Co-Expression of T- and B-Cell Markers in a Canine Intestinal Lymphoma: A Case Report
Abstract
Simple Summary
Abstract
1. Introduction
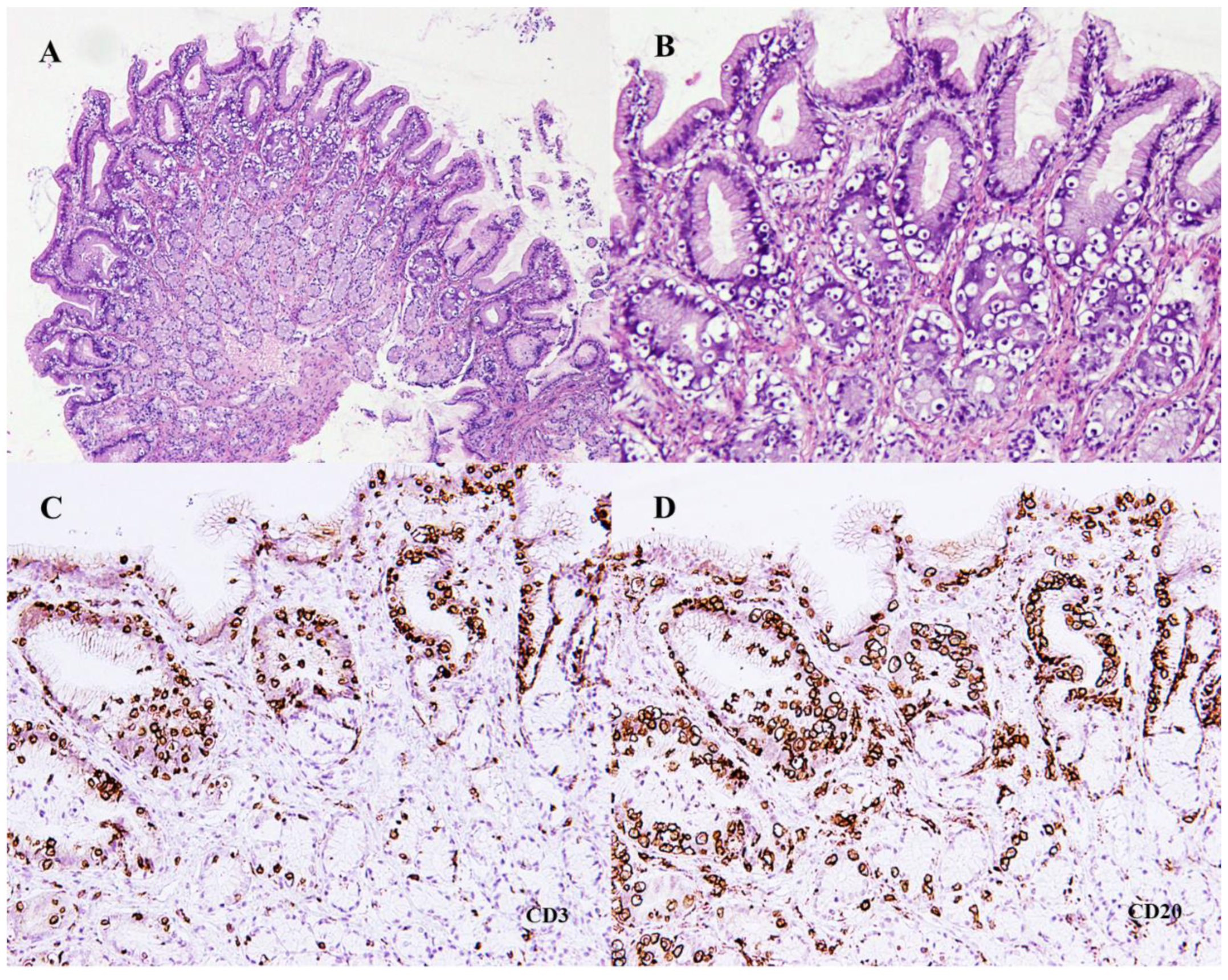
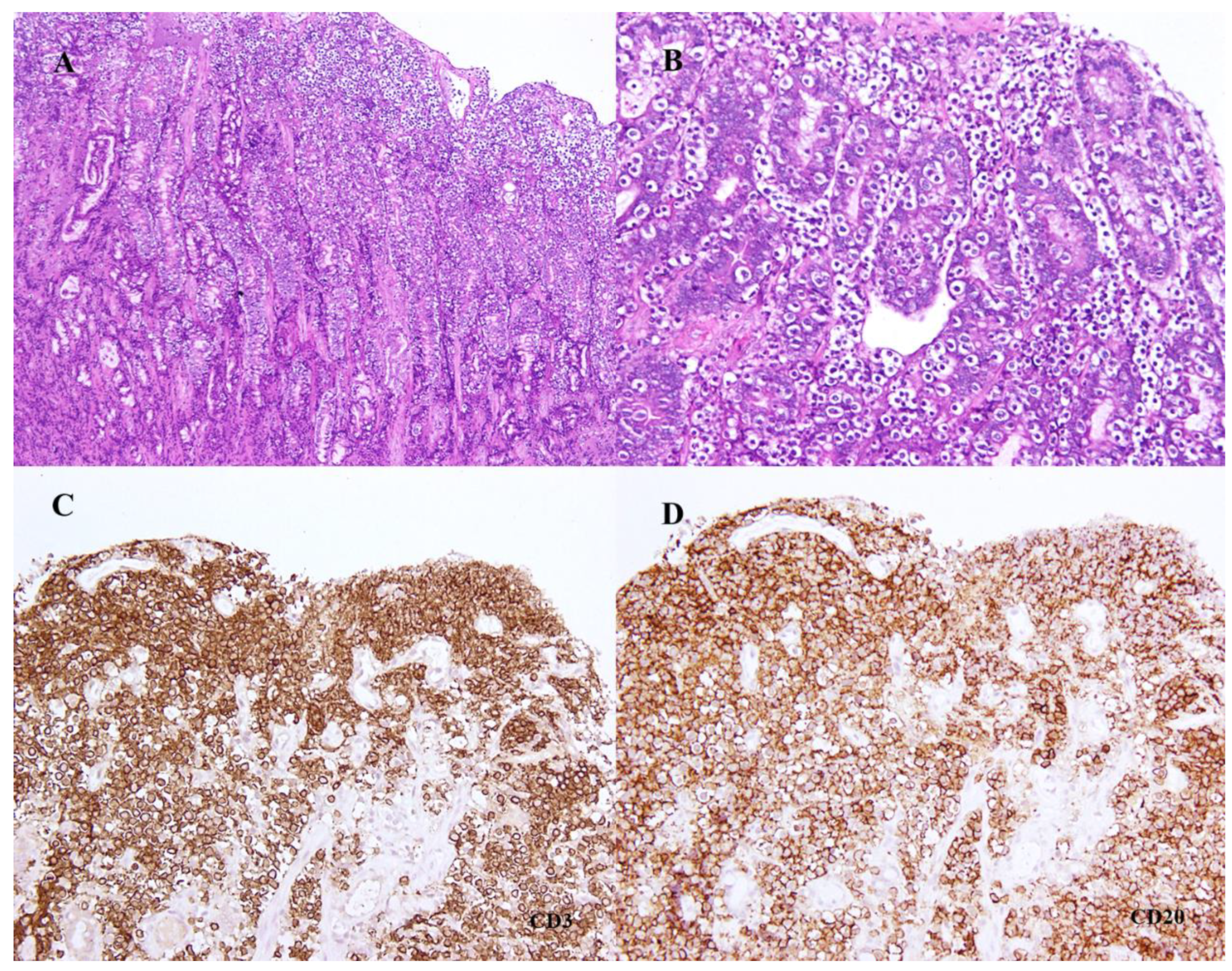
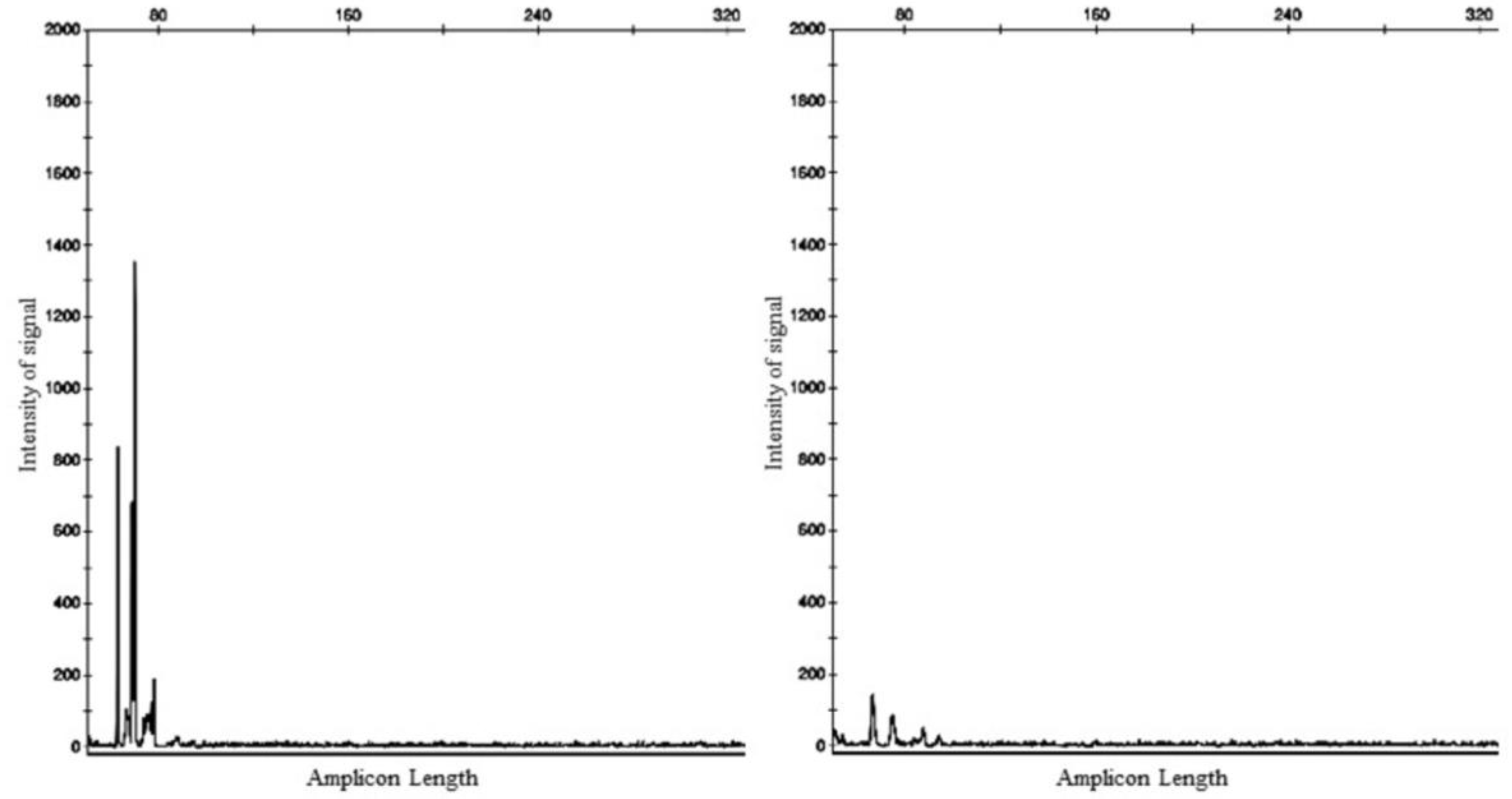
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vail, D.M.; Pinkerton, M.E.; Young, K.M. Hematopoietic Tumors. In Withrow & MacEwen’s Small Animal Clinical Oncology, 5th ed.; Elsevier: St. Louis, MI, USA, 2013; pp. 622–678. [Google Scholar]
- Munday, J.S.; Lohr, C.V.; Kiupel, M. Tumors of the alimentary tract. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 499–601. [Google Scholar]
- Coyle, K.A.; Steinberg, H. Characterization of lymphocytes in canine gastrointestinal lymphoma. Vet. Pathol. 2004, 41, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Gieger, T. Alimentary lymphoma in cats and dogs. Vet. Clin. North. Am. Small. Anim. Pract. 2011, 41, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Uzal, F.A.; Plattner, B.L.; Hostetterin, J.M. Alimentary System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals, 6th ed.; Grant Maxie, M., Ed.; Elsevier: St. Louis, MI, USA, 2016; Volume 2, pp. 16–257. [Google Scholar]
- Carrasco, V.; Rodrıguez-Bertos, A.; Rodrıguez-Franco, F.; Wise, A.G.; Maes, R.; Mullaney, T.; Kiupel, M. Distinguishing intestinal lymphoma from inflammatory bowel disease in canine duodenal endoscopic biopsy samples. Vet. Pathol. 