Simple Summary
Coccidiosis, a parasitic disease caused by protozoa of the genus Eimeria, is one of the most frequently investigated enteric poultry diseases, primarily due to its ubiquity and severe negative effects on the economic efficiency of the poultry industry. Immunoprophylaxis with live anticoccidial vaccines is regarded as an effective tool to control this parasitic disease; however, there is a great reluctance to use this approach in broilers, primarily because of reports of transient reduced performance due to the state of ‘mild coccidial infection’ associated with anticoccidial vaccines. In this context, the administration of some feed additives may be useful as supplementation to improve the health status and immune response. Therefore, the present study aimed to evaluate the effect of dietary supplementation with a probiotic on growth performance, oocyst shedding, and selected blood parameters in broilers vaccinated with a live oocyst vaccine.
Abstract
A total of 256 male Ross 308 chickens were assigned to four treatments in a 2 × 2 factorial design with two levels of the anticoccidial vaccine (ACV) Livacox T (none or 1 × dose) with or without dietary supplementation with the probiotic Protexin® (P). The growth performance parameters for the test periods (1–21, 22–42, and 1–42 d) and oocyst per gram (OPG) at weekly intervals were analysed. Blood samples were collected at 16 post-vaccination (pv) days to measure selected haematological, biochemical, redox, and immunological parameters. ACV administration worsened the performance parameters of the chickens for 1–21 d pv, while supplementation with P reduced this negative effect with a significant improvement in 1–21 d body weight gain and feed conversion ratio. ACV administration increased % phagocytic cells (%PC), phagocytic index (PI), respiratory burst activity, proportion of monocytes, and activities of aspartate aminotransferase (AST) and lactate dehydrogenase, while it decreased the catalase activity and concentration of malondialdehyde and peroxides. The dietary administration of P significantly increased counts of red blood cells and white blood cells and increased %PC and PI, while it decreased the heterophil proportion, heterophil/lymphocyte ratio (p = 0.059), and alanine aminotransferase and AST activities. The oocyst counts were comparable in all sampling periods, except on 14 d pv, as supplementation with P significantly decreased 14 d OPG, thus indicating a positive influence of P on immunity development. In conclusion, dietary supplementation with P led to improved performance, better immunity, and benefits in health status in broilers vaccinated with the ACV, without interfering with the circulating vaccine strains.
1. Introduction
Coccidiosis, caused by the intestinal protozoan parasite Eimeria species, has long been recognised as one of the most common and economically significant parasitic diseases in chickens [1,2,3,4]. The multiplication and growth of the coccidian parasite in the intestinal epithelium during infection cause diverse symptoms such as diarrhoea, haemorrhage, or even death in the severe form of coccidiosis or malabsorption, reduced performance, and enteritis in the subclinical form [1,5,6]. All of these effects compromise the welfare of birds and reduce the economic effectiveness of poultry production. According to a recent report by Blake et al. [1], the global cost of coccidiosis in chickens is estimated at approximately GBP 10.4 billion at 2016 prices, equivalent to GBP 0.16/chicken produced. Therefore, several efforts are being made globally to develop effective strategies to prevent or mitigate the negative effects of coccidiosis. Presently, the primary methods for controlling coccidiosis in chickens include the routine use of coccidiostats or anticoccidial vaccines (ACVs) based on live Eimeria strains [3]. However, both of these methods have limitations. For coccidiostats, the emergence of resistance of Eimeria strains to anticoccidials and the growing demand of customers for chemoprophylaxis-free poultry production have prompted the need to identify, develop, and implement other strategies to control coccidiosis.
Immunoprophylaxis of broilers with live ACVs, even though regarded as an effective approach to control coccidiosis and commonly used in the production of layers and breeders, still raises concerns of transient performance deterioration that may not be compensated during the relatively short lifespan of chickens [7,8,9]. ACVs function by inoculating a small number of oocysts of the specified species, which undergo their life cycle in the chicken gut. The second or third subsequent infection with the circulating oocysts should result in the development of acquired immunity to Eimeria species included in the vaccine, as the immunity is species-specific [10]. This process is sometimes accompanied by a state similar to mild subclinical coccidiosis, as the intestinal integrity may be compromised following replication of the vaccine oocysts in the intestinal epithelium; this results in a diminished absorptive intestinal surface area, malabsorption, and inflammation, and it can also be a predisposing factor for bacterial secondary enteritis [6,8].
In this context, nutritional methods as a supplementation approach to maintaining a healthy intestinal tract with balanced microflora might mitigate the abovementioned side effects of ACVs [11,12]. Probiotics, also known as direct-fed microbials, consist of non-pathogenic bacteria or fungi that beneficially act on animal health and growth performance through different modes of action such as stimulation of the immune system, modulation of the gut microbial ecosystem through the production of primary and secondary metabolites with antibacterial properties, and competitive exclusion of pathogens; these effects support the development of beneficial microflora, together with having a positive impact on the structural modulation of the intestinal epithelium and maintenance of the intestinal barrier integrity as well as increasing digestive enzyme activity and improving digestion [13,14,15]. Probiotics used in poultry are composed of one or multi-strain microbial species, which mainly belong to the genera Lactobacillus, Streptococcus, Bacillus, Enterococcus, Pediococcus, Aspergillus, and Saccharomyces [15,16]. The effectiveness of single [17,18,19,20] or multi-strain probiotics [21] was confirmed in broilers challenged with Eimeria and in birds vaccinated against coccidiosis [16,22]. However, most studies on probiotics and ACVs included a subsequent challenge with Eimeria to evaluate the combined effects of a probiotic and vaccine as a protective measure against coccidiosis. There is less research on the impact of probiotics in vaccinated birds that are not exposed to coccidiosis outbreaks or are experimentally challenged, although this situation is frequently encountered in poultry production conditions.
