Revisiting the Timing of Insemination at Spontaneous Estrus in Dairy Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. The Timing of Insemination
3. Is End-Estrus the Best Guide for the Timing of Insemination?
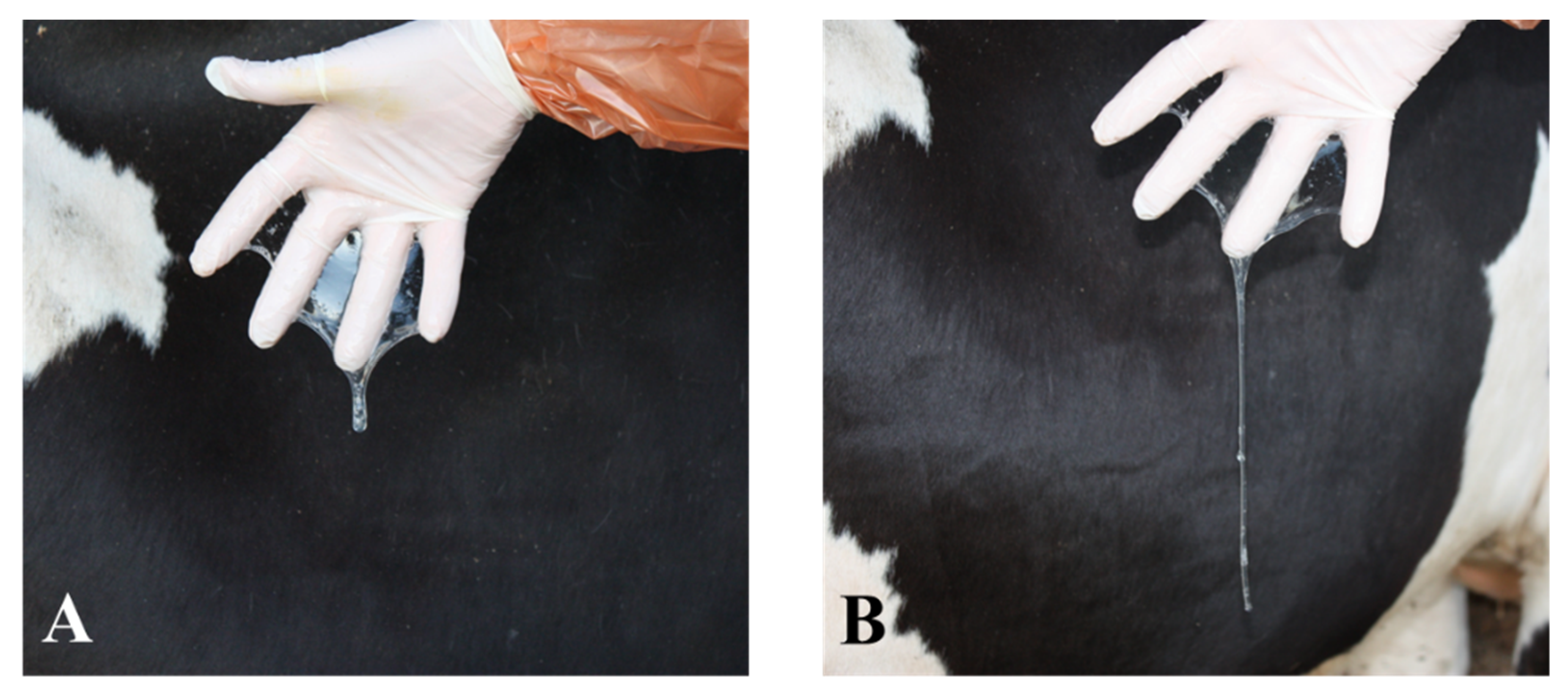
4. Physiological Indicators of Late Estrus
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeuner, F.E. The history of the domestication of cattle. In Man and Cattle; Mourant, A.E., Zeuner, E.F., Eds.; Royal Antropological Institute: London, UK, 1963; pp. 9–19. [Google Scholar]
- Zeuner, F.E. A History of Domesticated Animals; Hutchinson: London, UK, 1963; pp. 15–35. [Google Scholar]
- López-Gatius, F.; Hunter, R.H.F. Fertility, fecundity and the creative instinct. Gynecol. Obstet. Hum. Reprod. 2018, 47, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Curry, A. Archaeology: The milk revolution. Nature 2013, 500, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A. Edward Jenner and the small pox vaccine. Front. Immunol. 2011, 2, 21. [Google Scholar] [CrossRef]
- Verberckmoes, S.; Van Soom, A.; de Kruif, A. Intra-uterine insemination in farm animals and humans. Reprod. Domest. Anim. 2004, 39, 195–204. [Google Scholar] [CrossRef]
- Ombelet, W.; Van Robays, J. Artificial insemination history: Hurdles and milestones. Facts Views Vis. Obgyn 2015, 7, 137–143. [Google Scholar] [PubMed]
- Hunter, R.H.F.; López-Gatius, F. From sperm to embryos; lessons learnt from Tim Rowson’s career. Theriogenology 2021, 172, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Foote, R.H. Review: Dairy cattle reproductive physiology research and management--past progress and future prospects. J. Dairy Sci. 1996, 79, 980–990. [Google Scholar] [CrossRef]
- Funk, D.A. Major advances in globalization and consolidation of the artificial insemination industry. J. Dairy Sci. 2006, 89, 1362–1368. [Google Scholar] [CrossRef]
- Lewis, N.M.; Canedo-Ribeiro, C.; Rathje, C.C.; Jennings, R.L.; Danihel, M.; Bosman, L.M.; Silvestri, G.; Griffin, D.K. The economic burden of chromosome translocations and the benefits of enhanced screening for cattle breeding. Animals 2022, 12, 1982. [Google Scholar] [CrossRef]
- Foote, R.H. The artificial insemination. In New Technologies in Animal Breeding; Brackett, B.G., Seidel, G.E., Seidel, S.M., Eds.; Academic Press: New York, NY, USA, 1981; pp. 13–39. [Google Scholar]
- López-Gatius, F. Factors of a noninfectious nature affecting fertility after artificial insemination in lactating dairy cows. A review. Theriogenology 2012, 77, 1029–1041. [Google Scholar] [CrossRef]
- Dalton, J.C.; Robinson, J.Q.; Price, W.J.; DeJarnette, J.M.; Chapwanya, A. Artificial insemination of cattle: Description and assessment of a training program for veterinary students. J. Dairy Sci. 2021, 104, 6295–6303. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Wilkowska, M.; Kwasniewicz, A.; Graczyk, S.; Grzeczka, A.; Kulus, J.; Gehrke, M.; Kolomak, I.; Jaskowski, J.M. Progress in acquiring skills in cattle rectal examination by veterinary students consulting their acquired experience and professional motivation. Med. Weter. 2022, 78, 450–455. [Google Scholar] [CrossRef]
