harris, L.R. Normal reproductive organ development in CF-1 mice following prenatal exposure to bisphenol A. Toxicol Sci. 1999, 50, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, A.; Li, C.; Xin, F.; Duemler, A.; Li, W.; Rashid, C.; Bartolomei, M.S.; Simmons, R.A. Transgenerational effects of maternal bisphenol: A exposure on offspring metabolic health. J. Dev. Orig. Health Dis. 2019, 10, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Fujii, S.; Furukawa, M.; Kiguchi, M.; Ikka, T.; Harazono, A. Rat two-generation reproductive toxicity study of bisphenol A. Reprod. Toxicol. 2001, 15, 505–523. [Google Scholar] [CrossRef]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Arevalo, M.; Alonso-Magdalena, P.; Rebelo Dos Santos, J.; Quesada, I.; Carneiro, E.M.; Nadal, A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS ONE 2014, 9, e100214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manukyan, L.; Dunder, L.; Lind, P.M.; Bergsten, P.; Lejonklou, M.H. Developmental exposure to a very low dose of bisphenol A induces persistent islet insulin hypersecretion in Fischer 344 rat offspring. Environ. Res. 2019, 172, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Dabeer, S.; Afjal, M.A.; Ahmad, S.; Fatima, M.; Habib, H.; Parvez, S.; Raisuddin, S. Transgenerational effect of parental obesity and chronic parental bisphenol A exposure on hormonal profile and reproductive organs of preadolescent Wistar rats of F1 generation: A one-generation study. Hum. Exp. Toxicol. 2020, 39, 59–76. [Google Scholar] [CrossRef]
- Li, G.; Chang, H.; Xia, W.; Mao, Z.; Li, Y.; Xu, S. F0 maternal BPA exposure induced glucose intolerance of F2 generation through DNA methylation change in Gck. Toxicol. Lett. 2014, 228, 192–199. [Google Scholar] [CrossRef]
- Moustafa, G.G.; Ahmed, A.A.M. Impact of prenatal and postnatal exposure to bisphenol A on female rats in a two generational study: Genotoxic and immunohistochemical implications. Toxicol. Rep. 2016, 3, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Delclos, K.B.; Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; Latendresse, J.R.; Olson, G.R.; Davis, K.J.; Patton, R.E.; Gamboa da Costa, G.; Woodling, K.A.; et al. Toxicity Evaluation of Bisphenol A Administered by Gavage to Sprague Dawley Rats From Gestation Day 6 Through Postnatal Day 90. Toxicol. Sci. 2016, 153, 212. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Moro, G.; Abril, N.; Jara-Biedma, R.; Ramírez-Acosta, S.; Gómez-Ariza, J.L.; García-Barrera, T. Metabolic Impairments Caused by a “Chemical Cocktail” of DDE and Selenium in Mice Using Direct Infusion Triple Quadrupole Time-of-Flight and Gas Chromatography-Mass Spectrometry. Chem. Res. Toxicol. 2019, 32, 1940–1954. [Google Scholar] [CrossRef] [PubMed]

