Changes in the Populations of Two Lymnaeidae and Their Infection by Fasciola hepatica and/or Calicophoron daubneyi over the Past 30 Years in Central France
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farms Studied
2.2. Animals
2.3. Protocol for Snail Investigations
2.4. Protocol for Detecting Parasite Larval Forms in Snails
2.5. Meteorological Data
2.6. Statistics
3. Results
3.1. Snail Populations
3.2. Prevalence of Parasite Infection in Snails
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Fascioliasis and other plant-borne trematode zoonoses. Int. J. Parasitol. 2005, 35, 1255–1278. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009, 69, 41–146. [Google Scholar] [PubMed]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fascioliasis. Adv. Exp. Med. Biol. 2019, 1154, 71–103. [Google Scholar] [PubMed]
- Cwiklinski, K.; O’Neill, S.M.; Donnelly, S.; Dalton, J.P. A prospective view of animal and human fasciolosis. Parasite Immunol. 2016, 38, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.; Chandra, R.; Schmitt, E.K.; Chen, C.W.; Samantray, S.; Venishetty, V.K.; Hughes, D. Global impact of human fasciolosis. In Fasciolosis; Dalton, J.P., Ed.; CABI Publishing: Wallingford, UK, 2022; pp. 461–491. [Google Scholar]
- Iglesias-Piñeiro, J.; González-Warleta, M.; Castro-Hermida, J.A.; Córdoba, M.; González-Lanza, C.; Manga-González, Y.; Mezo, M. Transmission of Calicophoron daubneyi and Fasciola hepatica in Galicia (Spain): Temporal follow-up in the intermediate and definitive hosts. Parasit. Vectors 2016, 9, 610. [Google Scholar] [CrossRef]
- Szmidt-Adjidé, V.; Abrous, M.; Adjidé, C.C.; Dreyfuss, G.; Lecompte, A.; Cabaret, J.; Rondelaud, D. Prevalence of Paramphistomum daubneyi infection in cattle in central France. Vet. Parasitol. 2000, 7, 133–138. [Google Scholar] [CrossRef]
- Jones, R.; Williams, H.; Dalesman, S.; Brophy, P. Confirmation of Galba truncatula as an intermediate host snail for Calicophoron daubneyi in Great Britain, with evidence of alternative snail species hosting Fasciola hepatica. Parasit. Vectors 2015, 8, 656. [Google Scholar] [CrossRef]
- Jones, R.A.; Brophy, P.M.; Mitchell, E.S.; Williams, H.W. Rumen fluke (Calicophoron daubneyi) on Welsh farms: Prevalence, risk factors and observations on co-infection with Fasciola hepatica. Parasitology 2017, 144, 237–247. [Google Scholar] [CrossRef]
- Naranjo-Lucena, A.; Munita Corbalán, M.P.; Martínez-Ibeas, A.M.; McGrath, G.; Murray, G.; Casey, M.; Good, B.; Sayers, R.; Mulcahy, G.; Zintl, A. Spatial patterns of Fasciola hepatica and Calicophoron daubneyi infections in ruminants in Ireland and modelling of C. daubneyi infection. Parasit. Vectors 2018, 11, 531. [Google Scholar] [CrossRef]
- Huson, K.M.; Olivier, N.A.M.; Robinson, M.W. Paramphistomosis of ruminants: An emerging parasitic disease in Europe. Trends Parasitol. 2017, 33, 836–844. [Google Scholar] [CrossRef]
- Fenemore, C.; Floyd, T.; Mitchell, S. Rumen fluke in Great Britain. J. Comp. Pathol. 2021, 184, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Mage, C.; Bourgne, H.; Toullieu, J.M.; Rondelaud, D.; Dreyfuss, G. Fasciola hepatica and Paramphistomum daubneyi: Changes in prevalences of natural infections in cattle and in Lymnaea truncatula from central France over the past 12 years. Vet. Res. 2002, 33, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Rondelaud, D.; Vignoles, P.; Dreyfuss, G. La Limnée Tronquée, un Mollusque D’Intérêt Médical et Vétérinaire; PULIM: Limoges, France, 2009. [Google Scholar]
- Vignoles, P.; Dreyfuss, G.; Rondelaud, D. Ecologie et Parasitisme de la Limnée Étroite (Omphiscola glabra); PULIM: Limoges, France, 2017. [Google Scholar]
