Simple Summary
Locomotor training (LT) is a main strategy for functional neurorehabilitation that can promote stepping relearning, leading to a coordinated and modulated ambulation pattern, and can be applied to a wide range of neurological diseases, namely after spinal cord injury. This review article offers a neurophysiological explanation and an overview of current evidence on LT. Additionally, it provides the knowledge on how to perform this exercise through land and underwater treadmill training with primary guidelines and feasible protocols for small animals that may be meaningful on a day-to-day basis for implementation in the veterinary setting.
Abstract
Neurorehabilitation has a wide range of therapies to achieve neural regeneration, reorganization, and repair (e.g., axon regeneration, remyelination, and restoration of spinal circuits and networks) to achieve ambulation for dogs and cats, especially for grade 1 (modified Frankel scale) with signs of spinal shock or grade 0 (deep pain negative), similar to humans classified with ASIA A lesions. This review aims to explain what locomotor training is, its importance, its feasibility within a clinical setting, and some possible protocols for motor recovery, achieving ambulation with coordinated and modulated movements. In addition, it cites some of the primary key points that must be present in the daily lives of veterinarians or rehabilitation nurses. These can be the guidelines to improve this exciting exercise necessary to achieve ambulation with quality of life. However, more research is essential in the future years.
1. Introduction
Functional neurorehabilitation (FNR) is based on neuroanatomy and neurophysiology principles, allowing the development of intensive protocols that are implemented in human medicine [1]. It is mandatory to understand the function of spinal cord interneural circuits and the neural mechanisms necessary for locomotion control, which is the only way to support the progress of new targeted locomotor rehabilitation strategies. Thus, the physiological knowledge in animals and humans allows the optimization and evolution of neurorehabilitation protocols [1,2].
The evidence of signal transmission through a spinal cord injury (SCI), both caudally and rostrally to it, was the starting point for continuous stimulation of the remained and residual central axons pathways [3,4,5]. Experiments in animal models, such as cats with transected spinal cord, showed that treadmill training was effective to promote gate restoration [6]. Thus, functional training may promote the reorganization of neural locomotor networks after the loss of motor ability and following neuronal injury [2,7].
One of the main strategies for this is locomotor training (LT) because it could promote multi-stimulation from different manners, remaining the question of how to perform this training in a beneficial and safe way. An accurate LT may promote postural control and postural standing ability (assisted or not) by a neuromuscular organization that depends on spinal cord changes, usually biochemical alterations [8,9].
These exercises allow the dynamic interaction between afferent inputs from all functioning receptors, such as proprioceptive and biomechanical, located on: the intrafusal fibers of the muscle and the Golgi tendinous organ (muscle fibers group Ia and Ib) [10]; hip joint (mechanoreceptors); other joints and skin (stretch and sensitive mechanoreceptors) [11].
The LT recruits and adjusts the number of motoneurons that generate rhythmic movements and, at the same time, stimulates the peripheral receptors that provide nearly 30% of motor output [1].
The central pattern generators (CPG), located in the lumbar segments of the spinal cord [12,13,14,15], may be triggered by external stimuli provided by the treadmill belt movement, which is a common exercise performed in complete SCI dogs, cats, and rats to achieve normal stepping [6,16,17,18,19,20].
Therefore, one tool that provides this type of exercise is treadmill training, which allows stepping relearning, depending on the exercise’s practice. The performance success is highly variable, a fundamental feature of the neural movement control [21] based on plasticity improvement on spinal neuronal circuits, already established in different studies with “complete” SCI cats [6,21,22,23,24,25].
Those quadrupedal animals exhibited clear evidence that the spinal network, grounded on the CPG and motor interneurons, may be the start of generating locomotor rhythm [26]. Moreover, in human medicine, active assisted movements are studied concerning their potential benefit on motor control and spasticity [27].
Thus, treadmill LT allows the neuronal mechanism stimulation of the spinal segments caudally to the injury by stimulus of serotonin and/or noradrenergic pathways, promoting neural plasticity. This phenomenon potentiates spinal reflex excitability, which in part relies on glutamate action at N-methyl-d-aspartate (NDMA) receptors and brain-derived neurotrophic factor acting at TrKB receptors, possibly modulated by the treadmill training [28].
The main objective of this review was to obtain a neurophysiological explanation for locomotor training. In addition, address the applicability and primary guidelines for the clinical setting. Finally, accomplish protocols for possible motor recovery with coordinated and modulated movements necessary for ambulation.
1.1. Body-Weight-Supported Treadmill Training
Body-weight supported treadmill training (BWSTT), which usually refers to 60% to 80% of supported weight, is a method that allows activity-dependent plasticity enhancement and the residual pathways and networks [29,30,31,32]. In particular, the BWSTT aims to improve coordination and ambulation, focusing on the lumbar locomotor CPG circuits [8,33,34,35,36,37,38].
In human patients, these BWSTT exercises have been shown to reduce spasticity by replacing abnormal hyper-excitable sensory firing with functional afferent signaling, which decreases muscle spasm and co-contraction [39,40]. Moreover, the LT repetitive movements potentially improve glutamatergic dorsal root ganglia (DRG) sensory feedback, contributing to the primary extrinsic source stimulation and entering the spinal cord below the injury to engage the local circuits, mechanisms already evidenced in animal models [41].
1.2. Proprioceptive Sensory System
It is essential to consider the muscle spindle work, which is innervated by proprioceptive sensory neurons and conducts the muscle contraction information to the spinal cord. They are the proprioceptors that exhibit the most widespread central projection of all DRG sensory neurons and establish synaptic connections with motor neurons and interneurons of the intrinsic circuits [42]. Therefore, the muscle spindle afferent is mainly the key to the direct excitation of spinal circuits needed for motor control upregulation, mostly under disconnected descending input situations [43].
The cholinergic propriospinal cells significant for the CPG control and the coordinated locomotor output may be stimulated by the LT, contributing to the recovery of locomotion in the absence of descending locomotor control [44]. Thus, descending propriospinal neurons may provide neural networks excitation, regardless of supraspinal control. This activation of interneural networks could potentially promote locomotor coordination and is an effective strategy for stepping recovery [45,46] (Table 1).

Table 1.
Key point n° 1—The contribution of the proprioceptive system for locomotor training.
2. When to Perform the Locomotor Training?
The time to initiate LT is also of primary concern. Early treadmill training after severe SCI has been shown to promote the possibility of an increase in muscle electromyography signal and modulate activity over the step cycle, similar to healthy human patients. Furthermore, surface electromyography findings are shown to correlate with motor recovery [47,48].
There is a diminished noradrenergic input to neural networks in SCI, decreasing signal amplitude [49]. Moreover, dendritic persistent inward currents (PICs) and the motoneuron dendrites activation [50,51,52,53] may be restored with monoamines, which are released during exercise and have several effects on motoneurons and the PICs [54,55].
Post-operative rehabilitation may be safely used in the first 24 h after surgery, provided it does not worsen spinal hyperesthesia or decrease the neurological status [19,56,57]. Also, moderate-level evidence research has supported the implementation of LT within the first three days after surgery, in contrast with activity restriction (e.g., cage rest) that was supported by low-level evidence, demonstrating that this restriction does not mean annulment of LT and rehabilitation exercises [56] (Table 2).

Table 2.
Key point n° 2—Importance of early implementation of locomotor training.
