Brazilian Horses from Bahia State Are Highly Infected with Sarcocystis bertrami
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Equine Samples
2.2. Macroscopic Examination
2.3. Microscopical Analysis Using Fresh and Stained Tissues
2.4. Histology and Transmission Electron Microscopy
2.5. Tissue Digestion in Acid Pepsin
2.6. Molecular Analysis
3. Results
3.1. Macroscopic Evaluation
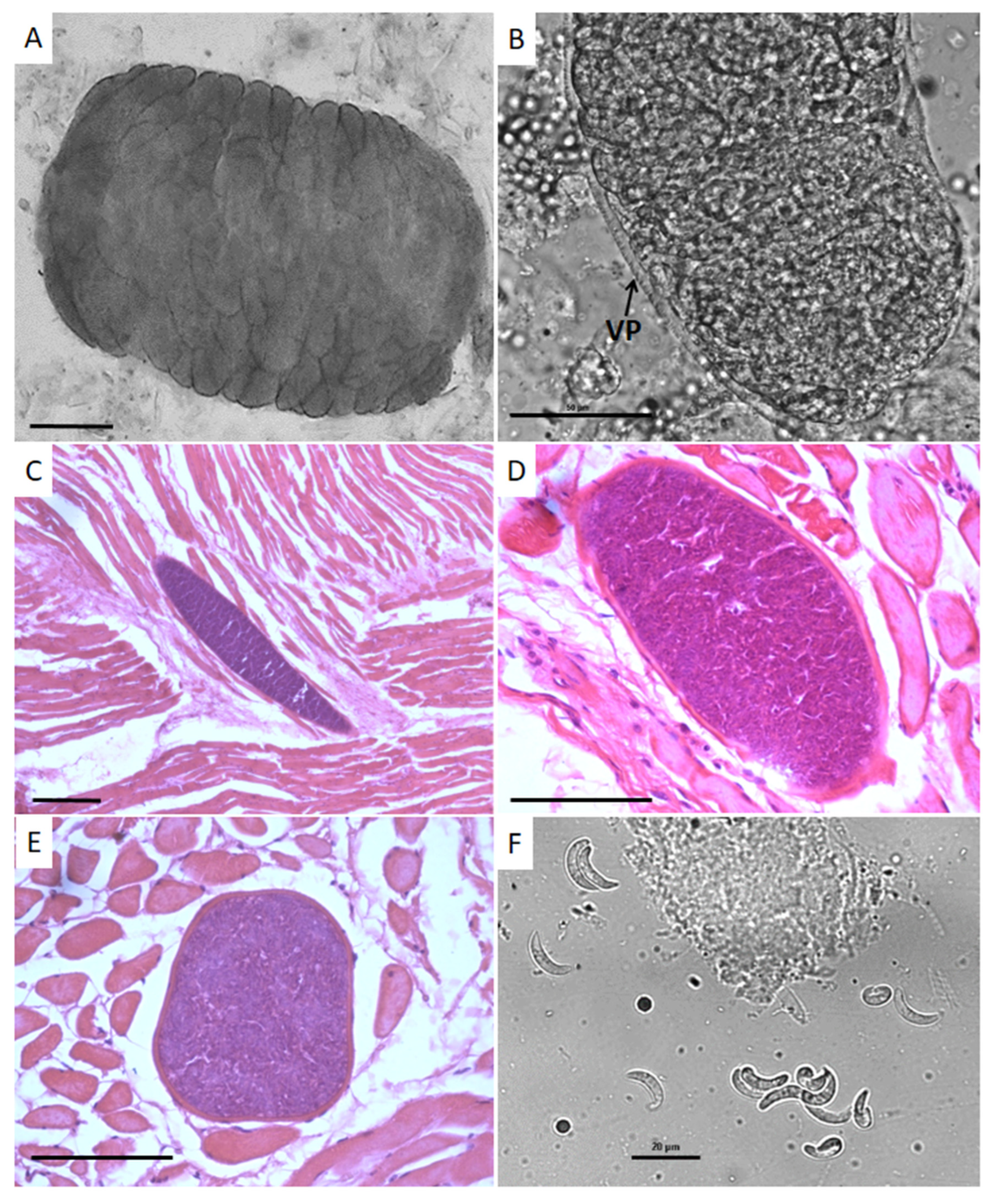
3.2. Microscopic Examination
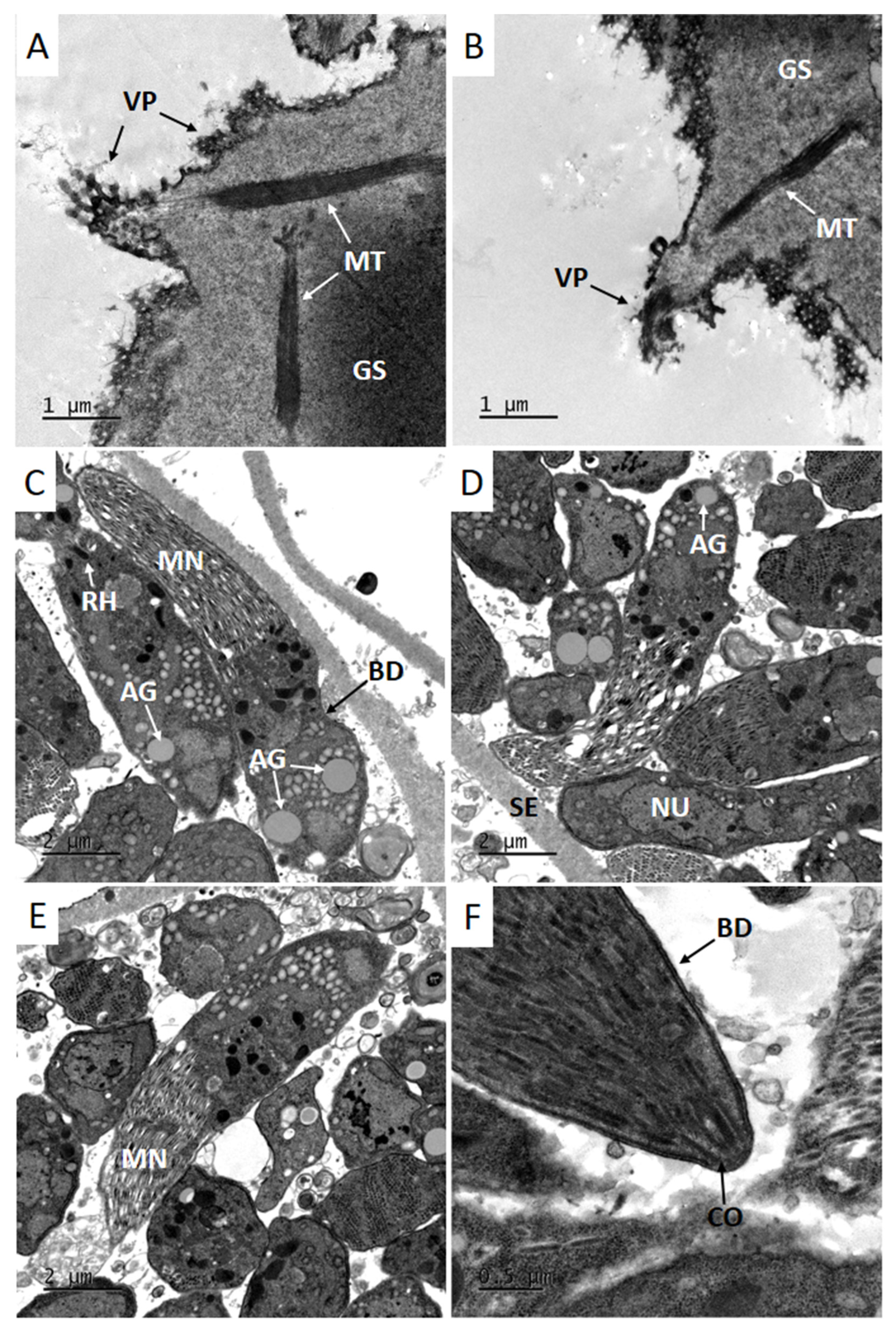
3.3. Transmission Electron Microscopy (TEM)
3.4. Bradyzoites in Pepsin Digested Samples
3.5. Molecular Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dupouy-Camet, J. History of trichinellosis in the history of the catalog of the French National Library. Hist. Sci. Med. 2015, 49, 411–420. [Google Scholar] [PubMed]
- Murrell, K.D.; Djordjevic, M.; Cuperlovic, K.; Sofronic, L.; Savic, M.; Djordjevic, M.; Damjanovic, S. Epidemiology of Trichinella infection in the horse: The risk from animal product feeding practices. Vet. Parasitol. 2004, 123, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Sarries, M.V.; Tateo, A.; Polidori, P.; Franco, D.; Lanza, M. Carcass characteristics, meat quality and nutritional value of horsemeat: A review. Meat Sci. 2014, 96, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Rommel, M.; Geisel, O. Studies on the incidence and life cycle of a sarcosporidian species of the horse (Sarcocystis equicanis n. spec). Berl. Munch. Tierarztl. Wochenschr. 1975, 88, 468–471. [Google Scholar]
- Dubey, J.P.; Streitel, R.H.; Stromberg, P.C.; Toussant, M.J. Sarcocystis fayeri sp. n. from the horse. J. Parasitol. 1977, 63, 443–447. [Google Scholar] [CrossRef]
- Hinaidy, H.; Loupal, G. Sarcocystis bertrami Doflein, 1901, ein Sarkosporid des Pferdes, Equus caballus. Zent. Veterinärmed. B 1982, 29, 681–701. [Google Scholar] [CrossRef]
- Schnieder, T.; Zimmermann, U.; Matuschka, F.; Bürger, H.J.; Rommel, M. Zur Klinik, Enzymaktivität und Antikörperbildung bei experimentell mit Sarkosporidien infizierten Pferden 1. Zent. Veterinärmed. B 1985, 32, 29–39. [Google Scholar] [CrossRef]