2015, 52, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Chandesris, M.O.; Malamut, G.; Verkarre, V.; Meresse, B.; Macintyre, E.; Delarue, R.; Rubio, M.T.; Suarez, F.; Deau-Fischer, B.; Cerf-Bensussan, N.; et al. Enteropathy-associated T-cell lymphoma: A review on clinical presentation, diagnosis, therapeutic strategies and perspectives. Gastroenterol. Clin. Biol. 2010, 34, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Zinzani, P.L.; Govi, S.; Pileri, S.A. Enteropathy-associated T-cell lymphoma. Crit. Rev. Oncol. Hematol. 2011, 79, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Brachelente, C.; Affolter, V.K.; Fondati, A.; Porcellato, I.; Sforna, M.; Lepri, E.; Mechelli, L.; Bongiovanni, L. CD3 and CD20 coexpression in a case of canine cutaneous epitheliotropic T-cell lymphoma (Mycosis Fungoides). Vet. Pathol. 2016, 53, 563–566. [Google Scholar] [CrossRef]
- Noland, E.L.; Kiupel, M. Coexpression of CD3 and CD20 in canine enteropathy-associated T-cell lymphoma. Vet. Pathol. 2018, 55, 241–244. [Google Scholar] [CrossRef]
- Nicoletti, A.; Aresu, L.; Marino, M.; Massaro, M.; Martignani, E.; Caporali, E.; Capuccini, S.; Bonfanti, U.; Gola, C. CD3-CD20–positive nodal lymphoma with cross-lineage rearrangement in a dog. J. Vet. Diagn. Investig. 2020, 32, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Waugh, E.M.; Gallagher, A.; Haining, H.; Johnston, P.E.J.; Marchesi, F.; Jarrett, R.F.; Morris, J.S. Optimisation and validation of a PCR for antigen receptor rearrangement (PARR) assay to detect clonality in canine lymphoid malignancies. Vet. Immunol. Immunopathol. 