| F0 Initial Mean Weigh ± SD | Dose Group |
|---|---|
| 21.93 ± 3.26 | CONTROL |
| 23.20 ± 3.79 | 0.5 µg/Kg BW/day |
| 22.75 ± 3.25 | 2 µg/kg BW/day |
| 23.59 ± 3.66 | 4 µg/kg BW/day |
| 21.58 ± 2.74 | 50 µg/kg BW/day |
| 22.63 ± 3.48 | 100 µg/kg BW/day |
| Parental Generation | Dose Group | % Survival |
|---|---|---|
| F0 | Control | 100 |
| 0.5 µg/kg BW/day | 100 | |
| 2 µg/kg BW/day | 90 | |
| 4 µg/kg BW/day | 100 | |
| 50 µg/kg BW/day | 90 | |
| 100 µg/kg BW/day | 100 | |
| F1 | Control | 100 |
| 0.5 µg/kg BW/day | 93.3 | |
| 2 µg/kg BW/day | 100 | |
| 4 µg/kg BW/day | 100 | |
| 50 µg/kg BW/day | 91.6 | |
| 100 µg/kg BW/day | 88.8 | |
| F2 | Control | 100 |
| 0.5 µg/kg BW/day | 100 | |
| 2 µg/kg BW/day | 100 | |
| 4 µg/kg BW/day | 100 | |
| 50 µg/kg BW/day | 100 | |
| 100 µg/kg/d | 100 | |
| F3 | Control | 100 |
| 0.5 µg/kg BW/day | 90.9 | |
| 2 µg/kg BW/day | 100 | |
| 4 µg/kg BW/day | 100 | |
| 50 µg/kg BW/day | 100 | |
| 100 µg/kg BW/day | 100 |
| Generation | Dose Group | Mean% Males ± SD | Mean% Females ± SD | Sex Ratio |
|---|---|---|---|---|
| F0 | Control | 56.3 ± 0.088 | 43.7 ± 0.088 | 56:44 |
| 0.5 µg/kg BW/day | 47.9 ± 0.206 | 52.1 ± 0.206 | 48:52 | |
| 2 µg/kg BW/day | 50.0 ± 0.181 | 50.0 ± 0.181 | 50:50 | |
| 4 µg/kg BW/day | 70.8 ± 0.058 | 29.2 ± 0.589 | 71:29 | |
| 50 µg/kg BW/day | 44.4 ± 0.181 | 55.6 ± 0.181 | 44:56 | |
| 100 µg/kg BW/day | 75.0 ± 0.014 | 25.0 ± 0.111 | 75:25 | |
| F1 | Control | 55.0 ± 0.070 | 45.0 ± 0.070 | 55:45 |
| 0.5 µg/kg BW/day | 50.0 ± 0.101 | 50.0 ± 0.101 | 50:50 | |
| 2 µg/kg BW/day | 53.6 ± 0.050 | 46.4 ± 0.050 | 54:46 | |
| 4 µg/kg BW/day | 59.8 ± 0.037 | 40.2 ± 0.037 | 60:40 | |
| 50 µg/kg BW/day | 28.3 ± 0.164 | 71.7 ± 0.164 | 28:72 | |
| 100 µg/kg BW/day | 28.6 ± 0.404 | 71.4 ± 0.404 | 29:71 | |
| F2 | Control | 50.0 ± 0.303 | 50.0 ± 0.303 | 50:50 |
| 0.5 µg/kg BW/day | 45.0 ± 0.070 | 55.0 ± 0.070 | 45:55 | |
| 2 µg/kg BW/day | 63.1 ± 0.286 | 36.9 ± 0.286 | 63:37 | |
| 4 µg/kg BW/day | 57.1 ± 0.181 | 42.9 ± 0.181 | 57:43 | |
| 50 µg/kg BW/day | 24.3 ± 0.060 | 75.7 ± 0.060 | 24:76 | |