- Fukuyo, M.; Battsetseg, G.; Byambaa, B. Prevalence of Sarcocystis infection in horses in Mongolia. Southeast Asian J. Trop. Med. Public Health 2002, 33, 718–719. [Google Scholar]
- Edwards, G. Prevalence of equine sarcocystis in British horses and a comparison of two detection methods. Vet. Rec. 1984, 115, 265–267. [Google Scholar] [CrossRef]
- Erber, M.; Geisel, O. Vorkommen und entwicklung von 2 sarkosporidienarten des pferdes. Z. Parasitenkd. 1981, 65, 283–291. [Google Scholar] [CrossRef]
- Kirmse, P. Sarcosporidiosis in equines of Morocco. Br. Vet. J. 1986, 142, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, R.J.; Clark, M.; Hudson, R.; Friesen, D. Histological and ultrastructural appearance of severe Sarcocystis fayeri infection in a malnourished horse. J. Vet. Diagn. Investig. 1990, 2, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Kamata, Y.; Saito, M.; Irikura, D.; Yahata, Y.; Ohnishi, T.; Bessho, T.; Inui, T.; Watanabe, M.; Sugita-Konishi, Y. A toxin isolated from Sarcocystis fayeri in raw horsemeat may be responsible for food poisoning. J. Food Prot. 2014, 77, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Herd, H.R.; Sula, M.M.; Starkey, L.A.; Panciera, R.J.; Johnson, E.M.; Snider, T.A.; Holbrook, T.C. Sarcocystis fayeri-Induced Granulomatous and Eosinophilic Myositis in 2 Related Horses. Vet. Pathol. 2015, 52, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Van Wilpe, E.; Verma, S.K.; Hilali, M. Ultrastructure of Sarcocystis bertrami sarcocysts from a naturally infected donkey (Equus asinus) from Egypt. Parasitology 2016, 143, 18–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murata, R.; Suzuki, J.; Hyuga, A.; Shinkai, T.; Sadamasu, K. Molecular identification and characterization of Sarcocystis spp. in horsemeat and beef marketed in Japan. Parasite 2018, 25, 27. [Google Scholar] [CrossRef]