2016, 182, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.D.; Reimer, S.B.; Kass, P.H.; Kiupel, M. Clinical outcomes of 30 cases (1997–2004) of canine gastrointestinal lymphoma. J. Am. Anim. Hosp. Assoc. 2007, 43, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sogame, N.; Risbon, R.; Burgess, K.E. Intestinal lymphoma in dogs: 84 cases (1997–2012). J. Am. Vet. Med. Assoc. 2018, 252, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Stockham, S.L.; Scott, M.A. Função Hepática. In Fundamentos da Patologia Clínica Veterinária, 2nd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2011; pp. 562–588. [Google Scholar]
- Siegel, A.; Wiseman, M. The Liver. In Cowell & Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat, 5th ed; Elsevier: St. Louis, MI, USA, 2020; pp. 347–578. [Google Scholar]
- Fernandes, N.C.; Guerra, J.M.; Réssio, R.A.; Wasques, D.G.; Etlinger-Colonelli, D.; Lorente, S.; Nogueira, E.; Dagli, M.L. Liquid-based cytology and cell block immunocytochemistry in veterinary medicine: Comparison with standard cytology for the evaluation of canine lymphoid samples. Vet. Comp. Oncol. 2016, 14, 107–116. [Google Scholar] [CrossRef]
- Heinrich, D.A.; Avery, A.C.; Henson, M.S.; Overmann, J.A.; Rendahl, A.K.; Walz, J.Z.; Seelig, D.M. Cytology and the cell block method in diagnostic characterization of canine lymphadenopathy and in the immunophenotyping of nodal lymphoma. Vet. Comp. Oncol. 2019, 17, 365–375. [Google Scholar] [CrossRef]