| 100 µg/kg BW/day | 66.0 ± 0.304 | 34.0 ± 0.304 | 66:34 | |
| F3 | Control | 46.4 ± 0.050 | 53.6 ± 0.050 | 46:54 |
| 0.5 µg/kg BW/day | 20.8 ± 0.058 | 79.2 ± 0.058 | 21:79 | |
| 2 µg/kg BW/day | 35.7 ± 0.101 | 64.3 ± 0.101 | 36:64 | |
| 4 µg/kg BW/day | 26.7 ± 0.094 | 73.3 ± 0.094 | 27:73 | |
| 50 µg/kg BW/day | 87.5 ± 0.176 | 12.5 ± 0.176 | 87:13 | |
| 100 µg/kg BW/day | 64.3 ± 0.303 | 35.7 ± 0.303 | 64:36 |
| Parental Group and Generation | Glucose | Triglycerides | Total Protein | Cholesterol | Creatinine | Albumin | Urea | Alkaline Phosphatase | |
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| F0 | Control | 261.175 ± 47.056 | 101.75 ± 11.814 | 57.200 ± 2.431 | 100.250 ± 14.407 | 0.375 ± 0.058 | 20.500 ± 1.290 | 46.450 ± 4.981 | 193.500 ± 44.003 |
| 0.5 µg/kg BW/day | 239.975 ± 15.462 | 92.25 ± 20.105 | 52.100 ± 1.283 | 84.500 ± 14.888 | 0.387 ± 0.027 | 19.000 ± 1.414 | 60.525 ± 18.717 | 321.000 ± 167.610 | |
| 2 µg/kg BW/day | 235.800 ± 64.924 | 95.50 ± 36.336 | 56.200 ± 3.844 | 96.000 ± 16.206 | 0.393 ± 0.055 | 21.000 ± 2.160 | 46.950 ± 6.289 | 448.750 ± 309.303 | |
| 4 µg/kg BW/day | 214.525 ± 20.475 | 110.500 ± 44.970 | 52.825 ± 1.203 | 111.500 ± 26.134 | 0.372 ± 0.036 | 19.250 ± 1.258 | 43.250 ± 5.952 | 378.250 ± 257.123 | |
| 50 µg/kg BW/day | 180.567 ± 20.296 | 114.00 ± 40.632 | 52.733 ± 3.600 | 112.888 ± 11.372 | 0.470 ± 0.040 | 20.000 ± 2.000 | 65.067 ± 34.184 | 442.000 ± 359.654 | |
| 100 µg/kg BW/day | 205.100 ± 35.522 | 80.750 ± 7.135 | 54.625 ± 1.858 | 95.250 ± 6.849 | 0.417 ± 0.032 | 19.000 ± 1.825 | 50.400 ± 14.886 | 427.000 ± 259.212 | |
| F1 | Control | 213.850 ± 16.205 | 103.000 ± 48.297 | 54.475 ± 6.367 | 84.000 ± 15.769 | 0.433 ± 0.095 | 21.000 ± 2.708 | 45.250 ± 10.883 | 176.500 ± 95.695 |
| 0.5 µg/kg BW/day | 234.200 ± 27.361 | 108.250 ± 39.601 | 57.625 ± 9.560 | 88.250 ± 17.613 | 0.412 ± 0.012 | 24.000 ± 5.354 | 47.225 ± 8.484 | 200.250 ± 151.856 | |
| 2 µg/kg BW/day | 174.675 ± 42.685 | 52.500 ± 10.661 | 53.875 ± 2.590 | 81.250 ± 29.341 | 0.415 ± 0.017 | 20.500 ± 1.732 | 45.738 ± 14.399 | 328.250 ± 75.177 | |
| 4 µg/kg BW/day | 231.550 ± 47.088 | 83.250 ± 40.729 | 53.175 ± 3.978 | 80.000 ± 29.040 | 0.375 ± 0.024 | 21.000 ± 2.708 | 42.225 ± 14.442 | 177.250 ± 102.024 | |
| 50 µg/kg BW/day | 263.825 ± 67.902 | 89.500 ± 28.687 | 54.650 ± 6.739 | 90.000 ± 17.907 | 0.377 ± 0.046 | 22.750 ± 2.629 | 47.088 ± 10.334 | 183.250 ± 37.295 | |