Thus, automated locomotor training is an essential tool already implemented in human patients with severe motor impairment. The choice of the device to assist training depends on the sensorimotor deficits and the cardiopulmonary restrictions [58], leading to a bodyweight-bearing training and a high repetitive rhythmic pattern with the help of a body harness [59]. In SCI human patients, this exercise can be performed through manual assistance by therapists or, mostly in cases of severe deficits and cardiorespiratory restrains, continued by robotic exoskeleton assistance or robotic assisted treadmill training (e.g., Lokomat®) [60,61]. On the other hand, in the veterinary field, spinalized animals presented good results when performing treadmill training [18,19,20,62,63], also in comparison with walking on different floors [64]. The alternative to robotic assistance in small animals that require body weight support has been the use of continuous wheelchair training, or in heavier dogs through a passive standing device above the treadmill [62].
Long-term locomotor training has been correlated to promote bone and muscle mass increase along with positive cardiovascular effects [45,65,66,67,68], intralimb hip-knee coordination [69], activation of propriospinal pathways that mediate interlimb coordination [70], improve muscle force output and endurance [71].
Repetitions Number
The number of repetitions and the functional consequences of locomotor training on neuronal plasticity is motor learning principles that must be implemented to increase performance [8] (Table 3).

Table 3.
Key point n° 3—Exercise repetitions contribution to locomotor training.
Research studies have shown that most known protocols, particularly 82%, are based on one daily training session [72,73,74,75,76,77], and only 18% were based on two to three daily sessions [78,79,80]. In the same studies, the duration of each session may vary, from 5–15 min (53%) to 20–30 min (47%), and the most frequent exercise implemented was treadmill training (33%), followed by the BWSTT (17%). The total rehabilitation time varies, from 1–36 weeks, with 15 studies reporting training five days/week [73,77,81,82,83,84]. The specific amount of time needed for standing or stepping also depends on each subject’s level of exercise-induced fatigue [67].
In translation for small animals, there are few reports of rehabilitation sessions manly with underwater treadmill locomotor training [85,86]. Recently some studies have been published describing locomotor exercises on land and underwater treadmill, in acute and chronic intervertebral disc disease (IVDD) dogs [19,20], SCI contusion cats, cervical IVDD dogs and in acute non-compressive nucleus pulposus extrusion (ANNPE) dogs [62,63].
In human patients, intensive rehabilitation therapy was performed five times/week for 90 min sessions, with step training for a minimum of 20 min and long-term follow-up evaluations for 6–12 months [9]. After intensive training, motor recovery in incomplete motor SCI patients was registered within the first two months of rehabilitation [87].
3. How to Perform the Locomotor Training?
The locomotor training implementation depends on the cause of the problem and has to consider the strict criteria of each situation. For example, in rehabilitation following SCI, it is mandatory to ensure stabilization of the spinal cord, performing the exercises without oscillations of the vertebral column.
Thus, the application of treadmill training is based on the possibility of guaranteeing the step cycle repetition in an easier and faster way. If the patient has monoplegia/monoparesis, paraplegia/paraparesis, or tetraplegia/tetraparesis, the adaptation to the treadmill surface is necessary to obtain voluntary or automatic movement. For that, the surrounding environment must be calm and quiet with classical music [19,20,62,63,88], but also with enough energy to promote motivation to start the step cycle, autonomously or with assisted exercises, such as the established bicycle movements, that may require perineal or tail stimulation [18,64,89].
Regarding bicycle movements, and in the case of hypotonic muscles, they should be performed with stretching limbs and vigorous stimulation of cutaneous afferent receptors on the treadmill surface [63]. However, if the dog or cat demonstrates hyperreflexia and increased muscle tonus, bicycle movements have to be smoother, and it is not advised the stretching of intrafusal fibers group Ia/Ib.
Locomotor Exercises
For neurologic dogs after SCI, the LT initiation may start with passive kinesiotherapy exercises for adaptation to the treadmill belt, essentially in deep pain negative (DPN) patients, resorting to the BWSTT step training (Figure 1). Next, on the bipedal treadmill training, the forelimbs remain stationary on a fixed platform above the belt [89]. At the same time, the perineal area is stimulated by suspending and crimping the tail or with assisted bicycle hindlimbs movements [5,19] (Figure 1A).

Figure 1.
Land treadmill bipedal step training on a cat (A) and quadrupedal step training on a dog (B).
For each training session, variables such as the walking speed and duration may start from 0.8 km/h (0.5 mph) to a maximum of 1.9 km/h (1.2 mph) [71,90,91], over 5 min (4–6 times/day, six days/week), until achieving 20 min (2 times/day, six days/week) [92] (Table 4).

Table 4.
Locomotor training protocols on the land treadmill and underwater treadmill according to etiology.
After quadrupedal training starts, patients may receive similar stimulation regarding speed and frequency, aiming to achieve 30–40 min (2 to 3 times/day, six days/week) [93]. Moreover, the treadmill slope should be elevated from 10° [70] to 25° [42] to encourage forelimb–hindlimb coordination [94] (Figure 1B).
Patients should perform quadrupedal training even in an early stage for complete stimulation, mainly of the residual descending pathways. This training allows a possibility of greater stimulation of the propriospinal system and the brain–brain stem-pelvic limb loop stimulation [18].
The previous guidelines should be implemented in patients with acute post-surgical compressive myelopathy, where patients should be managed with stabilization of the vertebral column and minimum oscillations. The standard protocol is the same for the same type of patient but in a chronic situation. However, the primary kind of exercise should be quadrupedal-step training.
Furthermore, the underwater treadmill (UWTM) (Figure 2) training could be applied from the second day of admission, with water temperature ~26 °C [95], beginning in 5 min until one hour per day (5 days/week) and speed from 1 km/h (0.28 m/s) to 3.5 km/h (0.97 m/s) [49,96], always looking for signs of overtraining. With regard to the UWTM, patients should have a rest of 48 h, due to the increase of volume of oxygen (VO2), heart and respiratory effort, and body energy consumption, which is higher compared to walking on land [97].

Figure 2.
Underwater treadmill quadrupedal training on a dog.
In chronic or acute SCI patients, LT performed on the UWTM should be initiated as early as possible. Different protocols are described in Table 4, considering each disease, post-operative or conservative management option and disease progression stage [19,56]. All guidelines are dependent on the cardiovascular and motor ability of each patient, and training should not be performed without monitorization of vital parameters (mucous membranes; capillary refill time, heart rate, respiratory rate; blood pressure, electrocardiogram, muscle fasciculations, spinal hyperesthesia and neurological grade), especially in intensive exercise with increasing time, speed, and slope (Figure 3). The same approach could be applied in patients with degenerative myelopathy and geriatric vestibular syndrome.

Figure 3.
Monitorization of a dog after the performance of locomotor training.
The many variables associated with the training protocol implementing exercise to any patient a challenge, differences in intensity, duration, pattern and frequency of training sessions, and the time post-injury when the exercise begins are of great importance [98].
4. Muscle Fatigue
Remple and colleagues (2001) [99] suggested that LT has the potential to induce changes in neural function within the spinal cord, promoting increased motor unit recruitment and resistance training, which increases excitability potential [100,101] and recruitment of spinal motor neurons [101,102,103].
In human patients, motor training was increased over six weeks until stimulation could be maintained for 30 min, similar to what may be achieved in dogs and cats, except adding 1 kg of weight bearing once the 30 min could be performed without fatigue at a given resistance [104]. The effects of leg weights, when adding 1% to 2% of the dog’s total body weight depending on the limb strength and recovery stage, at the level of the carpus/tarsus, are related to the muscle activity increase in the back muscles, promoting stabilization of the vertebral column, particularly when the contralateral limb is in the swing phase [105,106,107]. These types of eccentric exercises may also reduce intracortical inhibition and increase corticospinal excitability by 37–51% [108].