- Zeng, W.; Sun, L.; Xiang, Z.; Li, N.; Zhang, J.; He, Y.; Li, Q.; Yang, F.; Song, J.; Morris, J.; et al. Morphological and molecular characteristics of Sarcocystis bertrami from horses and donkeys in China. Vet. Parasitol. 2018, 252, 89–94. [Google Scholar] [CrossRef]
- Ma, C.L.; Ye, Y.L.; Wen, T.; Huang, Z.M.; Pan, J.; Hu, J.J.; Tao, J.P.; Song, J.L. Prevalence and morphological and molecular characteristics of Sarcocystis bertrami in horses in China. Parasite 2020, 27, 1. [Google Scholar] [CrossRef]
- Passantino, G.; Lia, R.P.; Latrofa, S.; Annoscia, G.; Slapeta, J.; Otranto, D.; Rossi, R.; Zizzo, N. Sarcocystis bertrami in skeletal muscles of donkeys (Equus africanus asinus) from Southern Italy. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100283. [Google Scholar] [CrossRef]
- Lunde, M.N.; Fayer, R. Serologic test for antibody to Sarcocystis in cattle. J. Parasitol. 1977, 63, 222–225. [Google Scholar] [CrossRef]
- Gjerde, B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013, 43, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Sarcocystis species in red deer revisited: With a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitology 2014, 141, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.S.; Mitchell, S.M.; Vianna, M.C.; Dubey, J.P. Sarcocystis neurona (Protozoa: Apicomplexa): Description of oocysts, sporocysts, sporozoites, excystation, and early development. J. Parasitol. 2004, 90, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.; Calero-Bernal, R.; Rosenthal, B.; Speer, C.; Fayer, R. Sarcocystosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- More, G.; Abrahamovich, P.; Jurado, S.; Bacigalupe, D.; Marin, J.C.; Rambeaud, M.; Venturini, L.; Venturini, M.C. Prevalence of Sarcocystis spp. in Argentinean cattle. Vet. Parasitol. 2011, 177, 162–165. [Google Scholar] [CrossRef]
- Willekes, C. The Donkey in Human History: An Archaeological Perspective by Peter Mitchell. Phoenix 2018, 72, 403–405. [Google Scholar] [CrossRef]