- Dreyfuss, G.; Vignoles, P.; Rondelaud, D. Current decline in the number and size of populations of Galba truncatula and Omphiscola glabra, intermediate hosts of Fasciola hepatica, on the acidic soils of central France. Parasite 2016, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, G.; Vignoles, P.; Rondelaud, D. Galba truncatula and Omphiscola glabra (Gastropoda, Lymnaeidae): Present decline in populations living on sedimentary soils in central France. Ann. Limnol. Int. J. Limnol. 2018, 54, 11. [Google Scholar] [CrossRef]
- Vignoles, P.; Dreyfuss, G.; Rondelaud, D. Decline in the number and size of populations of two Lymnaeidae living in central France over the last years. Int. J. Limnol. 2022, 58, 11. [Google Scholar] [CrossRef]
- Guy, F.; Rondelaud, D.; Botineau, M.; Dreyfuss, G.; Ghestem, A. Étude de relations entre les plantes les plus fréquentes et l’abondance de Lymnaea truncatula Müller, vecteur de Fasciola hepatica Linné dans les prairies marécageuses sur sol acide. Rev. Med. Vet. 1996, 147, 465–470. [Google Scholar]
- Rondelaud, D.; Hourdin, P.; Vignoles, P.; Dreyfuss, G.; Cabaret, J. The detection of snail host habitats in liver fluke infected farms by use of plant indicators. Vet. Parasitol. 2011, 181, 166–173. [Google Scholar] [CrossRef]
- Interbev Nouvelle-Aquitaine. Elevage Bovin en Territoire Limousin, Esition 2018; Chambres d’Agriculture de la Corrèze, de la Creuse et de la Haute-Vienne: Limoges, France, 2019. [Google Scholar]
- Maytie, B.; Grisot, L.; Baduel, L. Modalités Pratiques et Contraintes de L’Emploi des Fasciolicides en Conditions Terrain; La Dépêche Vétérinaire: Paris, France, 2020; pp. 21–27. [Google Scholar]
- Vareille-Morel, C.; Dreyfuss, G.; Rondelaud, D. The characteristics of habitats colonized by three species of Lymnaea in swampy meadows on acid soil: Their interest for fasciolosis control. Ann. Limnol. Int. J. Lim. 1999, 35, 173–178. [Google Scholar] [CrossRef]
- Rondelaud, D.; Vignoles, P.; Dreyfuss, G. Larval trematode infections in Lymnaea glabra populations from the Brenne Regional Natural Park, central France. Parasite 2015, 22, 38. [Google Scholar] [CrossRef]
- Rondelaud, D.; Vignoles, P.; Dreyfuss, G. Larval trematode infections in Galba truncatula (Gastropoda, Lymnaeidae) from the Brenne Regional Natural Park, central France. J. Helminthol. 2016, 90, 256–261. [Google Scholar] [CrossRef]
- Météo Concept. Normales Climatiques à Bellac. Available online: https://www.meteo-concept.fr/climatologie/normales/station/BELLAC (accessed on 14 November 2022).
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Johnson, C.N. Past and future decline and extinction of species. Available online: https://royalsociety.org/topics-policy/projects/biodiversity/decline-and-extinction (accessed on 15 November 2021).
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet. Parasitol. 2009, 163, 264–280. [Google Scholar] [CrossRef]
- Van Dijk, J.; Sargison, N.D.; Kenyon, F.; Skuce, P.J. Climate change and infectious disease: Helminthological challenges to farmed ruminants in temperate regions. Animals 2010, 4, 377–392. [Google Scholar] [CrossRef]
- Fox, N.J.; White, P.C.L.; McClean, C.J.; Marion, G.; Evans, A.; Hutchings, M.R. Predicting impacts of climate change on Fasciola hepatica risk. PLoS ONE 2011, 6, e16126. [Google Scholar] [CrossRef]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate change: Contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. Res. Treat. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef]
- Knubben-Schweizer, G.; Rössler, A.S.; Schade-Weskott, E.; Torgerson, P.R. Epidemiology and control. In Fasciolosis; Dalton, J.P., Ed.; CABI Publishing: Wallingford, UK, 2022; pp. 180–210. [Google Scholar]
- Cordellier, M.; Pfenninger, A.; Streit, B.; Pfenninger, M. Assessing the effects of climate change on the distribution of pulmonate freshwater snail biodiversity. Mar. Biol. 2012, 159, 2519–2531. [Google Scholar] [CrossRef]
- Météo France. Bilan Climatique de L’Année 2018. Available online: https://www.francetvinfo.fr/meteo/climat/l-ete-2018-est-le-deuxieme-le-plus-chaud-enregistre-par-meteo-france-derriere-celui-de-2003_2914883.html (accessed on 29 November 2021).