Moreover, in humans, fatigue resistance has been shown to improve significantly after only three months of exercising, in agreement with other studies [109,110,111,112], probably due to the LT contribution in improving oxidative and glycolytic enzymes metabolism [113,114] (Figure 4A,B).
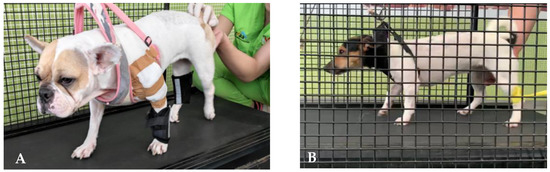
Figure 4.
Land treadmill training in an SCI dog with the addition of weight-bearing pads (A) and with thera-band resistance (B).
Thus, it is likely that the longer the daily training duration, the more fatigue resistance improves, achieving better results than previously reported, which could be associated with a type II to type I fiber conversion [104]. To Harness and colleagues (2008) [115], this type of approach was an intervention that might be useful to improve impairment and disability after SCI as a primary or adjuvant treatment [116,117]. Furthermore, some studies in dogs have reported the success on using the treadmill running as a modality of choice [118,119,120,121].
Dogs and cats that are DPN are the primary targets for this treatment strategy (Table 5), which may increase the level of motor pool activation and promote modulation [122] by performing steps backwards when the treadmill belt direction is reversed [123] (Figure 5A).

Table 5.
Key point n° 4—Locomotor training in deep pain positive and deep pain negative patients.

Figure 5.
Land treadmill training with a dog performing steps backwards (A) and stepping over obstacles (B).
One other training exercise that can be introduced is based on walking stimulation on a land treadmill with step-over obstacles attached to the belt (Figure 5B). This approach was previously presented in a series of experiments in cats with severe motor control deficits, suggesting that both corticospinal and rubrospinal pathways were damaged, and supported by the fact that the properties of neurons in both the motor cortex and red nucleus are compatible with modifications of gait, which are needed to step over obstacles [124].
Furthermore, human medicine research based on constraint-induced or forced-use training and treadmill training, with a particular interest in stroke and SCI patients, has allowed the development of a new field of rehabilitative restoration strategies that goes beyond task-specific training to include optimization of functional recovery through mechanisms of plasticity and regeneration [125].
5. Anti-Inflammatory Role
Locomotor training for motor recovery had a growing consensus among neuroscientists that plasticity and regeneration are not limited to the acute phase of injury and may also occur through the chronic phase [126,127]. The inflammatory process increases the release of nitric oxide due to the physiological role of neuronal nitric oxide synthase (nNOS) and endothelial NOS (eNOS), which produces nitric oxide following Ca2+ influx [128], causing tissue damage. Thus, a decrease in the inflammatory phase may contribute to more regeneration.
Treadmill exercises exerted their beneficial effects via a significant reduction of C-reactive protein and antioxidant ability [129,130], allowing a decrease in inflammation and restoration of anti-nociceptive inhibitory process, which suggests LT as a powerful exercise for management of neuropathic pain. There are evidence that the secondary anatomical and physical alterations may decrease inflammatory response and increase neutrophin levels, promoting neural regeneration [131]. In rats, it is hypothesized that early moderated exercise may be a therapeutic strategy for non-brain circulation, neuro-inflammation, and astrocytic coverage of brain vessels [132].
Moreover, the inflammatory disorder engages macrophages and/or perivascular cells that seem to play an essential role in producing nitric oxide via the inducible (iNOS) gene, expressed by microglial cells, increasing cytotoxic effect that was previously reported in association with the spinal cord cavitation phenomenon [128]. In the chronic phase, initial tissue necrosis develops into cavity formation, axonotomy, axonal demyelination, glial activation, and scarring [133].
Consequently, LT enhances the increase of M2 macrophages that promote angiogenesis [134,135,136] and matrix remodeling while suppressing destructive immunity, promoting functional recovery after SCI [137] depending on the volume of such cavities that can be estimated with 3D imaging [133,138]. In addition, the metabolic ability of muscle changes, measured by oxidative enzyme activity and concentrations of Na+/K+ ATPase, improves with LT, reducing fatigability after SCI in humans and rats. Thus, LT with or without electrical stimulation is likely required to improve muscle endurance via increasing muscle oxidative ability [114].
Findings also report the favorable effect of exercise in reducing the risk of neuroinflammatory disorders [139] possibly linked to the decrease of anti-inflammatory cytokines, such as IL1β, IL6, and TNFα [140], and adipokines via the muscle-adipose crosstalk [130,141,142].
LT is an example of intensive training based on PICs and voltage-sensitive modulation. The PICs require concomitant activation of serotonergic (5-HT) and noradrenergic (NA) receptors located on the motoneurons, which are modulated by 5-HT (2B/C) receptors specifically. The role of 5-HT and NA receptors in facilitating motoneurons PICs was first demonstrated in the decerebrate cat [143], but further studies are needed.
6. Regenerative Role
There are evidence that anatomical and physical changes may occur after LT, promoting neural regeneration [131]. Several studies in complete/incomplete SCI cats have shown step training on the treadmill [23,144,145] and a consequent increase in neurotrophin delivery [131].
Within the neurotrophins, the brain-derived neurotrophic factor (BDNF) plays a vital role in reorganizing the central nervous system as a potent neuromodulator for neural and nociceptive regeneration [59,146]. Moreover, the nerve growth factor (NGF), neurotrophin–3 (NT-3), and neurotrophin-4 (NT-4) are crucial for axonal growth and the remyelination of demyelinated axons, aiming to improve signal conduction across the injury site. Thus, LT may contribute to the disinhibition of the descending motor pathways inhibited with SCI, stimulating the pre-motor neuronal control and promoting intralimb and interlimb coordination [146,147,148].
Repeated cyclic exercises over time, every day and for several weeks, have been described to promote spinal reflex locomotion [18,19,20,149]. This approach allows plasticity in the reflex pathways [42,150], and it is known that limited residual descending inputs may be needed for significant functional improvements [9]. Therefore, motor training with a monotonous repetition of the same sensorimotor exercise could result in spinal learning and memorization [30].
Locomotor training helps to activate the substantial population of long ascending and descending propriospinal interneurons that connects the cervical and lumbar enlargements via the ventrolateral funiculus, contributing significantly to the neural coupling between cervical and lumbar spinal segments [1,46]; further research is needed.
For LT monitoring, it should be essential to measure the biomarkers concentrations, such as the glial fibrillary acid protein (GFAP) and phosphorylated neurofilament heavy chain (pNFH) [19,46,151], at the beginning of protocol and throughout the outcomes evaluations, which would allow early identification of progressive neuronal damage [19,152].
7. Conclusions
For Barrett et al. (2013) [153], neurorehabilitation has a wide range of treatments to achieve neural regeneration, repair, and dynamic reorganization of functional neural systems based on learning experience and neurophysiological stimulation. LT is essential to successful neurorehabilitation for veterinarians, nurses, and technicians, with sharp differences in evolution that are felt or observed every day/week. Thus, the performance of the LT should be different each day, with significant changes in gait patterns that can be observed in just one session. Research must be continued and staff education is necessary, promoting LT as an exciting, innovative, and not boring exercise.