| 100 µg/kg BW/day | 244.725 ± 20.672 | 118.500 ± 38.613 | 52.775 ± 4.313 | 86.750 ± 22.706 | 0.405 ± 0.046 | 20.250 ± 0.957 | 50.735 ± 11.092 | 122.250 ± 28.052 | |
| F2 | Control | 236.200 ± 34.523 | 97.250 ± 20.287 | 54.725 ± 1.087 | 96.000 ± 8.286 | 0.450 ± 0.047 | 21.000 ± 0.816 | 46.088 ± 2.418 | 283.500 ± 51.137 |
| 0.5 µg/kg BW/day | 192.375 ± 10.665 | 84.250 ± 22.246 | 55.275 ± 3.472 | 100.250 ± 16.720 | 0.445 ± 0.026 | 21.000 ± 0.816 | 47.925 ± 4.742 | 277.750 ± 40.901 | |
| 2 µg/kg BW/day | 268.925 ± 21.731 | 129.250 ± 23.796 | 54.725 ± 3.694 | 97.500 ± 26.664 | 0.385 ± 0.031 | 21.000 ± 1.414 | 38.450 ± 0.967 | 215.500 ± 24.569 | |
| 4 µg/kg BW/day | 229.085 ± 24.832 | 51.250 ± 16.560 | 54.275 ± 3.551 | 78.750 ± 28.016 | 0.405 ± 0.024 | 21.750 ± 1.258 | 48.775 ± 7.511 | 319.750 ± 148.153 | |
| 50 µg/kg BW/day | 266.800 ± 26.006 | 76.250 ± 17.346 | 52.750 ± 1.234 | 99.000 ± 13.976 | 5.555 ± 10.290 | 22.000 ± 1.414 | 41.700 ± 6.810 | 253.500 ± 33.669 | |
| 100 µg/kg BW/day | 235.075 ± 33.947 | 65.000 ± 30.188 | 55.875 ± 3.099 | 98.500 ± 13.127 | 0.457 ± 0.046 | 16.500 ± 2.449 | 44.925 ± 5.573 | 456.250 ± 472.867 | |
| F3 | Control | 260.850 ± 37.989 | 138.750 ± 16.800 | 51.875 ± 3.703 | 96.750 ± 13.841 | 0.383 ± 0.032 | 20.000 ± 0.000 | 40.513 ± 5.559 | 211.750 ± 93.343 |
| 0.5 µg/kg BW/day | 326.100 ± 32.489 | 148.000 ± 77.816 | 52.375 ± 1.519 | 91.500 ± 11.000 | 0.360 ± 0.029 | 20.250 ± 0.500 | 39.438 ± 4.893 | 211.500 ± 60.467 | |
| 2 µg/kg BW/day | 162.725 ± 42.410 | 105.000 ± 28.425 | 47.175 ± 14.590 | 83.500 ± 19.052 | 0.445 ± 0.143 | 20.000 ± 6.055 | 41.575 ± 15.491 | 149.750 ± 40.111 | |
| 4 µg/kg BW/day | 286.400 ± 78.460 | 107.500 ± 26.501 | 53.200 ± 3.576 | 84.250 ± 26.600 | 0.377 ± 0.049 | 20.750 ± 1.500 | 43.650 ± 7.093 | 185.250 ± 31.063 | |
| 50 µg/kg BW/day | 248.450 ± 72.523 | 116.000 ± 23.338 | 52.875 ± 1.504 | 95.500 ± 17.635 | 0.380 ± 0.029 | 20.500 ± 0.577 | 38.662 ± 7.214 | 219.250 ± 44.798 | |
| 100 µg/kg BW/day | 324.675 ± 59.572 | 123.500 ± 23.187 | 51.275 ± 1.967 | 82.000 ± 19.866 | 0.358 ± 0.023 | 19.000 ± 0.816 | 28.413 ± 7.542 | 223.500 ± 34.317 | |
| Parental Generation | Dose Group | PND0 | PND3 | PND7 | PND14 | PND21 |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| F0 | Control | 1.5 ± 0.169 | 1.956 ± 0.470 | 3.914 ± 0.168 | 6.642 ± 0.172 | 9.318 ± 0.414 |
| 0.5 µg/kg BW/day | 1.39 ± 0.628 | 2.55 ± 0.287 | 3.91 ± 0.497 | 6.43 ± 0.761 | 9.546 ± 0.205 | |