Protexin® (Probiotics International Ltd., Lopen Head, Somerset, UK) is a multi-strain probiotic preparation containing seven bacterial and two yeast strains. This bacterial and fungal combination preparation has been proven to enhance the immune response to Newcastle disease virus, lower the counts of caecal Escherichia coli [23], improve growth performance, and lower the levels of proinflammatory cytokines in broilers challenged with multiple bacterial species including avian pathogenic E. coli, Salmonella enteritidis, and Salmonella typhimurium [24]. However, this specific preparation has not been evaluated in chickens exposed to Eimeria oocysts.
Based on previous findings, we hypothesized that dietary supplementation with the probiotic Protexin® may reduce the potential negative side effects of ACVs on the growth performance of broiler chickens, without impairment of the circulating vaccine strain, which is crucial for the development of acquired immunity against coccidiosis. Thus, the present study aimed to evaluate the effects of Protexin® on broiler chickens vaccinated against coccidiosis in terms of their growth performance; profile of oocyst output of vaccine origin; and health status reflected by haematological, biochemical, redox, and immunological variables.
2. Materials and Methods
2.1. Birds, Diets, and Experimental Design
The animal experimental procedures were approved by the Second Local Ethical Committee on Animal Testing, Cracow, Poland. The study was designed as a 2 × 2 factorial experiment with 8 replicate pens of 8 male Ross 308 chickens per treatment. Treatments included a lack or a single dose of ACV (Livacox T®; Biopharm Co., Prague, Czech Republic, administered at 1 d of age) with or without dietary supplementation with the multi-strain probiotic Protexin® (Probiotics International Ltd., Lopen Head, Somerset, UK).
A total of 256 one-day-old male Ross 308 broiler chickens, obtained from a commercial hatchery (Daniela Kożuch Poultry Hatchery, Łężkowice, Poland), were randomly assigned to treatments at 1 d of age. The experimental period lasted for 42 days. The birds were reared under standard environmental conditions, with the temperature maintained from 32 °C at 1 d of age to 21 °C at 21 d of age.The birds were placed in floor pens with a total floor space of 0.76 m2 and equipped with 2 nipple-cup drinkers and a trough feeder. A bedding of wood shavings was used to ensure the recirculation of oocysts of vaccine origin. To avoid the transition of oocysts and reduce the risk of contamination of the unvaccinated groups, polyvinyl chloride sheets were used as barriers between the pens.
The birds were fed with maize–soybean meal basal diets in a mash form, and the diets were formulated to meet or exceed the nutritional requirements of broiler chickens for the starter (1–21 d of age) or grower-finisher (22–42 d of age) feeding phase (Table 1) [25]. The birds were provided water and fed ad libitum. The diets were free of antibiotic growth promoters and coccidiostats. Based on the chemical composition of raw feed materials, the content of nutrients in the basal diets was calculated, and the value of metabolizable energy was estimated according to equations from European Tables [26].

Table 1.
Composition of experimental diets for starter (1–21 d) and grower-finisher (22–42 d) feeding phases and nutrient content in basal feed mixtures.
2.2. Experimental Factors
At 1 d of age, 50% of the birds were administered a single, recommended dose of live, attenuated ACV (Livacox® T; Biopharm Research Institute of Biopharmacy and Veterinary Drugs, Prague, Czech Republic) before the chicks were placed in the designated pens. The single vaccine dose (0.01 mL), which contained 300–500 sporulated oocysts each of Eimeria acervulina, Eimeria maxima, and Eimeria tenella, was suspended in 0.24 mL distilled water and administered per os. Birds from the unvaccinated groups received an identical volume of distilled water.
In the treatment groups assigned to receive the probiotic bacteria, Protexin® was mixed with basal diets at the dose of 0.15 or 0.10 g/kg of feed in the starter or grower-finisher feeding phase, respectively. The composition of Protexin® was as follows: Lactobacillus plantarum, 1.89 × 1010 cfu/kg; Lactobacillus delbrueckii ssp. Bulgaricus, 3.09 × 1010 cfu/kg; Lactobacillus acidophilus, 3.09 × 1010 cfu/kg; Lactobacillus rhamnosus, 3.09 × 1010 cfu/kg; Bifidobacterium bifidum, 3.00 × 1010 cfu/kg; Streptococcus salivarius ssp. Thermophilus, 6.15 × 1010 cfu/kg; Enterococcus faecium, 8.85 × 1010 cfu/kg; Aspergillus oryzae, 7.98 × 109 cfu/kg; and Candida pintolopesii, 7.98 × 109 cfu/kg.
2.3. Sample Collection and Analytical Procedure
The standard AOAC methods [27] were used to analyse the basal diets for moisture (method 930.15), crude protein (method 984.13), crude fat (method 920.39), ash (method 942.05), amino acids (method 982.30), calcium (method 968.08), and total phosphorus content (method 965.17).
