Role of Bacteriophages for Optimized Health and Production of Poultry
Abstract
Simple Summary
Abstract
1. Introduction
2. Introduction to Bacteriophages in Biomedicine
2.1. History
2.2. Applications
3. Experimental Studies of Bacteriophages in Poultry
3.1. Campylobacter spp.
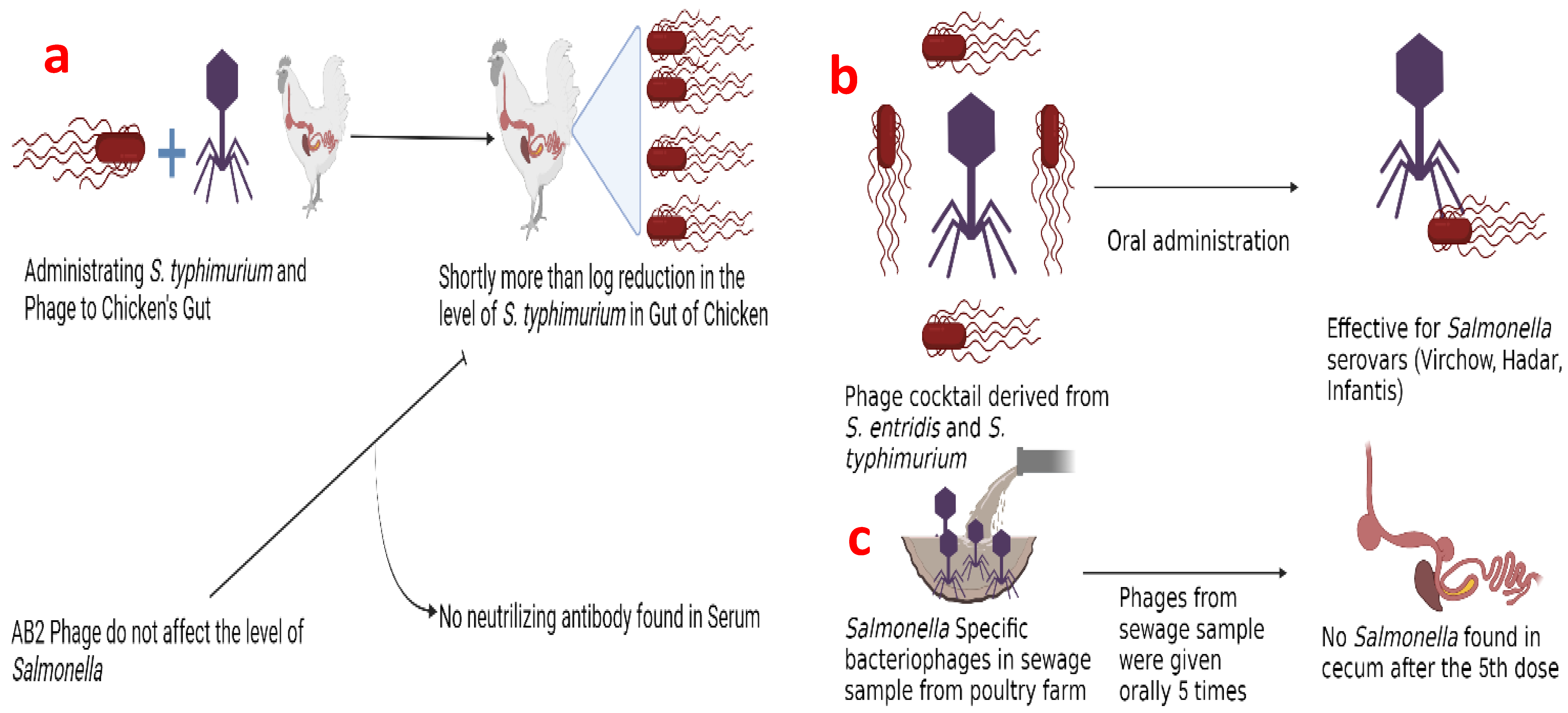
3.2. Salmonella spp.
3.3. Escherichia spp.
3.4. Clostridium spp.
4. Effect of Bacteriophages on Poultry Immunity against the Challenge of Salient Bacteria
4.1. Campylobacters
4.2. E. coli
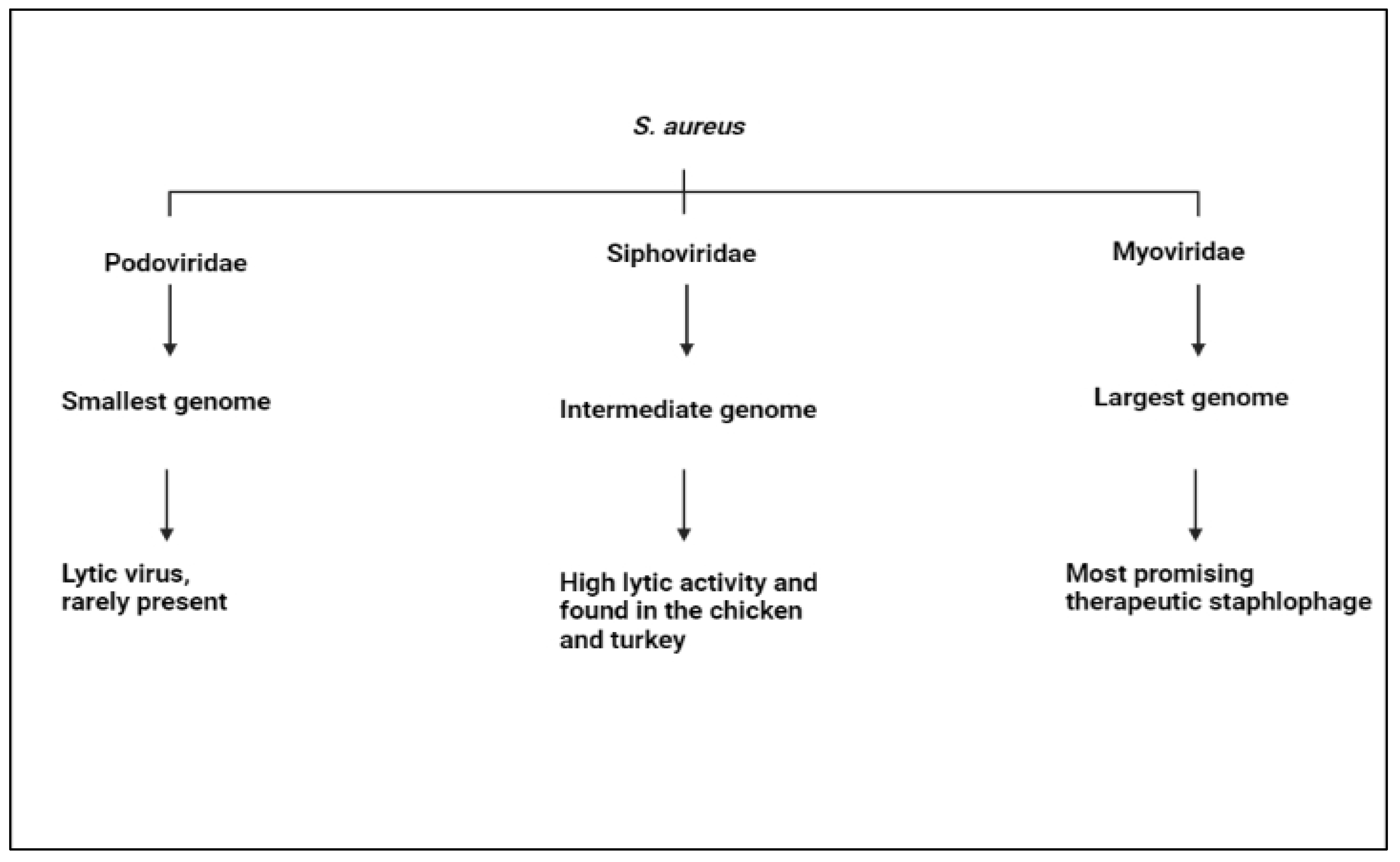
4.3. S. aureus
5. Products of Bacteriophages Used as Vaccines or Adjuvants
6. Bacteriophages and Food Safety/Security Regarding Poultry Meat
7. Biocontrol of Phages in Food Processing
8. Bacteriophages as Antiseptic and Disinfectants in the Poultry Industry
8.1. Role as Antiseptics
8.2. Role as Disinfectants
8.3. Limitations
9. Reduction of Food Contaminations
10. Adverse Effects of Bacteriophage in Poultry
11. Challenges in the Application of Bacteriophages in Poultry
12. Future Perspective
12.1. Regulatory Authority
12.2. Combination Therapies
12.3. Host Range Interactions
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). Overview of Global Meat Market Developments in 2020. 2021. Available online: https://www.fao.org/3/cb3700en/cb3700en.pdf.march2021.CB3700EN/1/03.2 (accessed on 1 October 2022).