| 2 µg/kg BW/day | 1.36 ± 0.127 | 1.47 ± 0.283 | 2.26 ± 0.332 | 5.805 ± 0.612 | 9.46 ± 0.547 | |
| 4 µg/kg BW/day | 1.406 ± 0.760 | 1.63 ± 0.086 | 3.023 ± 0.709 | 5.598 ± 1.242 | 9.24 ± 0.596 | |
| 50 µg/kg BW/day | 1.892 ± 0.127 | 2.72 ± 0.283 | 4.342 ± 0.332 | 5.899 ± 0.612 | 9.686 ± 0.547 | |
| 100 µg/kg BW/day | 1.549 ± 0.684 | 2.694 ± 0.125 | 4.151 ± 0.291 | 6.904 ± 1.027 | 10.13 ± 0.552 | |
| F1 | Control | 1.295 ± 0.100 | 1.805 ± 0.035 | 3.407 ± 0.038 | 6.296 ± 0.309 | 9.49 ± 0.427 |
| 0.5 µg/kg BW/day | 1.285 ± 0.039 | 1.751 ± 0.376 | 3.299 ± 0.568 | 6.173 ± 0.482 | 8.97 ± 1.126 | |
| 2 µg/kg BW/day | 1.317 ± 0.022 | 2.364 ± 0.283 | 4.278 ± 0.144 | 6.368 ± 0.313 | 8.91 ± 0.339 | |
| 4 µg/kg BW/day | 1.174 ± 0.159 | 1.594 ± 0.037 | 3.357 ± 0.284 | 6.354 ± 0.279 | 8.94 ± 0.531 | |
| 50 µg/kg BW/day | 1.308 ± 0.047 | 1.788 ± 0.130 | 3.64 ± 0.132 | 6.298 ± 0.271 | 8.86 ± 0.625 | |
| 100 µg/kg BW/day | 1.457 ± 0.230 | 1.764 ± 0.290 | 3.496 ± 0.245 | 6.43 ± 0.438 | 9.34 ± 0.874 | |
| F2 | Control | 1.482 ± 0.232 | 1.964 ± 0.656 | 4.201 ± 0.037 | 6.373 ± 0.309 | 8.634 ± 0.210 |
| 0.5 µg/kg BW/day | 1.64 ± 0.613 | 2.422 ± 0.543 | 4.162 ± 0.257 | 5.798 ± 0.441 | 7.69 ± 0.503 | |
| 2 µg/kg BW/day | 1.642 ± 0.247 | 1.903 ± 0.207 | 2.648 ± 0.438 | 5.862 ± 0.483 | 7.39 ± 0.606 | |
| 4 µg/kg BW/day | 1.487 ± 0.127 | 1.851 ± 0.283 | 4.463 ± 0.332 | 6.433 ± 0.612 | 9.03 ± 0.547 | |
| 50 µg/kg BW/day | 1.585 ± 0.024 | 2.39 ± 0.044 | 3.676 ± 0.653 | 7.479 ± 1.198 | 9.293 ± 0.402 | |
| 100 µg/kg BW/day | 1.501 ± 0.033 | 2.282 ± 0.516 | 4.288 ± 0.017 | 6.488 ± 0.158 | 8.37 ± 1.041 | |
| F3 | Control | 1.441 ± 0.180 | 1.905 ± 0.565 | 2.75 ± 0.155 | 4.603 ± 1.159 | 6.37 ± 0.366 |
| 0.5 µg/kg BW/day | 1.625 ± 0.261 | 1.898 ± 0.188 | 2.974 ± 0.036 | 5.918 ± 0.494 | 7.28 ± 0.535 | |
| 2 µg/kg BW/day | 1.329 ± 0.004 | 1.917 ± 0.072 | 3.719 ± 0.398 | 6.594 ± 0.152 | 7.42 ± 0.148 | |
| 4 µg/kg BW/day | 1.208 ± 0.022 | 1.663 ± 0.227 | 2.502 ± 0.033 | 5.446 ± 0.030 | 6.98 ± 0.256 | |
| 50 µg/kg BW/day | 1.273 ± 0.090 | 1.839 ± 0.067 | 2.639 ± 0.067 | 5.57 ± 0.127 | 6.74 ± 0.349 | |
| 100 µg/kg BW/day | 1.26 ± 0.157 | 1.684 ± 0.025 | 2.583 ± 0.266 | 5.819 ± 0.178 | 7.15 ± 0.167 |
| Offspring Group and Generation | Glucose | Tryglicerides | Total Protein | Cholesterol | Creatinine | Albumin | Urea | Alkaline Phosphatase | |