The feed intake was recorded weekly, and the birds were weighed at the age of 1, 21, and 42 d. The body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) were calculated for the starter, grower-finisher, and entire experimental period (1–42 d). FCR was calculated as kg feed/kg BWG, and the data were corrected for mortality. All growth performance data were analysed on a pen basis (n = 8).
To demonstrate the profile of oocyst shedding, the number of oocysts per gram of excreta (OPG) was determined using a concentration McMaster technique with a McMaster counting chamber [28]. OPG was calculated using pooled faecal samples collected from each replicate pen (n = 8) at 7, 14, 21, 28, and 35 d post-vaccination (pv). To obtain the pooled faecal sample, several fresh faecal samples from different locations in the pen were collected and homogenized for the analysis of OPG from each pen (replicate) separately. The OPG values were logarithmically transformed [log10 (OPG + 1)] to create a normal distribution.
At 16 d of age, the blood samples from 6 chickens per experimental group (1 chicken/replicate; n = 6) were collected from the wing vein into heparinized tubes and tubes without the anti-coagulant. The blood samples were then centrifuged at 3000× g for 10 min to obtain plasma and serum.
The following haematological parameters were analysed: haematocrit (Ht) and haemoglobin (Hb) levels, and red blood cell (RBC) and total white blood cell (WBC) count with leukograms [29]. The heterophil-to-lymphocyte ratio (H/L) was calculated as a parameter for the stress response [30,31].
Immunological analyses included the determination of phagocytic activity of leukocytes against the strain Staphylococcus aureus 209P, represented as the proportion of phagocytic cells (% PC) and as the phagocytic index (PI) [32]. The test of nitroblue tetrazolium (NBT) reduction to formazan was used to assess the respiratory burst activity of heterophils [33]. The turbidimetric method [32] was used to determine the lysozyme concentration.
The following biochemical indices were determined in the blood serum samples by using commercial kits developed by Cormay Co. (PZ Cormay Inc., Lomianki, Poland): total protein (TP), total cholesterol (TC), triacylglycerols (TG), and glucose (GLU) concentrations as well as enzyme activities: aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP).
As reported previously [34], the following indicators of redox status were determined in chicken blood plasma samples: concentrations of lipid peroxides (LOOH); malondialdehyde (MDA); ferric reducing ability of plasma (FRAP), as a parameter of the antioxidant potential; and activities of superoxide dismutase (SOD) and catalase (CAT).
2.4. Statistical Analysis
The data were analysed using STATISTICA version 13.3 (StatSoft Inc., Tulsa, OK, USA) software. Two-way analysis of variance (ANOVA) was used to determine the main effects of treatments such as ACV and dietary supplementation with the probiotic as the main factors and their interactions. One-way ANOVA was used to analyse the effect of probiotic supplementation on OPG results in vaccinated chickens. Duncan’s multiple range post hoc test was used to determine the differences between the treatments, and the effects were considered significant at a probability level of p ≤ 0.05.
3. Results
Performance results obtained in the starter, grower-finisher, and entire rearing period are presented in Table 2. The effects of ACV, probiotic supplementation, and their interaction on growth performance were significant only in the starter period. Regarding the independent effects, ACV significantly lowered FI, reduced BWG, and led to a higher FCR for the 1–21 d period, while dietary supplementation with probiotics significantly increased BWG for 1–21 d regardless of the vaccination status. The interaction of ACV × P was significant for 1–21 d FCR. Although P supplementation had no effect on FCR in unvaccinated birds, it improved FCR in the vaccinated ones to the level obtained in the unvaccinated groups.

Table 2.
Effects of the experimental factors on the performance in the starter (1–21 d) and grower-finisher (21–42 d) feeding phases and the entire experimental period (1–42 d).
Figure 1 presents the profile of oocyst shedding throughout the experimental period in the vaccinated groups of birds. The largest increase in OPG for the unsupplemented group was observed on 14 d pv, and the OPG value gradually decreased in the subsequent sampling periods. The highest value of OPG in the supplemented group was found on 7 d pv. On 14 d pv, the OPG value was significantly lower in the supplemented group than in the unsupplemented group. For all other sampling periods, the OPG was not affected by P supplementation (p > 0.05).
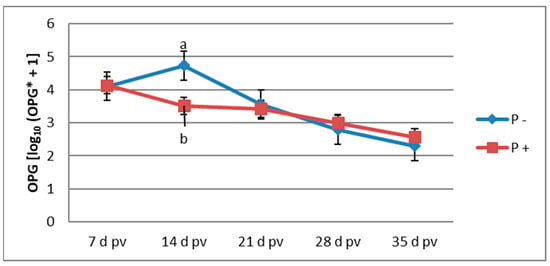
Figure 1.
Oocyst shedding in the vaccinated groups [log10 (OPG + 1]; (n = 6); a, b—mean values followed by different letters differ significantly at p ≤ 0.05; OPG*—oocysts per gram; pv—post-vaccination; P—probiotic preparation.
Table 3 presents the results for haematological parameters analysed from blood samples collected at 16 d of age. The unvaccinated birds receiving P showed a significant increase in RBC counts, while in vaccinated birds, the effect of P supplementation had no significant effect on this parameter (ACV × P; p ≤ 0.05). Regardless of ACV administration, P supplementation significantly increased the WBC count, decreased the proportion of heterophils in the leukogram, and decreased the H/L ratio (p = 0.059). The birds of both vaccinated groups showed an increase in the monocyte proportion in the leukogram (p ≤ 0.05). Haematocrit and haemoglobin levels and the proportions of lymphocytes, basophils, and eosinophils in the leukogram were not significantly influenced by the studied factors.