- Météo France. Le Bilan Climatique 2019. 2019 au 3e Rang des Années les Plus Chaudes en France Depuis le Début du XXe Siècle. Available online: https://meteofrance.com/actualites/climat/france-2019-au-3e-rang-des-annees-les-plus-chaudes (accessed on 29 November 2021).
- Météo France. Bilan Climatique 2020. 2020: L’Année la Plus Chaude en France Depuis 1900. Available online: https://meteofrance.com/actualites-et-dossiers-0/2020-lannee-la-plus-chaude-en-france-depuis-1900 (accessed on 29 November 2021).
- Moens, R. Factors affecting Lymnaea truncatula populations and related control measures. J. Med. Appl. Malacol. 1991, 3, 73–84. [Google Scholar]
- Boray, J.C.; Crowfoot, P.D.; Strong, M.B.; Allison, J.R.; Schellenbaum, M.; Von Orelli, M.; Sarasin, G. Treatment of immature and mature Fasciola hepatica infections in sheep with triclabendazole. Vet. Res. 1983, 113, 315–317. [Google Scholar] [CrossRef]
- Alvarez, L.I.; Lanusse, C.E.; Williams, D.J.L.; Fairweather, I. Flukicidal drugs: Pharmaco-therapeutics and drug resistance. In Fasciolosis; Dalton, J.P., Ed.; CABI Publishing: Wallingford, UK, 2022; pp. 211–255. [Google Scholar]
- Reynal, J.L. Enquête Épidémiologique sur les Traitements Appliqués Contre la Fasciolose et la Paramphistomose Bovine Dans le Sud-Ouest de la Corrèze, Pharm.D. Thesis. University of Limoges, Limoges, France, 2001.
- Vignoles, P.; Hourdin, P.; Dreyfuss, G.; Rondelaud, D. Epidémiologie de la fasciolose dans le Limousin: Bilan des recherches effectuées depuis les années 1970. Ann. Sci. Limousin 2019, 28, 39–66. [Google Scholar] [CrossRef]
- Abrous, M.; Rondelaud, D.; Dreyfuss, G.; Cabaret, J. Infection of Lymnaea truncatula and Lymnaea glabra by Fasciola hepatica and Paramphistomum daubneyi in farms of central France. Vet. Res. 1999, 30, 113–118. [Google Scholar]