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| F1 | Control | 265.283 ± 31.796 | 130.665 ± 51.852 | 44.734 ± 16.027 | 85.83 ± 33.700 | 0.335 ± 0.495 | 18.33 ± 3.295 | 39.2 ± 11.455 | 407.15 ± 38.395 |
| 0.5 µg/kg BW/day | 277.95 ± 38.537 | 108.25 ± 24.274 | 45.785 ± 1.718 | 87.33 ± 10.366 | 0.375 ± 0.007 | 18.83 ± 0.240 | 43.35 ± 0.777 | 531.15 ± 21.001 | |
| 2 µg/kg BW/day | 243 ± 1.414 | 106 ± 11.313 | 42.5 ± 3.535 | 91.1 ± 1.555 | 0.325 ± 0.035 | 19 ± 1.414 | 40.6 ± 11.313 | 492 ± 9.899 | |
| 4 µg/kg BW/day | 335.25 ± 42.431 | 138.5 ± 30.712 | 47.5 ± 5.280 | 77 ± 19.413 | 0.37 ± 1.870 | 20.5 ± 2.300 | 36.1 ± 10.381 | 448.5 ± 157.867 | |
| 50 µg/kg BW/day | 203.967 ± 47.988 | 93.915 ± 39.011 | 40.04 ± 8.640 | 75.665 ± 33.000 | 0.315 ± 0.035 | 17 ± 2.828 | 46.3 ± 0.89 | 411.75 ± 18.031 | |
| 100 µg/kgBW/day | 309.5 ± 42.431 | 118.5 ± 30.712 | 70.35 ± 5.280 | 110.75 ± 19.413 | 0.49 ± 1.870 | 34.5 ± 2.300 | 49.42 ± 10.381 | 335 ± 157.867 | |
| F2 | Control | 240.05 ± 16.334 | 92 ± 16.970 | 45.2 ± 6.788 | 95 ± 8.899 | 0.325 ± 0.007 | 20 ± 0.000 | 37.1 ± 1.979 | 624 ± 50.911 |
| 0.5 µg/kg BW/day | 235 ± 8.202 | 90.5 ± 4.949 | 52.15 ± 3.323 | 95.5 ± 6.363 | 0.355 ± 0.021 | 22 ± 1.414 | 41.4 ± 7.778 | 583.15 ± 15.344 | |
| 2 µg/kg BW/day | 235.5 ± 7.778 | 97.5 ± 16.263 | 47.6 ± 1.979 | 84.3 ± 4.666 | 0.32 ± 0.028 | 20 ± 1.414 | 34.05 ± 3.747 | 552.5 ± 4.949 | |
| 4 µg/kg BW/day | 204.8 ± 14.142 | 75 ± 1.414 | 55.5 ± 0.424 | 95.5 ± 6.363 | 0.39 ± 1.870 | 24 ± 0.000 | 52.3 ± 4.666 | 731.5 ± 54.447 | |
| 50 µg/kg BW/day | 248.4 ± 20.364 | 74 ± 2.828 | 45 ± 7.071 | 81.5 ± 9.192 | 0.37 ± 0.084 | 18.5 ± 3.535 | 34.35 ± 6.717 | 548.5 ± 173.241 | |
| 100 µg/kg BW/day | 214.25 ± 90.014 | 64 ± 36.769 | 53.55 ± 2.899 | 79.5 ± 24.748 | 0.37 ± 0.183 | 16.5 ± 7.778 | 33.55 ± 16.051 | 557 ± 200.818 | |
| F3 | Control | 325.95 ± 41.224 | 71 ± 25.455 | 51.8 ± 5.515 | 100 ± 33.941 | 0.37 ± 0.042 | 21.5 ± 2.121 | 35.55 ± 4.454 | 707.5 ± 127.986 |
| 0.5 µg/kg BW/day | 301.9 ± 21.920 | 55 ± 5.656 | 46.65 ± 1.767 | 90.5 ± 10.606 | 0.38 ± 0.042 | 19 ± 0.000 | 33.9 ± 2.262 | 795.5 ± 75.660 | |
| 2 µg/kg BW/day | 268.5 ± 13.435 | 88.5 ± 41.719 | 47.95 ± 3.747 | 83.5 ± 4.949 | 0.34 ± 0.028 | 19 ± 1.414 | 39.9 ± 13.010 | 695.5 ± 164.755 | |
| 4 µg/kg BW/day | 311.9 ± 42.431 | 94 ± 30.712 | 51.8 ± 5.280 | 98 ± 19.413 | 0.54 ± 1.870 | 21 ± 2.300 | 43 ± 10.381 | 579 ± 157.867 | |
| 50 µg/kg BW/day | 236.3 ± 42.431 | 74 ± 30.712 | 48 ± 5.280 | 90 ± 19.413 | 0.23 ± 0.323 | 20 ± 2.300 | 32.8 ± 10.381 | 802 ± 157.867 | |