Table 3.
Haematological parameters and leukogram in the chicken blood collected at 16 d of age.
Significant effects of ACV administration were observed on the increased values of % PC, PI, and NBT in the vaccinated birds. Similarly, P supplementation increased the % PC. The unvaccinated birds receiving P supplementation in the diet showed a higher PI, while in the vaccinated ones, P supplementation did not further increase the PI values (Table 4).

Table 4.
Immunological indices of chicken blood collected at 16 d of age.
Table 5 shows the effects of experimental factors on the biochemical indices. ACV × P interactions were significant for AST activity and TP and GLU concentrations. Although vaccination resulted in a significantly higher AST activity and TP concentration in the unsupplemented group, P supplementation in vaccinated birds decreased the AST and TP values to levels comparable to those observed in both unvaccinated groups in whom P supplementation did not affect these parameters. In the vaccinated group, P supplementation increased the serum GLU concentration, while the feed additive did not cause a significant difference in unvaccinated birds. Regarding independent effects, ACV administration increased the LDH activity and serum TG concentration, but it lowered the ALP activity and CHOL concentration. P supplementation also had significant effects on the ALT activity and CHOL concentration, as in both vaccinated and unvaccinated birds, P supplementation decreased the values of these parameters.

Table 5.
Biochemical indices of chicken blood collected at 16 d of age.
Regarding the redox status parameters (Table 6), ACV administration significantly decreased the CAT activity, reduced the blood LOOH concentration, and increased the FRAP values (p = 0.054). P supplementation showed a significant effect on the CAT activity, as both vaccinated and unvaccinated chickens receiving P supplementation showed lower CAT activity. The ACV × P interaction affected the MDA concentration. P supplementation decreased the MDA concentration in the unvaccinated birds but led to a significantly higher MDA concentration in the vaccinated ones. None of the experimental factors significantly affected the SOD activity.

Table 6.
Redox status indices of chicken blood collected at 16 d of age.
4. Discussion
Similar to the results of our previous study [35], the performance data obtained for 1–21 d pv in the present experiment, i.e., reduced FI and BWG and increased FCR, support the possible deterioration of growth performance in this period due to ACV administration. However, no significant differences regarding growth performance were observed at the end of the experiment, thus indicating that the vaccinated birds underwent compensatory growth following the initial decrease in growth performance after vaccination. Dietary supplementation with P resulted in improved BWG and FCR for the 1–21 d period, thus alleviating the negative side effects of ACV administration. Ritzi et al. [16] reported comparable effects of ACV administration and probiotic supplementation, as birds vaccinated with Immucox I (CEVA Animal Health Inc., Guelph, Ontario, Canada) and supplemented with a probiotic product (PoultryStar, BIOMIN GmbH, Herzogenburg, Austria) containing Enterococcus, Bifidobacterium, Pediococcus, and Lactobacillus species showed significantly greater weight gain as compared to vaccinated and non-supplemented ones; this result suggests that the addition of probiotics helped the birds to counter growth deterioration associated with ACV administration.
In the current study, we expected that dietary supplementation with the probiotic preparation would not exert a coccidiostatic effect, which could interfere with the circulation of oocysts of the vaccine strains and impair the development of acquired immunity. The observed OPG values in the group of vaccinated chickens supplemented with P indicate that this feed additive lacks coccidiostatic or coccidiocidal mode of action; this makes it suitable for use in birds vaccinated with live oocysts. According to the description given by the vaccine manufacturer, immunity should be acquired within 14 d pv. In the present study, this was reflected in the oocyst shedding profile that steadily declined from 14 d pv in the unsupplemented vaccinated group. In the P-supplemented group, the highest OPG value was observed at 7 d pv and then continuously decreased with significantly lower OPG at 14 d pv when compared with the unsupplemented group. This finding indicates that P supplementation can accelerate the development of immunity. The improved production of Eimeria-specific antibodies following P supplementation could be the mechanism underlying this effect. This hypothesis can be confirmed based on the results of the study of Lee et al. [36], where the supplementation of a probiotic consisting of Pediococcus acidilactici and Saccharomyces boulardii in birds infected with E. acervulina and E. tenella increased the level of serum Eimeria-specific antibodies along with decreased oocyst shedding. In the current study, the enhanced humoral immunity probably reduced inflammation and intestinal tissue damage by replicating a lower number of oocysts of the vaccine strains, which could result in improved functionality of the intestinal absorptive surface area and eventually in improved growth performance. Moreover, ACV administration may affect the intestinal microflora, which could also be linked to decreased growth performance. Orso et al. [9] demonstrated that, in ceca, ACV administration lowered the percentage of Bacteroidetes phylum that comprises genera producing short-chain fatty acids known for improving intestinal health and increased the percentage of deleterious Proteobacteria phylum. Probiotic supplementation could help maintain homeostasis of the intestinal microflora. In chickens challenged with Eimeria, the probiotic product of the whole cell wall of the yeast Pichia guilliermondii ameliorated the coccidial infection-induced caecal microflora shift by decreasing the population of Salmonella and E. coli and preventing a decrease in Lactobacillus population [37]. Wang et al. [38] performed 20 × overdosed ACV administration to simulate coccidial infection, and dietary supplementation with Bacillus subtilis increased bacterial species number in non-vaccinated broilers but decreased bacterial species in vaccinated broilers. B. subtilis positively altered the microbial profile by promoting Rikenella microfusus abundance, which decreased the proportion of other bacteria in the Firmicutes phylum and caused a lower abundance of Faecalibacterium, Blautia, Alkaliphilus, Ruminococcus, and Clostridium.