- Koch, J.; Weber, L.P.; Heppelmann, M.; Freise, F.; Klingelmann, M.; Bachmann, L. Effect of different thawing methods for frozen bull semen and additional factors on the conception rate of dairy cows in artificial insemination. Animals 2022, 12, 2330. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Treviño, L.M.; Chavez, M.I.; Véliz, F.G.; Macías-Cruz, U.; Avendaño-Reyes, L.; García, J.E. Fertility of Holstein cows and heifers submitted to timed artificial insemination and receiving one or two doses (12 h apart) of semen. Reprod. Domest. Anim. 2022. Advance online publication. [Google Scholar] [CrossRef]
- Foote, R.H. Estrus detection and estrus detection aids. J. Dairy Sci. 1975, 58, 248–256. [Google Scholar] [CrossRef]
- Senger, P.L. The estrus detection problem: New concepts, technologies, and possibilities. J. Dairy Sci. 1994, 77, 2745–2753. [Google Scholar] [CrossRef]
- Diskin, M.G.; Sreenan, J.M. Expression and detection of oestrus in cattle. Reprod. Nutr. Dev. 2000, 40, 481–491. [Google Scholar] [CrossRef]
- Roelofs, J.; López-Gatius, F.; Hunter, R.H.F.; van Eerdenburg, F.J.C.M.; Hanzen, C.H. When is a cow in estrus? Clinical and practical aspects. Theriogenology 2010, 74, 327–344. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Tanaka, T.; Kamomae, H. An investigation of the time period within which frozen-thawed semen delivers a high conception rate in lactating dairy cows. J. Reprod. Dev. 2020, 66, 277–280. [Google Scholar] [CrossRef]
- Hammond, J. The Physiology of Reproduction in the Cow; Cambridge University Press: Cambridge, UK, 1927; pp. 9–111. [Google Scholar]
- Laing, J. Observations on the survival time of the spermatozoa in the genital tract of the cow and its relation to fertility. J. Agric. Sci. 1945, 35, 72–83. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; Wilmut, I. Sperm transport in the cow: Peri-ovulatory redistribution of viable cells within the oviduct. Reprod. Nutr. Dev. 1984, 24, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F. Sperm release from oviduct epithelial binding is controlled hormonally by peri-ovulatory graafian follicles. Mol. Reprod. Dev. 2008, 75, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 2008, 52, 455–462. [Google Scholar] [CrossRef]
- Hunter, R.H.F. Ageing of the unfertilized cow egg in vivo: How soon is fertility compromised? Vet. Rec. 1989, 124, 489–490. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; Greve, T. Could artificial insemination of cattle be more fruitful? Penalties associated with ageing eggs. Reprod. Domest. Anim. 1997, 32, 137–141. [Google Scholar] [CrossRef]
- Saacke, R.G. Insemination factors related to timed AI in cattle. Theriogenology 2008, 70, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, A.R.; Lean, I.J.; Stevenson, M.A. Efficacy of Ovsynch program on reproductive performance in dairy cattle: A meta-analysis. J. Dairy Sci. 2005, 88, 2754–2770. [Google Scholar] [CrossRef]
- Macmillan, K. L Recent advances in the synchronization of estrus and ovulation in dairy cows. J. Reprod. Dev. 2010, 56, S42–S47. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Santos, V.G.; Giordano, J.O.; Wiltbank, M.C.; Fricke, P.M. Development of fertility programs to achieve high 21-day pregnancy rates in high-producing dairy cows. Theriogenology 2018, 114, 165–172. [Google Scholar] [CrossRef]