- Peleteiro, M.C. Citobloco. In Atlas de Citologia Veterinária, 1st ed.; Lidel: Lisbon, Portugal, 2011; pp. 297–302. [Google Scholar]
- Nakagun, S.; Horiuchi, N.; Watanabe, K.; Matsumoto, K.; Tagawa, M.; Shimbo, G.; Kobayashi, Y. CD3 and CD20 co-expression in a case of canine peripheral T-cell lymphoma with prominent cardiac and peripheral nerve involvement. J. Vet. Diagn. Investig. 2018, 30, 779–783. [Google Scholar] [CrossRef]
- Granum, L.; Gorman, E.; Ruaux, C.; Vernau, W. Biphenotypic B-cell lymphoma in 2 cats. Vet. Clin. Pathol. 2015, 44, 320–325. [Google Scholar] [CrossRef]
- Misra, D.S.; Bhardwaj, M.; Bahuguna, G.; Malhotra, V. An unusual case of enteropathy associated T-cell lymphoma with CD20 positivity. Indian J. Pathol. Microbiol. 2014, 57, 658–659. Available online: https://journals.sagepub.com/servlet/linkout?suffix=bibr6-0300985817747326&dbid=16&doi=10.1177%2F0300985817747326&key=10.4103%2F0377-4929.142727 (accessed on 27 November 2022). [CrossRef]
- Rahemtullah, A.; Longtine, J.A.; Harris, N.L.; Dorn, M.; Zembowicz, A.; Quintanilla-Fend, L.; Preffer, F.I.; Ferry, J.A. CD20þ T-cell lymphoma: Clinicopathologic analysis of 9 cases and a review of the literature. Am. J. Surg. Pathol. 2008, 32, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Schuh, E.; Berer, K.; Mulazzani, M.; Feil, K.; Meinl, I.; Lahm, H.; Krane, M.; Lange, R.; Pfannes, K.; Subklewe, M.; et al. Features of human CD3+CD20+ T cells. J. Immunol. 