Heterophils and lymphocytes are the most abundant WBC types in birds, and they play an essential role in innate and adaptative immunity, respectively. Heterophils are highly phagocytic and are involved in the acute inflammatory response as the first line of immune defence, mainly during the first hours following an immunological challenge [39,40]. In contrast, lymphocytes are involved in humoral adaptive immunity (B cells) and cell-mediated adaptive immunity (T cells). An elevated H/L ratio is generally regarded as an indicator of physiological stress in birds. Moreover, birds with a lower H/L ratio show better survival rates or resistance to Salmonella infection [41,42]. In this context, the haematological parameters observed at 16 d pv confirmed the beneficial effects of P supplementation, as it caused a significant increase in RBC and WBC counts and lowered the proportion of heterophils in the leukogram. Furthermore, in groups supplemented with P, the H/L ratio showed a tendency to decrease (p = 0.059), and the lymphocyte proportion increased. This profile of haematological variables obtained in the present study suggests that the probiotic participates in the development of better adaptive immune response and a reduction in stress. Similarly, Mohsin et al. [4] reported that the supplementation of Lactobacillus plantarum to broilers receiving a subunit vaccine containing E. maxima immune mapped protein-1 and subsequently challenged with coccidial infection resulted in increased WBC and RBC counts.
In the current study, ACV administration significantly elevated the values of parameters related to the phagocytic activity of leukocytes, such as % PC, PI, and NBT, at 16 d pv; this finding indicates an increase in the innate immunity response to the vaccine’s strains of oocysts. P supplementation significantly increased the % PC in both vaccinated and unvaccinated birds, but it increased PI only in the unvaccinated birds. Stringfellow et al. [22] reported that the effect of the probiotic PoultryStar and vaccine Coccivac B combination varied depending on the age of the birds and was the most pronounced at 14 d pv. At this period, ACV induced a significantly higher oxidative burst of heterophils, while the probiotic added to the water for vaccinated broilers caused a significantly higher oxidative burst of monocytes and heterophils and stimulated a cell-mediated immune response, as reflected in significantly higher lymphocyte proliferation. In the present study, the observed reduction in oocyst shedding at 14 d pv in birds receiving the probiotic may be linked to a higher immune response.
Coccidiosis often triggers an immune response that results in the generation of reactive free radicals such as nitric oxide (NO) and reactive oxygen species (ROS) [43,44]. An insufficient antioxidative defence system may negatively affect chickens and lead to intestinal necrosis because of oxidative damage. In broilers infected with E. acervulina [45] and E. tenella [31,32], oxidative stress was indicated as a factor because the increased NO and/or MDA concentration reduced SOD and increased CAT activities. As vaccination is regarded as a challenge similar to the course of mild coccidiosis, we expected that the corresponding profile of parameters would reflect the redox status. ACV administration significantly decreased the CAT activity, reduced the levels of serum peroxides, and increased the FRAP values (p = 0.054), with no effect on SOD activity. These results do not indicate the occurrence of oxidative stress during blood sampling. Moreover, P supplementation decreased the CAT activity and modulated the MDA concentration differently (by increasing and decreasing the MDA concentration in the vaccinated and unvaccinated groups, respectively); this result is difficult to explain. The hypothetical explanation for these unexpected results may be the fact that constant exposure to low doses of attenuated oocysts, along with the increase in the values of parameters related to the phagocytic activity, mainly in the respiratory burst activity of heterophils (NBT), may lead to a different adaptive mechanism that relies on CAT utilization to neutralize peroxides.
The activities of enzymes such as AST, ALT, LDH, or ALP are commonly used to verify whether the tested nutritional factors interfere negatively with the liver function [46]. In the present study, P supplementation significantly lowered the activity of AST and ALT in vaccinated birds, indicating the hepatoprotective properties of the probiotic. Similarly, supplementation with the probiotic L. plantarum (1 × 108 CFU) significantly decreased AST, ALT, and LDH activities in broilers infected with E. tenella [19].
P supplementation lowered the TC concentration and increased the GLU concentration in the serum of vaccinated birds. The positive cholesterolemic effect of Protexin® was reported previously [47,48], both in chickens challenged with Salmonella enteritidis [49] and in cockerels [23]. Among the proposed mechanisms, the one responsible for the hypocholesterolemic effect of probiotics may be the ability of certain lactic acid bacteria to produce the bile salt hydrolase enzyme that deconjugates bile salts. As deconjugated bile salts are less soluble at low pH, they are not absorbed efficiently in the intestine, leading to greater faecal excretion [23,50].
To accurately identify the mechanisms underlying the positive effects of the tested probiotics in vaccinated birds, further experiments are required to investigate their effects on gut histology, gut barrier integrity, the gut microbiota profile, and additional parameters of immunity.