- Abrous, M.; Rondelaud, D.; Dreyfuss, G. A field study of natural infections in three freshwater snails with Fasciola hepatica and/or Paramphistomum daubneyi in central France. J. Helminthol. 2000, 74, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Préveraud-Sindou, M.; Rondelaud, D. Fasciola hepatica L.: Données expérimentales sur l’attraction des miracidiums par Lymnaea truncatula Müller et des mollusques de familles voisines. Bull. Soc. Fr. Parasitol. 1990, 8, 277–282. [Google Scholar]
- Préveraud-Sindou, M.; Sindou, P.; Rondelaud, D. Nouvelles observations sur l’attraction des miracidiums de Fasciola hepatica L. par plusieurs espèces de limnées. Bull. Soc. Fr. Parasitol. 1989, 7, 55–60. [Google Scholar]
- Abrous, M.; Dreyfuss, G.; Rondelaud, D. L’aptitude de huit espèces de mollusques aquatiques à assurer le développement larvaire de Paramphistomum daubneyi Dinnik lors d’une infestation monospécifique ou d’une co-infestation avec Fasciola hepatica Linné. Rev. Med. Vet. 1999, 150, 727–732. [Google Scholar]
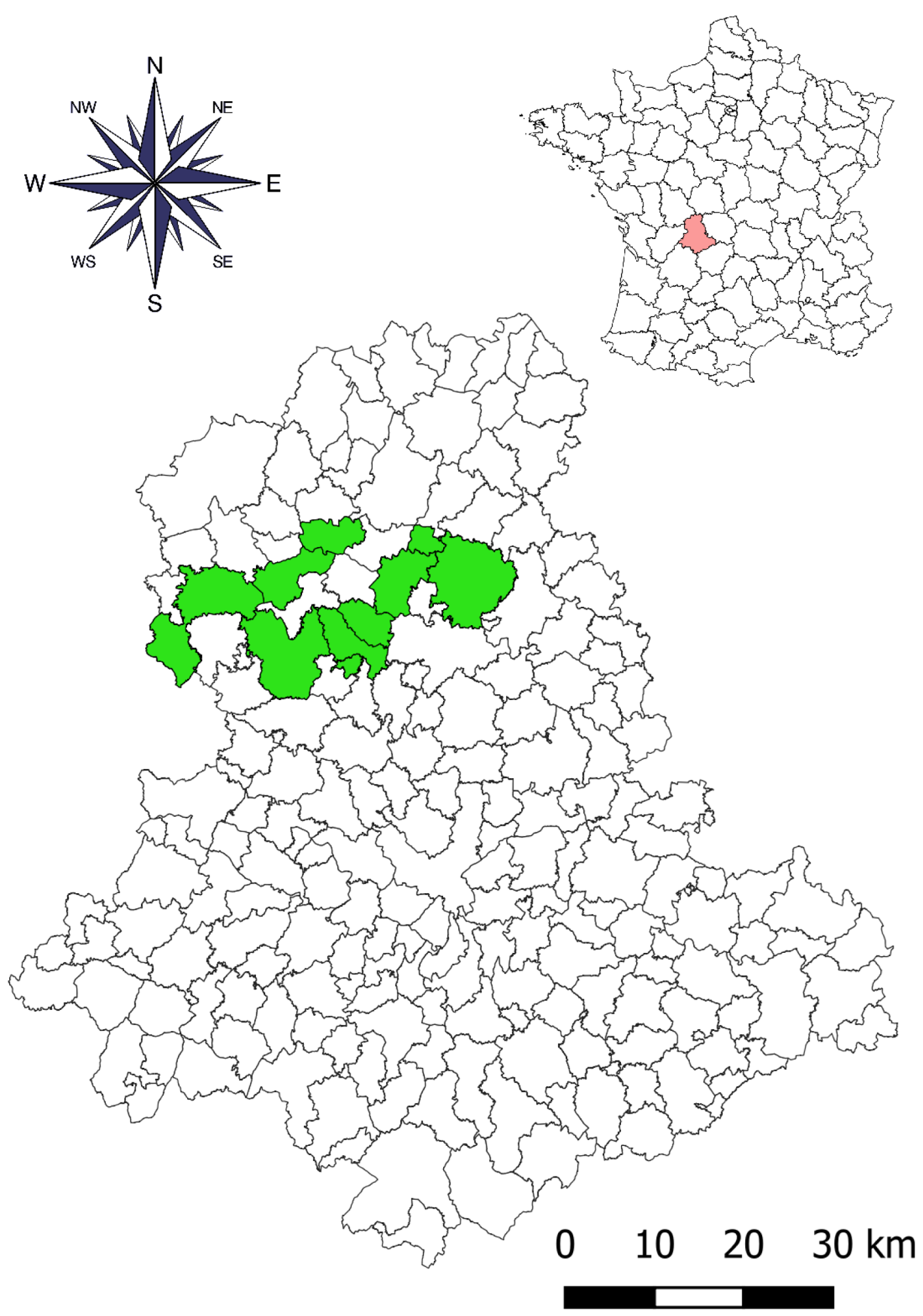
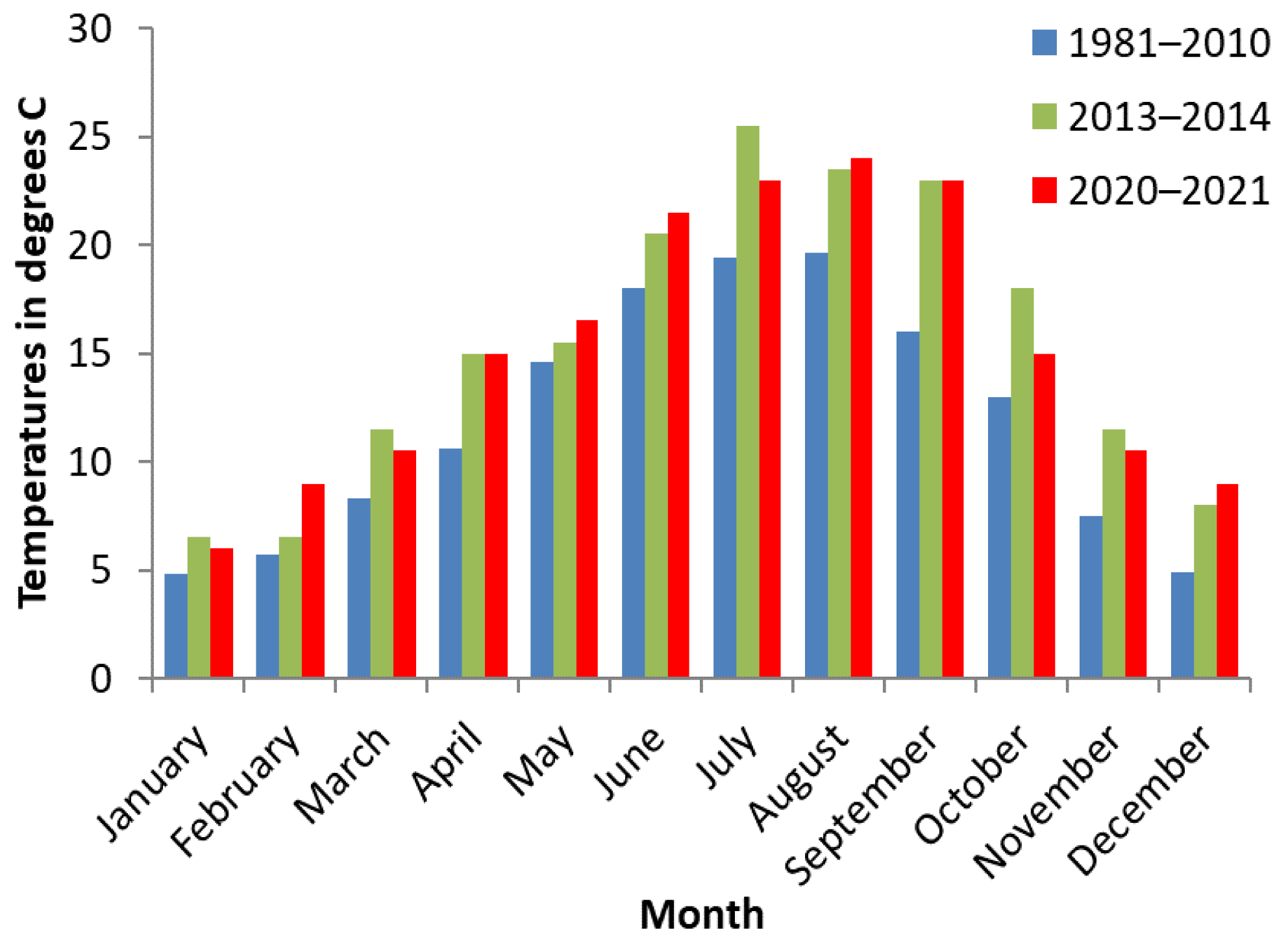
| Farm No. | Soil Geology | Number of Cattle in 2020–2021 | Year for the First Treatment with TBCZ | Total Area of Meadows (ha) * | Number of Snail Habitats ** | |
|---|---|---|---|---|---|---|
| Galba truncatula | Omphiscola glabra | |||||
| 1 | granitic | 41 | 1997 | 25 | 19 | 11 |
| 2 | gneissic | 49 | 1996 | 21 | 13 | 7 |
| 3 | granitic | 34 | 2001 | 28 | 19 | 9 |
| 4 | granitic | 51 | 1994 | 43 | 31 | 17 |
| 5 | granitic | 33 | 2003 | 21 | 16 | 11 |
| 6 | gneissic | 54 | 1994 | 31 | 14 | 9 |
| 7 | granitic | 57 | 1998 | 38 | 35 | 15 |
| 8 | granitic | 44 | 2002 | 34 | 22 | 13 |
| 9 | gneissic | 36 | 2003 | 19 | 17 | 9 |
| 10 | granitic | 48 | 1999 | 24 | 20 | 11 |