2016, 197, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Jullié, M.L.; Carlotti, M.; Vivot, A., Jr.; Beylot-Barry, M.; Ortonne, N.; Frouin, E.; Carlotti, A.; de Muret, A.; Balme, B.; Franck, F.; et al. CD20 antigen may be expressed by reactive or lymphomatous cells of transformed mycosis fungoides: Diagnostic and prognostic impact. Am. J. Surg. Pathol. 2013, 37, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Akalin, A.; Rodacker, M. CD20 positive T cell lymphoma: Is it a real entity? J. Clin. Pathol. 2004, 57, 442–444. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, P.C.L.G.; Peleteiro, M.C.; Carvalho, S.; Leal, R.O.; Pomba, C.; Duarte, A.; Correia, J. Co-Expression of T- and B-Cell Markers in a Canine Intestinal Lymphoma: A Case Report. Animals 2022, 12, 3531. https://doi.org/10.3390/ani12243531
Valente PCLG, Peleteiro MC, Carvalho S, Leal RO, Pomba C, Duarte A, Correia J. Co-Expression of T- and B-Cell Markers in a Canine Intestinal Lymphoma: A Case Report. Animals. 2022; 12(24):3531. https://doi.org/10.3390/ani12243531
Chicago/Turabian StyleValente, Pâmela Cristina Lopes Gurgel, Maria Conceição Peleteiro, Sandra Carvalho, Rodolfo Oliveira Leal, Constança Pomba, António Duarte, and Jorge Correia. 2022. "Co-Expression of T- and B-Cell Markers in a Canine Intestinal Lymphoma: A Case Report" Animals 12, no. 24: 3531. https://doi.org/10.3390/ani12243531
APA StyleValente, P. C. L. G., Peleteiro, M. C., Carvalho, S., Leal, R. O., Pomba, C., Duarte, A., & Correia, J. (2022). Co-Expression of T- and B-Cell Markers in a Canine Intestinal Lymphoma: A Case Report. Animals, 12(24), 3531. https://doi.org/10.3390/ani12243531