In addition, early intensive LT should be associated with multimodal modalities [18,19,20,63,85,88,123] in all neurological dogs and cats with or without pain perception after surgery or with conservative management, maintaining the stability of the SC. This early and intensive multimodal training can be applied across a diverse range of neural diseases, promoting research for years to come.
Author Contributions
Conceptualization, Â.M.; methodology D.G.; validation Â.M. and A.F.; formal analysis, Â.M. and A.F.; investigation D.G., A.C., C.C., Ó.G. and A.A. writing—original draft preparation, D.G., A.C. and C.C.; writing—review and editing Â.M. and A.F.; supervision Â.M.; project administration Â.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Côté, M.P.; Murray, L.M.; Knikou, M. Spinal Control of locomotion: Individual neurons, their circuits and functions. Front. Physiol. 2018, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Shmuelof, L.; Huang, V.S.; Haith, A.M.; Delnicki, R.J.; Mazzoni, P.; Krakauer, J.W. Overcoming motor “forgetting” through reinformcement of learned actions. J. Neurosci. 2012, 32, 14617–14621. [Google Scholar] [CrossRef]
- Tansey, K.E. Neural plasticity and locomotor recovery after spinal cord injury. PM R 2010, 2, 220–226. [Google Scholar]
- Dimitrijevic, M. Residual motor function after spinal cord injury. In Restorative Neurology of Spinal Cord Injury; Dimitrijevic, M., Kakulas, B., McKay, W., Vrbová, G., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 1–9. [Google Scholar]
- Kakulas, B.A.; Kaelan, C. The neuropathological foundations for the restorative neurology of spinal cord injury. Clin. Neurol. Neurosurg. 2015, 129, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, H.; Rossignol, S. Recovery of locomotion after chronic spinalisation in the adult cat. Brain Res. 1987, 412, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; van den Brand, R.; Roy, R.R.; Edgerton, V.R. Multisystem neurorehabilitation in rodents with spinal cord injury. In Neurorehabilitation Technology, 2nd ed.; Reinkensmeyer, D.J., Dietz, V., Eds.; Springer: Cham, Switzerland, 2016; pp. 59–79. [Google Scholar]
- Smith, A.C.; Knikou, M. A review on locomotor training after spinal cord injury: Reorganisation of spinal neuronal circuits and recovery of motor function. Neural. Plast. 2016, 1216258, 1–20. [Google Scholar] [CrossRef]
- Harkema, S.J.; Schmidt-Read, M.; Lorenz, D.J.; Edgerton, V.R.; Behrman, A.L. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys. Med. Rehabil. 2012, 93, 1508–1517. [Google Scholar] [CrossRef]
- Rossignol, S.; Frigon, A. Recovery of locomotion after spinal cord injury: Some facts and mechanisms. Annu. Rev. Neurosci. 2011, 34, 413–440. [Google Scholar] [CrossRef]
- Bouyer, L.J.G.; Rossignol, S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion in intact cats. J Neurophysiol. 2003, 90, 3625–3639. [Google Scholar] [CrossRef]
- Brown, T.G. On The Nature of The Fundamental Activity of The Nervous Centers; Together With an Analysis of The Conditioning of Rhythmic Activity in Progression, and a Theory of The Evolution of Functional in The Nervous System. J. Physiol. 1914, 48, 18–46. [Google Scholar] [CrossRef]
- Grillner, S.; Zangger, P. On the Central Generation of Locomotion in the Low Spinal Cat. Exp. Brain Res. 1979, 34, 241–261. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Wallen, P. Central pattern generators for locomotion, with special reference to vertebrates. Ann. Rev. Neurosci. 1985, 8, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Dubuc, R. Control of locomotion in vertebrates: Spinal and supraspinal mechanisms. Adv. Neurol. 1988, 47, 425–453. [Google Scholar] [PubMed]
- Robinson, G.A.; Goldberger, M.E. The development and recovery of motor function in spinal cats, I: The infant lesion effect. Exp. Brain Res. 1986, 62, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Howland, D.R.; Bregman, B.S.; Tessler, A.; Goldberger, M.E. Development of locomotor behaviour in the spinal kitten. Exp. Neurol. 1995, 135, 108–122. [Google Scholar] [CrossRef]
- Martins, Â.; Silva, C.; Gouveia, D.; Cardoso, A.; Coelho, T.; Gamboa, Ó.; Marcelino, E.; Ferreira, A. Spinal Locomotion in Cats Following Spinal Cord Injury: A Prospective Study. Animals 2021, 11, 1994. [Google Scholar] [CrossRef]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Carvalho, C.; Coelho, T.; Silva, C.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A controlled clinical study of intensive neurorehabilitation in post-surgical dogs with severe acute intervertebral disc extrusion. Animals 2021, 11, 3034. [Google Scholar] [CrossRef]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Carvalho, C.; Silva, C.; Coelho, T.; Gamboa, Ó.; Ferreira, A. Functional neurorehabilitation in dogs with an incomplete recovery 3 months following intervertebral disc surgery: A case series. Animals 2021, 11, 2442. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Courtine, G.; Gerasimenko, Y.P.; Lavrov, I.; Ichiyama, R.M.; Fong, A.J.; Cai, L.L.; Otoshi, C.K.; Tillakaratne, N.J.K.; Burdick, J.W.; et al. Training locomotor networks. Brain Res. Rev. 2008, 57, 241–254. [Google Scholar] [CrossRef]
- Lovely, R.G.; Gregor, R.J.; Roy, R.R.; Edgerton, V.R. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 1986, 92, 421–435. [Google Scholar] [CrossRef]
- De Leon, R.D.; Hodgson, J.A.; Roy, R.R.; Edgerton, V.R. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998, 80, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.L.; Courtine, G.; Fong, A.J.; Burdick, J.W.; Roy, R.R.; Edgerton, V.R. Plasticity of functional connectivity in the adult spinal cord. Philos Trans. R Soc. Land B Biol Sci. 2006, 361, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Hubli, M.; Dietz, V. The physiological basis of neurorehabilitation—locomotor training after spinal cord injury. J. Neuroeng. Rehabil. 2013, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, D.; Willerslev-Olsen, M.; Lundell, H.; Biering-Sorensen, F.; Bo Nielsen, J. Assessment of transmission in specific descending pathways in relation to gait and balance following spinal cord injury. Prog. Brain Res. 2015, 218, 79–101. [Google Scholar] [PubMed]
- Harel, N.Y.; Tansey, K.E. Spasticity. In Neurological Aspects of Spinal Cord Injury; Weidner, N., Ed.; Springer International: Cham, Switzerland, 2017; pp. 303–324. [Google Scholar]
- Bradbury, E.J.; McMahon, S.B. Spinal cord repair strategies: Why do they work? Nat. Rev. Neurosci. 2006, 7, 644–653. [Google Scholar] [CrossRef]
- Dobkin, B. Functional rewiring of brain and spinal cord after injury: The three R´s of neural repair and neurological rehabilitation. Curr. Opin. Neurol. 2000, 13, 655–659. [Google Scholar] [CrossRef]