| 100 µg/kg BW/day | 160.75 ± 128.622 | 31.5 ± 23.334 | 24.2 ± 18.384 | 49 ± 41.012 | 0.23 ± 1.870 | 15 ± 0.000 | 20.25 ± 4.313 | 473.5 ± 324.562 | |
| F4 | Control | 202.15 ± 4.454 | 131 ± 26.870 | 48.3 ± 2.262 | 78.5 ± 17.677 | 0.39 ± 0.056 | 19.5 ± 0.707 | 49.3 ± 0.565 | 740 ± 2.828 |
| 0.5 µg/kg BW/day | 271.05 ± 5.161 | 136.5 ± 10.606 | 52.05 ± 0.494 | 88 ± 16.970 | 0.37 ± 0.014 | 21.5 ± 0.707 | 46.7 ± 1.838 | 563.5 ± 21.920 | |
| 2 µg/kg BW/day | 241.45 ± 20.011 | 82.5 ± 0.707 | 41.75 ± 1.343 | 73.5 ± 9.192 | 0.34 ± 0.042 | 18 ± 1.414 | 29.95 ± 2.757 | 1049.5 ± 27.577 | |
| 4 µg/kg BW/day | 253.2 ± 4.949 | 113 ± 0.000 | 52.45 ± 1.626 | 72.5 ± 6.363 | 0.385 ± 0.007 | 22 ± 0.000 | 38 ± 3.959 | 579.5 ± 43.133 | |
| 50 µg/kg BW/day | 285.9 ± 36.628 | 101.5 ± 14.849 | 49 ± 1.272 | 66.5 ± 14.849 | 0.4 ± 0.014 | 20 ± 0.000 | 37.8 ± 8.343 | 532 ± 9.899 | |
| 100 µg/kg BW/day | 236.5 ± 51.123 | 125 ± 14.142 | 53.75 ± 3.889 | 79.5 ± 20.506 | 0.415 ± 0.007 | 21.5 ± 2.121 | 44.05 ± 10.960 | 461 ± 128.693 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujalance-Reyes, F.; Molina-López, A.M.; Ayala-Soldado, N.; Lora-Benitez, A.; Mora-Medina, R.; Moyano-Salvago, R. Analysis of Indirect Biomarkers of Effect after Exposure to Low Doses of Bisphenol A in a Study of Successive Generations of Mice. Animals 2022, 12, 300. https://doi.org/10.3390/ani12030300
Bujalance-Reyes F, Molina-López AM, Ayala-Soldado N, Lora-Benitez A, Mora-Medina R, Moyano-Salvago R. Analysis of Indirect Biomarkers of Effect after Exposure to Low Doses of Bisphenol A in a Study of Successive Generations of Mice. Animals. 2022; 12(3):300. https://doi.org/10.3390/ani12030300
Chicago/Turabian StyleBujalance-Reyes, Francisca, Ana M. Molina-López, Nahúm Ayala-Soldado, Antonio Lora-Benitez, Rafael Mora-Medina, and Rosario Moyano-Salvago. 2022. "Analysis of Indirect Biomarkers of Effect after Exposure to Low Doses of Bisphenol A in a Study of Successive Generations of Mice" Animals 12, no. 3: 300. https://doi.org/10.3390/ani12030300
APA StyleBujalance-Reyes, F., Molina-López, A. M., Ayala-Soldado, N., Lora-Benitez, A., Mora-Medina, R., & Moyano-Salvago, R. (2022). Analysis of Indirect Biomarkers of Effect after Exposure to Low Doses of Bisphenol A in a Study of Successive Generations of Mice. Animals, 12(3), 300. https://doi.org/10.3390/ani12030300