- Gameiro, M.B.P.; Clancy, C.; Zanella, A.J. Between freedom and abandonment: Social representations of free-roaming donkeys in the Brazilian Northeast. Anthrozoos 2022, 35, 335–354. [Google Scholar] [CrossRef]
- Saville, W.J.; Dubey, J.P.; Oglesbee, M.J.; Sofaly, C.D.; Marsh, A.E.; Elitsur, E.; Vianna, M.C.; Lindsay, D.S.; Reed, S.M. Experimental infection of ponies with Sarcocystis fayeri and differentiation from Sarcocystis neurona infections in horses. J. Parasitol. 2004, 90, 1487–1491. [Google Scholar] [CrossRef][Green Version]
- Portella, L.P.; Cadore, G.C.; Sangioni, L.A.; Pellegrini, L.F.; Fighera, R.; Ramos, F.; Vogel, F.S. Antibodies against Apicomplexa protozoa and absence sarcocysts in heart tissues from horses in southern Brazil. Rev. Bras. Parasitol. Vet. 2017, 26, 100–103. [Google Scholar] [CrossRef]
- Speer, C.A.; Dubey, J.P.; McAllister, M.M.; Blixt, J.A. Comparative ultrastructure of tachyzoites, bradyzoites, and tissue cysts of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 1999, 29, 1509–1519. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, C.; da Silva, B.; Nogueira, Y.; Bezerra, T.; Tavares, A.; Borges-Silva, W.; Gondim, L. Brazilian Horses from Bahia State Are Highly Infected with Sarcocystis bertrami. Animals 2022, 12, 3491. https://doi.org/10.3390/ani12243491
Marques C, da Silva B, Nogueira Y, Bezerra T, Tavares A, Borges-Silva W, Gondim L. Brazilian Horses from Bahia State Are Highly Infected with Sarcocystis bertrami. Animals. 2022; 12(24):3491. https://doi.org/10.3390/ani12243491
Chicago/Turabian StyleMarques, Caroline, Bruno da Silva, Yuri Nogueira, Taynar Bezerra, Aline Tavares, Waléria Borges-Silva, and Luís Gondim. 2022. "Brazilian Horses from Bahia State Are Highly Infected with Sarcocystis bertrami" Animals 12, no. 24: 3491. https://doi.org/10.3390/ani12243491
APA StyleMarques, C., da Silva, B., Nogueira, Y., Bezerra, T., Tavares, A., Borges-Silva, W., & Gondim, L. (2022). Brazilian Horses from Bahia State Are Highly Infected with Sarcocystis bertrami. Animals, 12(24), 3491. https://doi.org/10.3390/ani12243491