- López-Gatius, F. Ovarian response to prostaglandin F2α in lactating dairy cows: A clinical update. J. Reprod. Dev. 2022, 68, 104–109. [Google Scholar] [CrossRef]
- Vishwanath, R.; Moreno, J.F. Review: Semen sexing—Current state of the art with emphasis on bovine species. Animal 2018, 12, s85–s96. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.O.; Morotti, F.; Lorenzetti, E.; Bizarro-Silva, C.; Seneda, M.M. Intensified use of TAI and sexed semen on commercial farms. Anim. Reprod. 2018, 15, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.; Pirez, M.C.; Steele, H.; Kölle, S. The reproductive success of bovine sperm after sex-sorting: A meta-analysis. Sci. Rep. 2021, 11, 17366. [Google Scholar] [CrossRef]
- Seidel, G.E., Jr.; DeJarnette, J.M. Applications and world-wide use of sexed semen in cattle. Anim. Reprod. Sci. 2021. Advance online publication. [Google Scholar] [CrossRef]
- Thomas, J.M.; Locke, J.; Bonacker, R.C.; Knickmeyer, E.R.; Wilson, D.J.; Vishwanath, R.; Arnett, A.M.; Smith, M.F.; Patterson, D.J. Evaluation of SexedULTRA 4M™ sex-sorted semen in timed artificial insemination programs for mature beef cows. Theriogenology 2019, 123, 100–107. [Google Scholar] [CrossRef]
- Perry, G.A.; Walker, J.A.; Rich, J.; Northrop, E.J.; Perkins, S.D.; Beck, E.E.; Sandbulte, M.D.; Mokry, F.B. Influence of Sexcel™ (gender ablation technology) gender-ablated semen in fixed-time artificial insemination of beef cows and heifers. Theriogenology 2020, 146, 140–144. [Google Scholar] [CrossRef]
- Trimberger, G.W.; Davis, H.P. Breeding efficiency in dairy cattle bred at various stages of estrus by artificial insemination. J. Dairy Sci. 1943, 26, 757–759. [Google Scholar]
- Trimberger, G.W. Conception rate in dairy cattle by insemination at various intervals before and after ovulation. J. Dairy Sci. 1944, 27, 659–660. [Google Scholar]
- Hall, J.G.; Branton, C.; Stone, E.J. Estrus, estrous cycles, ovulation time, time of service, and fertility of dairy cattle in Louisiana. J. Dairy Sci. 1959, 42, 1086–1094. [Google Scholar] [CrossRef]
- Gwazdauskas, F.C.; Whitter, W.D.; Vinson, W.E.; Pearson, R.E. Evaluation of reproductive efficiency of dairy cattle with emphasis on time of breeding. J. Dairy Sci. 1986, 69, 290–297. [Google Scholar] [CrossRef]
- Rankin, T.A.; Smith, W.R.; Shanks, R.D.; Lodge, J.R. Timing of insemination in dairy heifers. J. Dairy Sci. 1992, 75, 2840–2845. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F.; Labèrnia, J.; Santolaria, P.; Rutllant, J.; López-Béjar, M. The relationship of rheological behavior of the vaginal fluid at the time of insemination to the pregnancy rate in dairy cows. Theriogenology 1997, 48, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Nebel, R.L.; Dransfield, M.G.; Jobst, S.M.; Bame, J.H. Automated electronic systems for the detection of oestrus and timing of AI in cattle. Anim. Reprod. Sci. 2000, 60, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.C.; Nadir, S.; Bame, J.H.; Noftsinger, M.; Nebel, R.L.; Saacke, R.G. Effect of time of insemination on number of accessory sperm, fertilization rate, and embryo quality in nonlactating dairy cattle. J. Dairy Sci. 2001, 84, 2413–2418. [Google Scholar] [CrossRef]