| 11 | granitic | 35 | 1994 | 35 | 16 | 10 |
| 12 | granitic | 41 | 1996 | 27 | 18 | 10 |
| 13 | granitic | 34 | 1995 | 29 | 20 | 14 |
| 14 | granitic | 52 | 1999 | 34 | 20 | 13 |
| 15 | granitic | 45 | 2000 | 27 | 11 | 8 |
| 16 | gneissic | 38 | 1995 | 20 | 15 | 11 |
| 17 | granitic | 49 | 2002 | 26 | 19 | 13 |
| 18 | gneissic | 44 | 2002 | 20 | 19 | 11 |
| 19 | granitic | 36 | 1996 | 31 | 17 | 13 |
| 20 | granitic | 42 | 1995 | 28 | 14 | 10 |
| 21 | granitic | 54 | 1994 | 50 | 33 | 21 |
| 22 | gneissic | 37 | 2000 | 23 | 19 | 12 |
| 23 | gneissic | 40 | 1998 | 34 | 15 | 8 |
| 24 | granitic | 47 | 2004 | 41 | 34 | 18 |
| 25 | granitic | 40 | 1994 | 33 | 16 | 10 |
| 26 | granitic | 38 | 1996 | 28 | 18 | 12 |
| 27 | granitic | 52 | 1995 | 38 | 26 | 13 |
| 28 | gneissic | 46 | 1996 | 35 | 28 | 15 |
| 29 | granitic | 40 | 2002 | 26 | 13 | 8 |
| 30 | granitic | 47 | 2000 | 49 | 39 | 17 |
| 31 | granitic | 35 | 1997 | 23 | 20 | 12 |
| 32 | granitic | 39 | 1995 | 42 | 32 | 15 |
| 33 | gneissic | 48 | 1997 | 34 | 14 | 10 |
| 34 | granitic | 42 | 2000 | 31 | 21 | 14 |
| 35 | granitic | 55 | 2001 | 41 | 27 | 13 |
| 36 | gneissic | 37 | 1997 | 24 | 17 | 13 |
| 37 | granitic | 42 | 1999 | 37 | 20 | 11 |
| 38 | granitic | 36 | 2002 | 31 | 15 | 10 |
| 39 | gneissic | 41 | 1998 | 42 | 27 | 12 |
| Parameter | Period of Snail Investigations | Overall Rate of Decline (%) | ||
|---|---|---|---|---|
| Before 1998 | 2013–2014 | 2020–2021 | ||
| Number of snail populations: | ||||
| Galba truncatula | 809 | 566 | 370 | 54.2 |
| Omphiscola glabra | 457 | 384 | 299 | 34.5 |
| Habitat area (m2): | ||||
| G. truncatula | 1.8 (0.5) | 1.6 (0.6) | 1.7 (0.6) | 0 |
| O. glabra | 8.1 (3.4) | 8.9 (2.8) | 5.4 (2.3) | 33.4 |
| Number of snails per population: | ||||