5. Conclusions
The concept of using probiotics as a supportive tool for immunoprophylaxis with live ACV is a promising approach, as shown by a recent study. Supplementation with a multi-strain probiotic mitigates the negative effects of ACV on the growth performance and indicates the stimulating effects of the probiotic on the immune response, as reflected in parameters related to phagocytic activity or OPG. Probiotic supplementation also provided health benefits by increasing RBC and WBC counts and reducing the concentration of TC or liver enzymatic activity. The probiotic used in the present study did not affect the circulation of the vaccine’s oocysts, which is crucial for immune development; thus, this probiotic is recommended to decrease the potential adverse effects associated with ACV administration.
Author Contributions
Conceptualization, A.A.-W., S.Ś. and D.J.; formal analysis, A.A.-W. and K.O.; investigation, A.A.-W. and S.Ś.; methodology, A.A.-W., S.Ś., K.O. and D.J.; project administration, A.A.-W.; resources, A.A.-W. and S.Ś.; supervision, A.A.-W. and S.Ś.; writing—original draft, A.A.-W.; writing—review and editing, A.A.-W., S.Ś., K.O. and D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Centre for Research and Development in Poland and conducted within the Biostrateg programme: ‘GUTFEED—innovative nutrition in sustainable poultry production’ BIOSTRATEG1/267659/7/NCBR/2015.
Institutional Review Board Statement
The protocols for this study were approved by the 2nd Local Ethical Committee on Animal Testing, Cracow, Poland (Authorization No. 1065/2013). This study was carried out in compliance with Directive 2010/63/EU and in accordance with the Act on Experiments on Animals of 21 January 2005 and the Regulation of the Minister of Science and Information Technology of 29 July 2005 on the National Committee for Animal Experimentation.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Li, Y.; Zhang, X.; Wang, Y.; Huang, Z.; Yin, G.; Zhang, Z. Development of CRISPR-CAS9 Based RNA Drugs against Eimeria tenella Infection. Genomics 2021, 113, 4126–4135. [Google Scholar] [CrossRef]
- Lin, X.; Mohsin, M.; Abbas, R.Z.; Li, L.; Chen, H.; Huang, C.; Li, Y.; Goraya, M.U.; Huang, Z.; Yin, G. Evaluation of Immunogenicity and Protective Efficacy of Eimeria maxima Immune Mapped Protein 1 with EDA Adjuvant in Chicken. Pak. Vet. J. 2020, 40, 209–213. [Google Scholar] [CrossRef]
- Mohsin, M.; Li, L.; Huang, X.; Aleem, M.T.; Habib, Y.J.; Ismael, A.; Afzal, M.Z.; Abbas, R.Z.; Abbas, A.; Yin, G. Immunogenicity and Protective Efficacy of Probiotics with EtIMP1C against Eimeria tenella Challenge. Pak. Vet. J. 2021, 41, 274–278. [Google Scholar] [CrossRef]
- Vermeulen, A.N.; Schaap, D.C.; Schetters, T.P.M. Control of Coccidiosis in Chickens by Vaccination. Vet. Parasitol. 2001, 100, 13–20. [Google Scholar] [CrossRef]
- Williams, R.B. Intercurrent Coccidiosis and Necrotic Enteritis of Chickens: Rational, Integrated Disease Management by Maintenance of Gut Integrity. Avian Pathol. 2005, 34, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Cherry, T.E.; Danforth, H.D.; Richards, G.; Shirley, M.W.; Williams, R.B. Sustainable Coccidiosis Control in Poultry Production: The Role of Live Vaccines. Int. J. Parasitol. 2002, 32, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Lehman, R.; Moran, E.T.; Hess, J.B. Response of Coccidiostat- versus Vaccination-Protected Broilers to Gelatin Inclusion in High and Low Crude Protein Diets. Poult. Sci. 2009, 88, 984–993. [Google Scholar] [CrossRef]
- Orso, C.; Stefanello, T.B.; Franceschi, C.H.; Mann, M.B.; Varela, A.P.M.; Castro, I.M.S.; Frazzon, J.; Frazzon, A.P.G.; Andretta, I.; Ribeiro, A.M.L. Changes in the Ceca Microbiota of Broilers Vaccinated for Coccidiosis or Supplemented with Salinomycin. Poult. Sci. 2021, 100, 100969. [Google Scholar] [CrossRef]
- Sharman, P.A.; Smith, N.C.; Wallach, M.G.; Katrib, M. Chasing the Golden Egg: Vaccination against Poultry Coccidiosis. Parasite Immunol. 2010, 32, 590–598. [Google Scholar] [CrossRef]
- Arczewska-Włosek, A.; Świątkiewicz, S. Nutrition as a Modulatory Factor of the Efficacy of Live Anticoccidial Vaccines in Broiler Chickens. Worlds Poult. Sci. J. 2014, 70, 81–92. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S. Recent Advances in Immunomodulation and Vaccination Strategies Against Coccidiosis. Avian Dis. 2005, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Probiotics in Poultry: Modes of Action. Worlds Poult. Sci. J. 1997, 53, 351–368. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.-M.E.; Alagawany, M. Probiotics in Poultry Feed: A Comprehensive Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef]