- Nebel, R.L.; Walker, W.L.; McGilliard, M.L.; Allen, C.H.; Heckman, G.S. Timing of artificial insemination of dairy cows: Fixed time once daily versus morning and afternoon. J. Dairy Sci. 1994, 77, 3185–3191. [Google Scholar] [CrossRef]
- Foote, R.H. Time of artificial insemination and fertility in dairy cattle. J. Dairy Sci. 1979, 62, 355–358. [Google Scholar] [CrossRef]
- At-Taras, E.E.; Spahr, S.L. Detection and characterization of estrus in dairy cattle with an electronic heatmount detector and an electronic activity tag. J. Dairy Sci. 2001, 84, 792–798. [Google Scholar] [CrossRef]
- Cavalieri, J.; Flinker, L.R.; Anderson, G.A.; Macmillan, K.L. Characteristics of oestrus measured using visual observation and radiotelemetry. Anim. Reprod. Sci. 2003, 76, 1–12. [Google Scholar] [CrossRef]
- Roelofs, J.B.; van Eerdenburg, F.J.; Soede, N.M.; Kemp, B. Pedometer readings for estrous detection and as predictor for time of ovulation in dairy cattle. Theriogenology 2005, 64, 1690–1703. [Google Scholar] [CrossRef]
- Borchers, M.R.; Bewley, J.M. An assessment of producer precision dairy farming technology use, prepurchase considerations, and usefulness. J. Dairy Sci. 2015, 98, 4198–4205. [Google Scholar] [CrossRef]
- Reith, S.; Hoy, S. Review: Behavioral signs of estrus and the potential of fully automated systems for detection of estrus in dairy cattle. Animal 2018, 12, 398–407. [Google Scholar] [CrossRef]
- Roelofs, J.B.; Graat, E.A.; Mullaart, E.; Soede, N.M.; Voskamp-Harkema, W.; Kemp, B. Effects of insemination-ovulation interval on fertilization rates and embryo characteristics in dairy cattle. Theriogenology 2006, 66, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Hockey, C.D.; Morton, J.M.; Norman, S.T.; McGowan, M.R. Improved prediction of ovulation time may increase pregnancy rates to artificial insemination in lactating dairy cattle. Reprod. Domest. Anim. 2010, 45, e239–e248. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, E.; Kanno, C.; Yanagawa, Y.; Katagiri, S.; Nagano, M. Relationship between the timing of insemination based on estrus detected by the automatic activity monitoring system and conception rates using sex-sorted semen in Holstein dairy cattle. J. Reprod. Dev. 2022, 68, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.L.; Nebel, R.L.; McGilliard, M.L. Time of ovulation relative to mounting activity in dairy cattle. J. Dairy Sci. 1996, 79, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.; Weitze, K.F.; Otto, F.; Maciel, S.; Tomo, P. Onset and duration of oestrus related to the time of ovulation and fertility in a herd of Nguni cattle in south Mozambique. Reprod. Domest. Anim. 1997, 32, 303–307. [Google Scholar] [CrossRef]
- Pinheiro, O.L.; Barros, C.M.; Figueiredo, R.A.; do Valle, E.R.; Encarnação, R.O.; Padovani, C.R. Estrous behavior and the estrus-to-ovulation interval in Nelore cattle (Bos indicus) with natural estrus or estrus induced with prostaglandin F2 alpha or norgestomet and estradiol valerate. Theriogenology 1998, 49, 667–681. [Google Scholar] [CrossRef]
- Saumande, J.; Humblot, P. The variability in the interval between estrus and ovulation in cattle and its determinants. Anim. Reprod. Sci. 2005, 85, 171–182. [Google Scholar] [CrossRef]
- Wishart, D.F. Observations on the oestrous cycle of the Friesian heifer. Vet. Rec. 1972, 90, 595–597. [Google Scholar] [CrossRef]