- Anonymous. EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control): The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef]
- Page, S.W.; Gautier, P. Use of antimicrobial agents in livestock. Rev. Sci. Tech. 2012, 31, 145–188. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Leger, D.; Carson, C. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can. Vet. J. 2012, 53, 1289–1300. [Google Scholar] [PubMed]
- Kirbis, A.; Krizmana, M. Spread of antibiotic-resistant bacteria from food of animal origin to humans and vice versa. Procedia Food Sci. 2015, 5, 148–151. [Google Scholar] [CrossRef]
- Ackermann, H.W. Bacteriophage observations and evolution. Res. Microbiol. 2003, 154, 245–251. [Google Scholar] [CrossRef]
- Fernandes, S.; Proença, D.; Cantante, C.; Silva, F.A.; Leandro, C.; Lourenço, S.; Milheiriço, C.; de Lencastre, H.; Cavaco-Silva, P.; Pimentel, M. Novel chimerical endolysins with broad antimicrobial activity against methicillin-resistant Staphylococcus aureus. Microb. Drug Resist. 2012, 18, 333–343. [Google Scholar] [CrossRef]
- Monk, A.B.; Rees, C.D.; Barrow, P.; Hagens, S.; Harper, D.R. Bacteriophage applications: Where are we now? Lett. Appl. Microbiol. 2010, 51, 363–369. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. European Food Safety Authority, European Centre for Disease Prevention and Control: The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, 5598. [Google Scholar]
- Brüssow, H. Phage therapy: The Escherichia coli experience. Microbiology 2005, 151, 2133–2140. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Kutateladze, M.; Adamia, R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 2010, 28, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Adhya, S.; Washart, P.; Paul, B.; Trostel, A.N.; Powell, B.; Carlton, R.; Merril, C.R. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 2002, 70, 204–210. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; DiRita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Atterbury, R.J.; Connerton, P.L.; Dodd, C.E.; Rees, C.E.; Connerton, I.F. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 2003, 69, 6302–6306. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.J.; Connerton, P.L.; Connerton, I.F. Phage biocontrol of Campylobacter jejuni in chickens does not produce collateral effects on the gut microbiota. Front. Microbiol. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Berchieri, A., Jr.; Lovell, M.A.; Barrow, P.A. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res. Microbiol. 1991, 142, 541–549. [Google Scholar] [CrossRef]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The use of bacteriophages in the poultry industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018, 8, 1539056. [Google Scholar] [CrossRef]
- Barrow, P.; Lovell, M.; Berchieri, A., Jr. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 1998, 5, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Huff, G.R.; Huff, W.E.; Rath, N.C.; Donoghue, A.M. Critical evaluation of bacteriophage to prevent and treat colibacillosis in poultry. J. Ark. Acad. Sci. 2009, 63, 93–98. [Google Scholar]