| G. truncatula | 25.7 (9.4) | 17.5 (7.3) | 5.4 (1.6) | 79.0 |
| O. glabra | 8.5 (3.2) | 5.4 (1.6) | 2.1 (1.2) | 75.3 |
| Parameter | Number of Snails (Prevalence of Infection in %) | ||
|---|---|---|---|
| Before 1998 | 2013–2014 | 2020–2021 | |
| Galba truncatula | |||
| Number of snails collected | 3893 | 3897 | 1950 |
| Number of snails with: | |||
| Fasciola hepatica | 198 (5.1) | 82 (2.1) | 19 (0.9) ** |
| Calicophoron daubneyi | 0 | 131 (3.4) | 142 (7.2) |
| Omphiscola glabra | |||
| Number of snails collected | 3900 | 3900 | 1948 |
| Number of snails with: | |||
| F. hepatica | 11 (0.28) * | 55 (1.4) | 12 (0.6) ** |
| C. daubneyi | 0 | 66 (1.6) | 42 (2.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondelaud, D.; Vignoles, P.; Dreyfuss, G. Changes in the Populations of Two Lymnaeidae and Their Infection by Fasciola hepatica and/or Calicophoron daubneyi over the Past 30 Years in Central France. Animals 2022, 12, 3566. https://doi.org/10.3390/ani12243566
Rondelaud D, Vignoles P, Dreyfuss G. Changes in the Populations of Two Lymnaeidae and Their Infection by Fasciola hepatica and/or Calicophoron daubneyi over the Past 30 Years in Central France. Animals. 2022; 12(24):3566. https://doi.org/10.3390/ani12243566
Chicago/Turabian StyleRondelaud, Daniel, Philippe Vignoles, and Gilles Dreyfuss. 2022. "Changes in the Populations of Two Lymnaeidae and Their Infection by Fasciola hepatica and/or Calicophoron daubneyi over the Past 30 Years in Central France" Animals 12, no. 24: 3566. https://doi.org/10.3390/ani12243566
APA StyleRondelaud, D., Vignoles, P., & Dreyfuss, G. (2022). Changes in the Populations of Two Lymnaeidae and Their Infection by Fasciola hepatica and/or Calicophoron daubneyi over the Past 30 Years in Central France. Animals, 12(24), 3566. https://doi.org/10.3390/ani12243566