- Roelofs, J.B.; van Eerdenburg, F.J.; Soede, N.M.; Kemp, B. Various behavioral signs of estrous and their relationship with time of ovulation in dairy cattle. Theriogenology 2005, 63, 1366–1377. [Google Scholar] [CrossRef]
- Cutullic, E.; Delaby, L.; Causeur, D.; Michel, G.; Disenhaus, C. Hierarchy of factors affecting behavioural signs used for oestrus detection of Holstein and Normande dairy cows in a seasonal calving system. Anim. Reprod. Sci. 2009, 113, 22–37. [Google Scholar] [CrossRef]
- Garcia, E.; Hultgren, J.; Fällman, P.; Geust, J.; Algers, B.; Stilwell, G.; Gunnarsson, S.; Rodriguez-Martinez, H. Intensity of oestrus signalling is the most relevant indicator for animal well-being in high-producing dairy cows. Vet. Med. Int. 2011, 2011, 540830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaude, I.; Kempf, A.; Strüve, K.D.; Hoedemaker, M. Comparison of visual and computerized estrous detection and evaluation of influencing factors. Anim. Reprod. Sci. 2017, 184, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Dobson, H.; Walker, S.L.; Morris, M.J.; Routly, J.E.; Smith, R.F. Why is it getting more difficult to successfully artificially inseminate dairy cows? Animal 2008, 2, 1104–1111. [Google Scholar] [CrossRef]
- Valenza, A.; Giordano, J.O.; Lopes, G.; Vincenti, L.; Amundson, M.C.; Fricke, P.M. Assessment of an accelerometer system for detection of estrus and treatment with gonadotropin-releasing hormone at the time of insemination in lactating dairy cows. J. Dairy Sci. 2012, 95, 7115–7127. [Google Scholar] [CrossRef]
- Tippenhauer, C.M.; Plenio, J.L.; Madureira, A.; Cerri, R.; Heuwieser, W.; Borchardt, S. Factors associated with estrous expression and subsequent fertility in lactating dairy cows using automated activity monitoring. J. Dairy Sci. 2021, 104, 6267–6282. [Google Scholar] [CrossRef]
- Denis-Robichaud, J.; Cerri, R.; Jones-Bitton, A.; LeBlanc, S.J. Survey of reproduction management on Canadian dairy farms. J. Dairy Sci. 2016, 99, 9339–9351. [Google Scholar] [CrossRef] [PubMed]
- Adenuga, A.H.; Jack, C.; Olagunju, K.O.; Ashfield, A. Economic viability of adoption of automated oestrus detection technologies on dairy farms: A review. Animals 2020, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, T.; Tanaka, T.; Kamomae, H. Relationships between the appearances and changes of estrous signs and the estradiol-17β peak, luteinizing hormone surge and ovulation during the periovulatory period in lactating dairy cows kept in tie-stalls. J. Reprod. Dev. 2014, 60, 106–114. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Endo, N.; Tanaka, T.; Kamomae, H. Evaluation of criteria for optimal time AI postulated by estrous signs in lactating dairy cows kept in tie-stalls. J. Reprod. Dev. 2017, 63, 597–604. [Google Scholar] [CrossRef][Green Version]
- López-Gatius, F.; Rutllant, J.; Labèrnia, J.; Ibarz, A.; López-Béjar, M.; Santolaria, P. Rheological behavior of the vaginal fluid of dairy cows at estrus. Theriogenology 1996, 46, 57–63. [Google Scholar] [CrossRef]
- Rutllant, J.; López-Béjar, M.; López-Gatius, F. Ultrastructural and rheological properties of bovine vaginal fluid and its relation to sperm motility and fertilization: A review. Reprod. Domest. Anim. 2005, 40, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sturman, H.; Oltenacu, E.A.; Foote, R.H. Importance of inseminating only cows in estrus. Theriogenology 2000, 53, 1657–1667. [Google Scholar] [CrossRef]