- Edgerton, V.; de Leon, R.; Harkema, S.; Hodgson, J.A.; London, N.; Reinkensmeyer, D.J.; Roy, R.R.; Talmadge, R.J.; Tillakaratne, N.J.; Timoszyk, W.; et al. Retraining the injured spinal cord. J. Physiol. 2001, 533, 15–22. [Google Scholar] [CrossRef]
- Edgerton, V.; Harkema, S.; Dobkin, B. I am retraining the human spinal cord. In Spinal Cord Medicine: Principles and Practice; Lin, V., Ed.; Demos Medical: New York, NY, USA, 2003; pp. 817–828. [Google Scholar]
- Barbeau, H.; Fung, J. The role of rehabilitation in the recovery of walking in the neurological population. Curr. Opin. Neurol. 2001, 14, 735–740. [Google Scholar] [CrossRef]
- Colombo, G.; Joerg, M.; Schreider, R.; Dietz, V. Treadmill training of paraplegic patients using a robotic orthosis. J. Rehabil. Res. Dev. 2000, 37, 693–700. [Google Scholar]
- Swinnen, E.; Duerinck, S.; Baeyens, J.-P.; Meeusen, R.; Kerckhofs, E. Effectiveness of robot-assisted gait training in persons with spinal cord injury: A systematic review. J. Rehabil. Med. 2010, 42, 520–526. [Google Scholar] [CrossRef]
- Adams, M.M.; Hicks, A.L. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J. Spinal Cord Med. 2011, 34, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Alexeeva, N.; Sames, C.; Jacobs, P.L.; Hobday, L.; Distasio, M.M.; Mitchell, S.A.; Calancie, B. Comparison of training methods to improve walking in persons with chronic spinal cord injury: A randomised clinical trial. J. Spinal Cord Med. 2011, 34, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Tefertiller, C.; Pharo, B.; Evans, N.; Winchester, P. Efficacy of rehabilitation robotics for walking training in neurological disorders: A review. J. Rehabil. Res. Dev. 2011, 48, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, C.; Moffat, F. Effects of locomotor training after incomplete spinal cord injury: A systematic review. Arch. Phys. Med. Rehabil. 2013, 94, 2297–2308. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L.; Gorassini, M.A. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J. Neurophysiol. 2005, 94, 2844–2855. [Google Scholar] [CrossRef]
- Norton, J.A.; Gorassini, M.A. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J. Neurophysiol. 2006, 95, 2580–2589. [Google Scholar] [CrossRef]
- Lavrov, I.; Courtine, G.; Dy, C.J.; Brand, R.; Fong, A.J.; Gerasimenko, Y.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Facilitation of stepping with epidural stimulation in spinal rats: Role of sensory input. J. Neurosci. 2008, 28, 7774–7780. [Google Scholar] [CrossRef]
- Rossignol, S.; Dubuc, R.; Gossard, J.P. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef]
- Takeoka, A.; Vollenweider, I.; Courtine, G.; Arber, S. Muscle Spindle Feedback Directs Locomotor Recovery and Circuit Reorganization after Spinal Cord Injury. Cell 2014, 159, 1626–1639. [Google Scholar] [CrossRef]
- Jordan, L.M.; McVagh, J.R.; Noga, B.R.; Cabaj, A.M.; Majczynski, H.; Slawinska, U.; Provencher, J.; Leblond, H.; Rossignol, S. Cholinergic mechanisms in spinal locomotion—potential target for rehabilitation approaches. Front. Neural. Circ. 2014, 8, 1–25. [Google Scholar]
- Behrman, A.L.; Harkema, S.J. Locomotor Training After Human Spinal Cord Injury: A Series of Case Studies. Phys. Ther. 2000, 80, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Sureddi, S.; Alam, M.; Zhong, H.; Roy, R.R.; Edgerton, V.R.; Gerasimenko, Y. Unique Spatiotemporal Neuromodulation of the Lumbosacral Circuitry Shapes Locomotor Success after Spinal Cord Injury. J. Neurotr. 2016, 33, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Atkinson, D.; Boakye, M.; Tolfo, C.Z.; Aslan, S.; Green, M.; Mckay, B.; Ovechkin, A.; Harkema, S.J. Quantitative and sensitive assessment of neurophysiological status after human spinal cord injury. J. Neurosurg. Spine 2012, 17, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mckay, W.B.; Ovechkin, A.V.; Vitaz, T.W.; De Paleville, D.G.L.T.; Harkema, S.J. Neurophysiological characterization of motor recovery in acute spinal cord injury. Spinal Cord 2011, 49, 421–429. [Google Scholar] [CrossRef]
- Barbeau, H.; Rossignol, S. Enhancement of locomotor recovery following spinal cord injury. Curr. Opin. Neurol. 1994, 7, 517–524. [Google Scholar] [CrossRef]
- Powers, R.K.; Turker, K.S.; Binder, M.D. What can be learned about motoneurone properties from studying firing patterns? Adv. Exp. Med. Biol. 2002, 508, 199–205. [Google Scholar]
- Binder, M.D. Intrinsic dendritic currents make a major contribution to the control of motoneurone discharge. J. Physiol. 2003, 552, 665. [Google Scholar] [CrossRef]
- Heckman, C.J.; Lee, R.H.; Brownstone, R.M. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behaviour. Trends Neurosci. 2003, 26, 688–695. [Google Scholar] [CrossRef]
- Heckman, C.J.; Gorassini, M.A.; Bennett, D.J. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve 2005, 31, 135–156. [Google Scholar] [CrossRef]
- Powers, R.K.; Binder, M.D. Input-output functions of mammalian motoneurons. Rev. Physiol. Biochem. Pharmacol. 2001, 143, 137–263. [Google Scholar]
- Alaburda, A.; Perrier, J.F.; Hounsgaard, J. Mechanisms causing plateau potentials in spinal motoneurones. Adv. Exp. Med. Biol. 2002, 508, 219–226. [Google Scholar] [PubMed]
- Olby, N.J.; Moore, S.A.; Brisson, B.; Fenn, J.; Flegel, T.; Kortz, G.; Lewis, M.; Tipold, A. ACVIM consensus statement on diagnosis and management of acute canine thoracolumbar intervertebral disc extrusion. J. Vet. Intern. Med. 2022, 36, 1570–1596. [Google Scholar] [CrossRef] [PubMed]
- Mojarradi, A.; Decker, S.D.; Backstrom, C.; Bergknut, N. Safety of early postoperative hydrotherapy in dogs undergoing thoracolumbar hemilaminectomy. J. Small Anim. Pract. 2021, 62, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Hensel, C.; Kaschuba, R.; Schmidt, E.-M. Neurological Aspects of Spinal Cord Injury; Weidner, N., Ed.; Springer International: Cham, Switzerland, 2017; pp. 649–688. [Google Scholar]
- Knikou, M. Plasticity of corticospinal neural control after locomotor training in human spinal cord injury. Neural Plast. 2012, 254948. [Google Scholar] [CrossRef]
- Harkema, S.; Behrman, A.; Barbeau, H. Evidence-based therapy for recovery of function after spinal cord injury. Handb. Clin. Neurol. 2012, 109, 259–274. [Google Scholar]
- Nooijen, C.F.; Ter Hoeve, N.; Field-Fote, E.C. Gate quality is improved by locomotor training in individuals with SCI regardless of training approach. J. Neuroeng. Rehabil. 2009, 6, 36. [Google Scholar] [CrossRef]
- Gouveia, D.; Carvalho, C.; Cardoso, A.; Gamboa, Ó.; Almeida, A.; Ferreira, A.; Martins, Â. Early locomotor training in tetraplegic post-surgical dogs with cervical intervertebral disc disease. Animals 2022, 12, 2369. [Google Scholar] [CrossRef]