- Dhama, K.; Verma, V.; Sawant, P.M.; Tiwari, R.; Vaid, R.; Chauhan, R. Applications of Probiotics in Poultry: Enhancing Immunity and Beneficial Effects on Production Performances and Health—A Review. J. Immunol. Immunopathol. 2011, 13, 1–19. [Google Scholar]
- Ritzi, M.M.; Abdelrahman, W.; van-Heerden, K.; Mohnl, M.; Barrett, N.W.; Dalloul, R.A. Combination of Probiotics and Coccidiosis Vaccine Enhances Protection against an Eimeria Challenge. Vet. Res. 2016, 47, 111. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, X.; Li, X.; Zhao, J.; Zhang, K.; Hao, X.; Liu, K.; Liu, H. Protective Effect of Lactobacillus plantarum P8 on Growth Performance, Intestinal Health, and Microbiota in Eimeria-Infected Broilers. Front. Microbiol. 2021, 12, 705758. [Google Scholar] [CrossRef] [PubMed]
- Aida, M.; Yamada, R.; Nakamura, S.; Imaoka, T.; Shimonishi, H.; Matsuo, T.; Taniguchi, I.; Tsukahara, T. The Effect of Supplementation with Weizmannia coagulans Strain SANK70258 to Coccidia-Infected Broilers Is Similar to That of a Coccidiostat Administration. Vet. Sci. 2022, 9, 406. [Google Scholar] [CrossRef]
- Mohsin, M.; Zhang, Z.; Yin, G. Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella. Vaccines 2022, 10, 97. [Google Scholar] [CrossRef]
- Awais, M.M.; Jamal, M.A.; Akhtar, M.; Hameed, M.R.; Anwar, M.I.; Ullah, M.I. Immunomodulatory and Ameliorative Effects of Lactobacillus and Saccharomyces Based Probiotics on Pathological Effects of Eimeriasis in Broilers. Microb. Pathog. 2019, 126, 101–108. [Google Scholar] [CrossRef]
- Dalloul, R.; Lillehoj, H.; Shellem, T.; Doerr, J. Enhanced Mucosal Immunity against Eimeria acervulina in Broilers Fed a Lactobacillus-Based Probiotic. Poult. Sci. 2003, 82, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Stringfellow, K.; Caldwell, D.; Lee, J.; Mohnl, M.; Beltran, R.; Schatzmayr, G.; Fitz-Coy, S.; Broussard, C.; Farnell, M. Evaluation of Probiotic Administration on the Immune Response of Coccidiosis-Vaccinated Broilers. Poult. Sci. 2011, 90, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Rehman, A.; Sardar, R.; Khawaja, T. The Effect of Probiotic Supplementation on the Growth Performance, Blood Biochemistry and Immune Response of Reciprocal F1 Crossbred (Rhode Island Red×Fayoumi) Cockerels. J. Appl. Anim. Res. 2013, 41, 417–426. [Google Scholar] [CrossRef]
- Sabry Abd Elraheam Elsayed, M.; Shehata, A.A.; Mohamed Ammar, A.; Allam, T.S.; Ali, A.S.; Ahmed, R.H.; Abeer Mohammed, A.B.; Tarabees, R. The Beneficial Effects of a Multistrain Potential Probiotic, Formic, and Lactic Acids with Different Vaccination Regimens on Broiler Chickens Challenged with Multidrug-Resistant Escherichia coli and Salmonella. Saudi J. Biol. Sci. 2021, 28, 2850–2857. [Google Scholar] [CrossRef]
- Smulikowska, S.; Rutkowski, A. Recommended Allowances and Nutritive Value of Feedstuffs. Poultry Feeding Standards, 4th ed.; The Kielanowski Institute of Animal Physiology and Nutrition, PAS, Polish Branch of WPSA: Jabłonna, Poland, 2005; ISBN 83-917097-7-9. (In Polish) [Google Scholar]
- World’s Poultry Science Association; Nutrition of the European Federation of Branches; Subcommittee Energy of the Working Group. European Table of Energy Values for Poultry Feedstuffs, 3rd ed.; WPSA: Beekbergen, The Netherlands, 1989; ISBN 90-71463-00-0. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005; ISBN 978-0935584752. [Google Scholar]
- FAO. Epidemiology, Diagnosis and Control of Poultry Parasites; Manuel FAO de Santé Animale; FAO: Rome, Italy, 1998; ISBN 92-5-104215-2. [Google Scholar]
- Feldman, B.V.; Zinkl, J.G.; Nemi, C.J. Schalm’s Veterinary Hematology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; ISBN 978-0-683-30692-7. [Google Scholar]
- Jahanian, R.; Rasouli, E. Dietary Chromium Methionine Supplementation Could Alleviate Immunosuppressive Effects of Heat Stress in Broiler Chicks. J. Anim. Sci. 2015, 93, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Lentfer, T.L.; Pendl, H.; Gebhardt-Henrich, S.G.; Fröhlich, E.K.F.; Borell, E.V. H/L Ratio as a Measurement of Stress in Laying Hens—Methodology and Reliability. Br. Poult. Sci. 2015, 56, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, A.K.; Anderson, D.P. Nonspecific Defense Mechanisms Assay in Fish: II. Potential Killing Activity of Neutrophils and Macrophages, Lysozyme Activity in Serum and Organs and Total Immunoglobulin (Ig) Level in Serum. In Fish Diseases Diagnosis and Prevention Methods; Inland Fisheries Institute: Olsztyn, Poland, 1993; pp. 105–112. [Google Scholar]