- Studer, E. Palpation of the genital tract for prediction of estrus in the cow. Vet. Med. Small Anim. Clin. 1975, 70, 1337–1341. [Google Scholar]
- Hanzen, C. Estrus—Behavior patterns and methods of detection—A review. Ann Méd. Vét. 1981, 125, 617–633. [Google Scholar]
- Keenan, L.R.J. Routine evaluation of the reproductive tract and pregnancy diagnosis in the cow. Irish Vet. J. 1984, 38, 132–135. [Google Scholar]
- López-Gatius, F. Feeling the ovaries prior to insemination. Clinical implications for improving the fertility of the dairy cow. Theriogenology 2011, 76, 177–183. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F. Is fertility declining in dairy cattle? A retrospective study in northeastern Spain. Theriogenology 2003, 60, 89–99. [Google Scholar] [CrossRef]
- López-Gatius, F.; Hunter, R.H.F. Clinical relevance of pre-ovulatory follicular temperature in heat-stressed lactating dairy cows. Reprod. Domest. Anim. 2017, 52, 366–370. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Endo, N.; Tanaka, T.; Kamomae, H. No adverse effect of confirmation of ovulation by rectal palpation and ultrasonography after artificial insemination on formation, development, and function of the corpus luteum and conception rate in cows. J. Reprod. Dev. 2022. Advance Online Publication. [Google Scholar] [CrossRef]
- Hays, R.L.; Vandemark, N.L. Effect of stimulation of the reproductive organs of the cow on the release of an oxytocin-like substance. Endocrinology 1953, 52, 634–637. [Google Scholar] [CrossRef]
- Vandemark, N.L.; Hays, R.L. Uterine motility responses to mating. Am. J. Physiol. 1952, 170, 518–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marion, G.B.; Smith, V.R.; Wiley, T.E.; Barrett, G.R. The effect of sterile copulation on time of ovulation in dairy heifers. J. Dairy Sci. 1950, 33, 885–889. [Google Scholar] [CrossRef]
| AI-ovulation Interval (a) | Conception (n = 132) [42] | Conception (n = 100) [22] | Good Quality Embryos (n = 122) [56] |
|---|---|---|---|
| Before ovulation | |||
| >24 h | 8/15 (53.3%) | 10/26 (38.5%) | 11/27 (40.7%) * |
| 12–24 h | 23/29 (79.3%) | 18/28 (64.3%) | 23/34 (67.6%) ** |
| 0–12 h | 19/28 (67.9%) | 16/31 (51.6%) | 12/29 (41.4%) *** |
| After ovulation | |||
| 0–12 h | 14/40 (35%) | 3/12 (25%) | 2/32 (6.3%) **** |
| Ovulation assessed | Every 2 h | Every 6 h | Every 4 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gatius, F. Revisiting the Timing of Insemination at Spontaneous Estrus in Dairy Cattle. Animals 2022, 12, 3565. https://doi.org/10.3390/ani12243565
López-Gatius F. Revisiting the Timing of Insemination at Spontaneous Estrus in Dairy Cattle. Animals. 2022; 12(24):3565. https://doi.org/10.3390/ani12243565
Chicago/Turabian StyleLópez-Gatius, Fernando. 2022. "Revisiting the Timing of Insemination at Spontaneous Estrus in Dairy Cattle" Animals 12, no. 24: 3565. https://doi.org/10.3390/ani12243565
APA StyleLópez-Gatius, F. (2022). Revisiting the Timing of Insemination at Spontaneous Estrus in Dairy Cattle. Animals, 12(24), 3565. https://doi.org/10.3390/ani12243565