- Gouveia, D.; Cardoso, A.; Carvalho, C.; Gonçalves, A.R.; Gamboa, Ó.; Canejo-Teixeira, R.; Ferreira, A.; Martins, Â. Influence of spinal shock on the neurorehabilitation of ANNPE dogs. Animals 2022, 12, 1557. [Google Scholar] [CrossRef]
- Martins, A.; Gouveia, D.; Cardoso, A.; Viegas, I.; Gamboa, O.; Ferreira, A. A comparison between body weight-supported treadmill training and conventional over-ground training in dogs with incomplete spinal cord injury. Front. Vet. Sci. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Wirz, M.; Colombo, G.; Dietz, V. Long-term effects of locomotor training in spinal humans. J. Neurol. Neurosurg. Psychiatry 2001, 71, 93–96. [Google Scholar] [CrossRef][Green Version]
- Hicks, A.L.; Adams, M.M.; Martin, G.K.; Giangregorio, L.; Latimer, A.; Phillips, S.M.; McCartney, N. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: Effects on functional walking ability and measures of subjective well-being. Spinal Cord 2005, 43, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.; Apple, D.; Barbeau, H.; Basso, M.; Behrman, A.; Deforge, D.; Ditunno, J.; Dudley, G.; Elashoff, R.; Fugate, L.; et al. Weight-supported treadmill vs. over-ground training for walking after acute incomplete SCI. Neurology 2006, 66, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.J.; Hillyer, J.; Schmidt-Read, M.; Ardolino, E.; Sisto, S.A.; Behrman, A.L. Locomotor training: As a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 1588–1597. [Google Scholar] [CrossRef]
- Field-Fote, E.C.; Tepavac, D. It improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys. Ther. 2002, 82, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Juvin, L.; Simmers, J.; Morin, D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J. Neurosci. 2005, 25, 6025–6035. [Google Scholar] [CrossRef] [PubMed]
- Escalona, M.; Delivet-Mongrain, H.; Kundu, A.; Gossard, J.P.; Rossignol, S. Ladder treadmill: A method to assess locomotion in cats with an intact or lesioned spinal cord. J. Neurosci. 2017, 37, 5429–5446. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.E.; Liu, M.; Bose, P.; O´Steen, W.A.; Thompson, F.J.; Anderson, D.K.; Vandenborne, K. Changes in soleus muscle function and fibre morphology with one week of locomotor training in spinal cord contusion injured rats. J. Neurotrauma 2006, 23, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.A.T.; Cunha, R.C.; Vialle, E.M.; Osiecki, R.; Moreira, G.H.G.; Simeoni, R.B.; Francisco, J.C.; Guarita-Souza, L.C.; Oliveira, L.; Zocche, L.; et al. Functional outcome of bone marrow stem cells (CD45(+)/CD34(-)) after cell therapy in acute spinal cord injury: In exercise training and in sedentary rats. Transplant 2008, 40, 847–849. [Google Scholar] [CrossRef]
- Heng, C.; de Leon, R.D. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp. Neurol. 2009, 216, 139–147. [Google Scholar] [CrossRef]
- Ichiyama, R.; Potuzak, M.; Balak, M.; Kalderon, N.; Edgerton, V.R. Enhanced motor function by training in spinal cord contused rats following radiation therapy. PLoS ONE 2009, 4, e6862. [Google Scholar] [CrossRef]
- Oh, M.J.; Seo, T.B.; Kwon, K.B.; Yoon, S.J.; Elzi, D.J.; Kim, B.G.; Namgung, U. Axonal outgrowth and Erk1/2 activation by training after spinal cord injury in rats. J. Neurotrauma 2009, 26, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Murray, M.; Houle, J.D. A training paradigm to enhance motor recovery in contused rats: Effects of staircase training. Neurorehabil. Neural. Repair. 2011, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Moshonkina, T.; Avelev, V.; Gerasimenko, Y.; Mathur, R.; Bijlani, R.L. Treadmill training accelerates restoration of locomotion after complete spinal cord transection in the rat. Indian J. Physiol. Pharmacol. 2002, 46, 499–503. [Google Scholar] [PubMed]
- Nothias, J.M.; Mitsui, T.; Shumsky, J.S.; Fischer, I.; Antonacci, M.D.; Murray, M. Combined effects of neurotrophin-secreting transplants, exercise, and serotonergic drug challenge improve function in spinal rats. Neurorehabil. Neural. Repair. 2005, 19, 296–312. [Google Scholar] [CrossRef]
- Foret, A.; Quertainmont, R.; Botman, O.; Bouhy, D.; Amabili, P.; Brook, G.; Schoenen, J.; Frazen, R. Stem cells in the adult rat spinal cord: Plasticity after injury and treadmill training exercise. J. Neurochem. 2010, 112, 762–772. [Google Scholar] [CrossRef]
- Engesser-Cesar, C.; Anderson, A.J.; Basso, D.M.; Edgerton, V.R.; Cotman, C.W. Voluntary wheel running improves recovery from a moderate spinal cord injury. J. Neurotrauma 2005, 22, 157–171. [Google Scholar] [CrossRef]
- Kuerzi, J.; Brown, E.H.; Shum-Siu, A.; Siu, A.; Burke, D.; Morehouse, J.; Smith, R.R.; Magnuson, D.S.K. Task-specificity vs ceiling effect: Step-training in shallow water after spinal cord injury. Exp. Neurol. 2010, 224, 178–187. [Google Scholar] [CrossRef]
- Lee, Y.S.; Zdunowski, S.; Edgerton, V.R.; Roy, R.R.; Zhong, H.; Hsiao, I.; Lin, V.W. Improvement of gait patterns in step-trained, complete spinal cord-transected rats treated with a peripheral nerve graft and acidic fibroblast growth factor. Exp. Neurol. 2010, 224, 429–437. [Google Scholar] [CrossRef]
- Tillakaratne, N.J.K.; Guu, J.J.; de Leon, R.D.; Bigbee, A.J.; London, N.J.; Zhong, H.; Ziegler, M.D.; Joynes, R.L.; Roy, R.R.; Edgerton, V.R. Functional recovery of stepping in rats after a complete neonatal spinal cord transection is not due to regrowth across the lesion site. Neuroscience 2010, 166, 23–33. [Google Scholar] [CrossRef]
- Zidan, N.; Sims, C.; Fenn, J.; Williams, K.; Griffith, E.; Early, P.J.; Mariani, C.; Munana, K.R.; Guevar, J.; Olby, N.J. A randomised, blinded, prospective clinical trial of postoperative rehabilitation in dogs after surgical decompression of acute thoracolumbar intervertebral disc herniation. J. Vet. Intern. Med. 2018, 32, 1133–1144. [Google Scholar] [CrossRef]
- Gallucci, A.; Dragone, L.; Menchetti, M.; Gagliardo, T.; Pietra, M.; Cardinali, M.; Gandini, G. Acquisition of involuntary spinal locomotion (spinal walking) in dogs with irreversible thoracolumbal spinal cord lesion: 81 dogs. J. Vet. Intern. Med. 2017, 31, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Kugler, J.; Pohl, M. Locomotor training for walking after spinal cord injury. Cochran. Data. Syst. Rev. 2008, 11, CD006676. [Google Scholar]
- Lewis, M.J.; Bowditch, J.; Laflen, B.; Perry, N.; Yoquelet, R.; Thomovsky, S.A. Pilot Study on Feasibility of Sensory-Enhanced Rehabilitation in Canine Spinal Cord Injury. Front. Vet. Sci. 2022, 9, 921471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-X.; Huang, F.; Gates, M.; White, J.; Holmberg, E.G. Tail nerve electrical stimulation induces body weight-supported stepping in rats with spinal cord injury. J. Neurosci. Methods 2010, 187, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing neuroplasticity for clinical applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Galea, M.P.; Gonzenbach, R.; Kesselring, J. Neurorehabilitation: Applied neuroplasticity. J. Neurol. 2017, 264, 603–615. [Google Scholar] [CrossRef]