- Park, B.H.; Fikrig, S.M.; Smithwick, E.M. Infection and Nitroblue-Tetrazolium Reduction by Neutrophils: A Diagnostic Aid. Lancet 1968, 292, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Ognik, K.; Wertelecki, T. Effect of Different Vitamin E Sources and Levels on Selected Oxidative Status Indices in Blood and Tissues as Well as on Rearing Performance of Slaughter Turkey Hens. J. Appl. Poult. Res. 2012, 21, 259–271. [Google Scholar] [CrossRef]
- Arczewska-Włosek, A.; Świątkiewicz, S.; Kowal, J.; Józefiak, D.; Długosz, J. The Effect of Increased Crude Protein Level and/or Dietary Supplementation with Herbal Extract Blend on the Performance of Chickens Vaccinated against Coccidiosis. Anim. Feed Sci. Technol. 2017, 229, 65–72. [Google Scholar] [CrossRef]
- Lee, S.; Lillehoj, H.S.; Park, D.W.; Hong, Y.H.; Lin, J.J. Effects of Pediococcus- and Saccharomyces-Based Probiotic (MitoMax®) on Coccidiosis in Broiler Chickens. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 261–268. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Sifri, M.; Selvaraj, R.K. Effect of Yeast Cell Product Supplementation on Broiler Cecal Microflora Species and Immune Responses during an Experimental Coccidial Infection. Poult. Sci. 2013, 92, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Farnell, Y.Z.; Kiess, A.S.; Peebles, E.D.; Wamsley, K.G.S.; Zhai, W. Effects of Bacillus subtilis and Coccidial Vaccination on Cecal Microbial Diversity and Composition of Eimeria-Challenged Male Broilers. Poult. Sci. 2019, 98, 3839–3849. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B. Avian Heterophils in Inflammation and Disease Resistance. Poult. Sci. 1998, 77, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Thiam, M.; Barreto Sánchez, A.L.; Zhang, J.; Wen, J.; Zhao, G.; Wang, Q. Investigation of the Potential of Heterophil/Lymphocyte Ratio as a Biomarker to Predict Colonization Resistance and Inflammatory Response to Salmonella enteritidis Infection in Chicken. Pathogens 2022, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Thiam, M.; Wang, Q.; Barreto Sánchez, A.L.; Zhang, J.; Ding, J.; Wang, H.; Zhang, Q.; Zhang, N.; Wang, J.; Li, Q.; et al. Heterophil/Lymphocyte Ratio Level Modulates Salmonella Resistance, Cecal Microbiota Composition and Functional Capacity in Infected Chicken. Front. Immunol. 2022, 13, 816689. [Google Scholar] [CrossRef] [PubMed]
- Al-Murrani, W.K.; Al-Rawi, I.K.; Raof, N.M. Genetic Resistance to Salmonella typhimurium in Two Lines of Chickens Selected as Resistant and Sensitive on the Basis of Heterophil/Lymphocyte Ratio. Br. Poult. Sci. 2002, 43, 501–507. [Google Scholar] [CrossRef]
- Perez-Carbajal, C.; Caldwell, D.; Farnell, M.; Stringfellow, K.; Pohl, S.; Casco, G.; Pro-Martinez, A.; Ruiz-Feria, C.A. Immune Response of Broiler Chickens Fed Different Levels of Arginine and Vitamin E to a Coccidiosis Vaccine and Eimeria Challenge. Poult. Sci. 2010, 89, 1870–1877. [Google Scholar] [CrossRef]
- Muthamilselvan, T.; Kuo, T.-F.; Wu, Y.-C.; Yang, W.-C. Herbal Remedies for Coccidiosis Control: A Review of Plants, Compounds, and Anticoccidial Actions. Evid. Based Complement. Alternat. Med. 2016, 2016, 9459047. [Google Scholar] [CrossRef]
- Koinarski, V.; Gabrashanska, M.; Georgieva, N.; Petkov, P. Antioxidant Parameters in Eimeria acervulina Infected Chicks after Treatment with a New Zinc Compound. Bull. Vet. Inst. Pulawy 2006, 50, 55–61. [Google Scholar]
- Ognik, K.; Krauze, M. The Potential for Using Enzymatic Assays to Assess the Health of Turkeys. Worlds Poult. Sci. J. 2016, 72, 535–550. [Google Scholar] [CrossRef]
- Azadegan Mehr, M.; Hassanabadi, A.; Nassiri Moghaddam, H.; Kermanshahi, H. Supplementation of Clove Essential Oils and Probiotic to the Broiler’s Diet on Performance, Carcass Traits and Blood Components. Iran. J. Appl. Anim. Sci. 2014, 4, 117–122. [Google Scholar]
- Pourakbari, M.; Seidavi, A.; Asadpour, L.; Martínez, A. Probiotic Level Effects on Growth Performance, Carcass Traits, Blood Parameters, Cecal Microbiota, and Immune Response of Broilers. An. Acad. Bras. Ciênc. 2016, 88, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Hamid, F.M.; El-Gohary, A.; Risha, E.F. Incorporation Efficacy Comparison of Probiotic and Antibiotic on Growth Performance, Some Immunological and Biochemical Parameters in Salmonella entertidis Challenged Chicks. Life Sci. J. 2013, 10, 3550–3558. [Google Scholar]
- Klaver, F.A.; van der Meer, R. The Assumed Assimilation of Cholesterol by Lactobacilli and Bifidobacterium bifidum Is Due to Their Bile Salt-Deconjugating Activity. Appl. Environ. Microbiol. 1993, 59, 1120–1124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).