- Rossignol, S.; Chau, C.; Giroux, N.; Brustein, E.; Bouyer, L.; Marcoux, J.; Langlet, C.; Barthel6my, D.; Provencher, J.; Leblond, H.; et al. The cat model of spinal injury. Prog. Brain Res. 2002, 137, 151–168. [Google Scholar]
- Muir, G.D.; Steeves, J.D. Sensorimotor stimulation to improve locomotor recovery after spinal cord injury. Trends Neurosci. 1997, 20, 72–77. [Google Scholar] [CrossRef]
- Engesser-Cesar, C.; Ichiyama, R.M.; Nefas, A.L.; Hill, M.A.; Edgerton, V.R.; Cotman, C.W.; Anderson, A.J. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fibre growth. Eur. J. Neurosci. 2007, 25, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M. Aquatic Therapy. In Essential Facts of Physical Medicine, Rehabilitation and and Sports Medicine in Companion Animal; Bockstahler, B., Wittek, K., Levine, D., Maierl, J., Millis, D., Eds.; VBS GmbH: Babenhausen, Germany, 2019; pp. 175–188. [Google Scholar]
- English, A.W.; Wilhelm, J.C.; Ward, P.J. Exercise, Neurotrophins, and Axon Regeneration in the PNS. Physiology 2014, 29, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Remple, M.S.; Bruneau, R.M.; VandenBerg, P.M.; Goertzen, C.; Kleim, J.A. Sensitivity of cortical movement representations to motor experience: Evidence that skill learning but not strength training induces cortical reorganisation. Behaviour. Brain Res. 2001, 123, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sale, D.G. Neural adaptation to resistance training. Med. Sci. Sports Exerc. 1988, 20, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Sale, D.G.; MacDougall, J.D.; Upton, A.R.; MacComas, A.J. Effect of strength training upon motoneuron excitability in man. Med. Sci. Sports Exerc. 1983, 15, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M. Muscle strength and its development. New perspectives. Sports Med. 1988, 6, 146–168. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M. Neural adaptations with chronic physical activity. J. Biomech. 1977, 30, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Duffell, L.D.; Donaldson, N.N.; Perkins, T.A.; Rushton, D.N.; Hunt, K.J.; Kakebeeke, T.H.; Newham, D.J. Long-term intensive electrically stimulated cycling by spinal cord-injured people: Effect on muscle properties and their relation to power output. Muscle Nerve 2008, 38, 1304–1311. [Google Scholar] [CrossRef]
- Schilling, N.; Carrier, D.R. Function of the epaxial muscles during trotting. J. Exp. Biol. 2009, 212, 1053–1063. [Google Scholar] [CrossRef][Green Version]
- Schilling, N.; Fischbein, T.; Yang, E.P.; Carrier, D.R. Function of the extrinsic hindlimb muscles in trotting dogs. J. Exp. Bio. 2009, 212, 1036–1052. [Google Scholar] [CrossRef]
- Millis, D.L.; Drum, M.; Levine, D. Therapeutic exercises: Joint motion, strengthening, endurance, and speed exercises. In Canine Rehabilitation and Physical Therapy, 2nd ed.; Millis, D.L., Levine, D., Eds.; Elsevier Saunders: Philadephia, PA, USA, 2014; pp. 506–525. [Google Scholar]
- Kidgell, D.J.; Frazer, A.K.; Daly, R.M.; Ruotsalainen, I.; Ahtiainen, J.; Avela, J.; Howatson, G. Increased cross-education of muscle strength and reduced corticospinal inhibition following eccentric strength training. Neuroscience 2015, 300, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, H.L.; de Hann, A.; Sargeant, A.J.; Dallmeijer, A.; Hopman, M.T.E. Altered contractile properties of the quadriceps muscle in people with spinal cord injury following functional electrical stimulated cycle training. Spinal Cord 2000, 98, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Harridge, S.D.R.; Anderson, J.L.; Hartkopp, A.; Zhou, S.; Biering-Sorensen, F.; Sandri, C.; Kjaer, M. Training by low-frequency stimulation of tibialis anterior in spinal cord injured men. Muscle Nerve 2002, 25, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Rochester, L.B.M.; Chandler, C.S.; Johnson, M.A.; Sutton, R.A.; Miller, S. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects.1. Contractile properties. Paraplegia 1995, 33, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.B.; Gordon, J.; Jefferson, A.; Sharfenberger, J.F.; Yang, J.; Totosy, D.Z.; Belanger, M. Optimal stimulation of paralysed muscle after human spinal cord injury. J. Appl. Physiol. 1992, 72, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Rochester, L.B.M.; Barrow, M.J.; Chandler, C.S.; Sutton, R.A.; Miller, S.; Johnson, M.A. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia 1995, 33, 514–522. [Google Scholar] [CrossRef]
- Gerrits, H.L.; Hopman, M.T.; Offringa, C.; Engelen, B.G.; Sargeant, A.J.; Jones, D.A.; De Haan, A. Variability in fibre properties in paralysed human quadriceps muscles and effects of training. Pflugers Arch. 2003, 445, 734–740. [Google Scholar] [CrossRef]
- Harness, E.T.; Yozbatiran, N.; Cramer, S.C. Effects of intense exercise in chronic spinal cord injury. Spinal Cord 2008, 46, 733–737. [Google Scholar] [CrossRef]
- Burns, A.S.; Ditunno, J.F. Establishing prognosis and maximising functional outcomes in rehabilitation management. Spine 2001, 26, S137–S145. [Google Scholar] [CrossRef]
- Jones, T.A.; Chu, C.J.; Grande, L.A.; Gregory, A.D. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J. Neurosci. 1999, 19, 10153–10163. [Google Scholar] [CrossRef]
- Billman, G.E.; Schwartz, P.J.; Gagnol, J.P.; Stone, H.L. Cardiac response to submaximal exercise in dogssusceptible to sudden cardiac death. J. Appl. Physiol. 1985, 59, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Musch, T.I.; Friedman, D.B.; Pitetti, K.H.; Haidet, G.C.; Stray-Gundersen, J.; Mitchell, J.H.; Ordway, G.A. Regional distribution of blood flow of dogs during graded dynamic exercise. J. Appl. Physiol. 1985, 63, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.S.; Rossi, N.F.; Churchill, P.C. Substantial cardiac parasympathetic activity exists during heavy dynamic exercise in dogs. Am. J. Physiol. Heart Circ. Physiol. 1997, 273, H2135–H2140. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Copp, S.W.; Colburn, T.D.; Craig, J.C.; Allen, D.L.; Sturek, M.; O´Leary, D.D.; Zucker, I.H.; Musch, T.I. Guidelines for animal exercise and training protocols for cardiovascular studies. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1100–H1138. [Google Scholar] [CrossRef]
- Moore, S.A.; Tipold, A.; Olby, N.J.; Stein, V.; Granger, N. Cansort- Canine Spinal Cord Injury Consortium (CANSORT SCI) Current Approaches to the Management of Acute Thoracolumbar Disc Extrusion in Dogs. Front. Veter. Sci. 2020, 7, 610. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Roy, R.R. Robotic training and spinal cord plasticity. Brain Res. Bull. 2009, 15, 4–12. [Google Scholar] [CrossRef][Green Version]
- Drew, T.; Jiang, W.; Widajewicz, W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res. Brain Res. Rev. 2002, 40, 178–191. [Google Scholar] [CrossRef]
- Sadowsky, C.L.; Hammond, E.R.; Strohl, A.B.; Commean, P.K.; Eby, S.A.; Damiano, D.L.; Wingert, J.R.; Bae, K.T.; McDonald, J.W. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J. Spinal Cord Med. 2013, 36, 623–631. [Google Scholar] [CrossRef]
- McDonald, J.; Sadowsky, C.; Stampas, A. The changing field of rehabilitation: Optimisation of spontaneous regeneration and recovery of function. In Handbook of Clinical Neurology. Spinal Cord Injury Edition; McDonald, J., Verhaagen, J., Eds.; Elsevier: New York, NY, USA, 2012; p. 109. [Google Scholar]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Satake, K.; Matsuyama, Y.; Kamiya, M.; Kawakami, H.; Iwata, H.; Adachi, K.; Kiuchi, K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Mol. Brain Res. 2000, 85, 114–122. [Google Scholar] [CrossRef]
- Salem, H.R.; Faried, M.A. Treadmill exercise training ameliorates functional and structural age-associated kidney changes in male albino rats. Sci. World J. 2021, 2021, 1393372. [Google Scholar] [CrossRef] [PubMed]
- Dugan, E.A.; Jergova, S.; Sagen, J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp. Neurol. 2020, 327, 113208. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.P.; Murray, M.; Lemay, M.A. Rehabilitation strategies after spinal cord injury: Inquiry into success and failure mechanisms. J. Neurotrauma 2017, 34, 1841–1857. [Google Scholar] [CrossRef] [PubMed]
- Leardini-Tristão, M.; Andrade, G.; Garcia, C.; Reis, A.P.; Lourenço, M.; Moreira, E.; Lima, F.; Castro-Faria-Neto, H.C.; Tibirica, E.; Estato, V. Physical exercise promotes astrocyte coverage of microvessels in a model of chronic cerebral hypoperfusion. J. Neuroinflammation 2020, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Granger, N. Spinal cord elasticity: A physical parameter to guide treatment. In Proceedings of the 2022 34th ESVN-ECVN Symposium Spinal Cord Injury-Taking Steps Forward, Maiorca, Spain, 23–24 September 2022; p. 48. [Google Scholar]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Sun, H.; Liu, D. Interleukin-6 gene transfer reverses body weight gain and fatty liver in obese mice. Biochim. Biophys. Acta 2015, 1852, 1001–1011. [Google Scholar] [CrossRef]
- Grisouard, J.; Bouillet, E.; Timper, K.; Radimerski, T.; Dembinski, K.; Frey, D.M.; Peterli, R.; Zulewski, H.; Keller, U.; Müller, B.; et al. Both inflammatory and classical lipolytic pathways are involved in lipopolysaccharide-induced lipolysis in human adipocytes. Innate Immun. 2012, 18, 25–34. [Google Scholar] [CrossRef]
- Geng, C.; Cao, H.; Ying, X.; Zhang, H.; Yu, H. The effects of hyperbaric oxygen on macrophage polarisation after rat spinal cord injury. Sci. Direct. 2015, 1606, 68–76. [Google Scholar]
- Prager, J.; Fenn, J.; Plested, M.; Escauriaza, L.; van der Merwe, T.; King, B.; Chari, D.; Wong, L.F.; Granger, N. Transplantation of encapsulated autologous olfactory ensheathing cell populations expressing chondroitinase for spinal cord injury: A safety and feasibility study in companion dogs. J. Tissue Eng. Regen. Med. 2022, 16, 788–798. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, J.; Yan, T.; Zhang, Y.; Chen, Y.; Chang, R.; Wong, G. Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer´s disease mice. J. Neuroinflamm. 2020, 17, 4. [Google Scholar] [CrossRef]
- Gomes da Silva, S.; Simões, P.; Mortara, R.A.; Scorza, F.A.; Cavalheiro, E.A.; Naffah-Mazzacoratti, M.G.; Arida, R.M. Exercise-induced hippocampal anti-inflammatory response in aged rats. J. Neuroinflamm. 2013, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Martin, B.; Maudsley, S. Anti-inflamma.atory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer’s disease. Curr. Alzheimer. Res. 2012, 9, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, N.; Motl, R.W.; Mokhtarzade, M.; Zimmer, P.; Ranjbar, R.; Keytsman, C.; Cullen, T.; Negaresh, R.; Baker, J.S. Acute responses of cytokines and adipokines to aerobic exercise in relapsing vs. remitting women with multiple sclerosis. Complement. Ther. Clin. Pract. 2018, 31, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.C.; Stephens, M.J.; Ballou, E.W.; Heckman, C.J.; Bennett, D.J. Motorneuronexcitability and muscle spasms are regulated by 5-HT2B and 5 HT2C receptor activity. J. Neurophysiol. 2011, 105, 731–748. [Google Scholar] [CrossRef]
- Dietz, V.; Colombo, G.; Jensen, L.; Baumgartner, L. Locomotor capacity of the spinal cord in paraplegic patients. Ann. Neurol. 1995, 37, 574–582. [Google Scholar] [CrossRef]
- Van de Crommert, H.W.; Mulder, T.; Duysens, J. Neural control of locomotion: Sensory control of the central pattern generator and its relation to treadmill training. Gait. Post. 1998, 7, 251–263. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Tufik, S.; Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol. Life Sci. 2015, 73, 975–983. [Google Scholar] [CrossRef]
- Knikou, M.; Mummidisetty, C.K. Locomotor training improves premotoneuronal control after chronic spinal cord injury. J. Neurophysiol. 2014, 111, 2264–2275. [Google Scholar] [CrossRef]
- Alen, J.F. Traumatic spinal cord injury repair and regeneration. In Proceedings of the 2022 34th ESVN-ECVN Symposium Spinal Cord Injury—Taking Steps Forward, Maiorca, Spain, 23–24 September 2022; pp. 38–39. [Google Scholar]
- Knikou, M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin. Neurophysiol. 2010, 121, 1655–1668. [Google Scholar] [CrossRef]
- Lavrov, I.; Gerasimenko, Y.P.; Ichiyama, R.M.; Courtine, G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. J. Neurophysiol. 2006, 96, 1699–1710. [Google Scholar] [CrossRef]
- Olby, N.J.; Lim, J.; Wagner, N.; Zidan, N.; Early, P.J.; Mariani, C.L.; Muñana, K.R.; Laber, E. Time course and prognostic value of serum GFAP, pNFH, and S100þ concentrations in dogs with complete spinal cord injury because of intervertebral disc extrusion. J. Vet. Intern. Med. 2019, 33, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Olby, N. Treatment of Paralysis in Dogs. In Proceedings of the 2022 34th ESVN-ECVN Symposium Spinal Cord Injury—Taking Steps Forward, Maiorca, Spain, 23–24 September 2022; pp. 45–47. [Google Scholar]
- Barrett, A.M.; Oh-Park, M.; Chen, P.; Ifejika, N.L. Neurorehabilitation: Five new things. Neurol. Clin. Pract. 2013, 3, 384–492. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).