- Leskinen, K.; Tuomala, H.; Wicklund, A.; Horsma-Heikkinen, J.; Kuusela, P.; Skurnik, M.; Kiljunen, S. Characterization of vB_SauM-fRuSau02, a Twort-like bacteriophage isolated from a therapeutic phage cocktail. Viruses 2017, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Marek, A.; Pyzik, E.; Stępień-Pyśniak, D.; Urban-Chmiel, R.; Nowaczek, A. Characterization of bacteriophages and their carriage in Staphylococcus aureus isolated from broilers in Poland. Br. Poult. Sci. 2019, 60, 373–380. [Google Scholar] [CrossRef]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- Sahin, O.; Kassem, I.I.; Shen, Z.; Lin, J.; Rajashekara, G.; Zhang, Q. Campylobacter in Poultry: Ecology and Potential Interventions. Avian Dis. 2015, 59, 185–200. [Google Scholar] [CrossRef]
- Wysok, B.; Pastuszczak-Frak, M.; Uradziński, J.; Gomółka-Pawlicka, M.; Dzisko, J.; Dziedziech, M.; Marko, A. Występowanie i antybiotykooporność szczepów Campylobacter spp. wyizolowanych od zwierząt rzeźnych i ludzi. Med. Weter. Vet. Med. Sci. Prac. 2015, 71, 801–806. [Google Scholar]
- Nowaczek, A.; Urban-Chmiel, R.; Dec, M.; Puchalski, A.; St ˛epie ´n-Py´sniak, D.; Marek, A.; Pyzik, E. Campylobacter spp. and bacteriophages from broiler chickens: Characterization of antibiotic susceptibility profiles and lytic bacteriophages. MicrobiologyOpen 2019, 8, e784. [Google Scholar] [CrossRef]
- Gast, R.K. Salmonella Infections. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V.L., Eds.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 677–736. [Google Scholar]
- Nolan, L.K.; Barnes, H.J.; Vaillancourt, J.P.; Abdul-Aziz, T.; Logue, C.M. Colibacillosis. In Diseases of Poultry; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 751–805. [Google Scholar]
- Andreasen, C.B. Staphylococcosis. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V.L., Eds.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 971–977. [Google Scholar]
- Feßler, A.T.; Kadlec, K.; Hassel, M.; Hauschild, T.; Eidam, C.; Ehricht, R.; Monecke, S.; Schwarz, S. Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Food and Food Products of Poultry Origin in Germany. Appl. Environ. Microbiol. 2011, 77, 7151–7157. [Google Scholar] [CrossRef]
- Glünder, G.; Spiering, N.; Hinz, K. Investigations on parenteral immunization of chickens with a Campylobacter mineral oil vaccine. In COST Action; Athogenic Micro-Organisms in Poultry and Eggs; 1997; European Commission: Budapest, Hungary, 1998; Volume 97. [Google Scholar]
- Ziprin, R.L.; Hume, M.E.; Young, C.R.; Harvey, R.B. Inoculation of chicks with viable non-colonizing strains of Campylobacter jejuni: Evaluation of protection against a colonizing strain. Curr. Microbiol. 2002, 44, 221–223. [Google Scholar] [CrossRef]
- Widders, P.R.; Thomas, L.M.; Long, K.A.; Tokhi, M.A.; Panaccio, M.; Apos, E. The specificity of antibody in chickens immunised to reduce intestinal colonisation with Campylobacter jejuni. Vet. Microbiol. 1998, 64, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.M.; Husband, A.J.; Widders, P.R. In ovo oral vaccination with Campylobacter jejuni establishes early development of intestinal immunity in chickens. Br. Poult. Sci. 1995, 36, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Neal-McKinney, J.M.; Samuelson, D.R.; Eucker, T.P.; Nissen, M.S.; Crespo, R.; Konkel, M.E. Reducing Campylobacter jejuni colonization of poultry via vaccination. PLoS ONE 2014, 9, e114254. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Yin, Y.X.; Pan, Z.M.; Zhang, G.; Zhu, A.P.; Liu, X.F.; Jiao, X.A. Intranasal immunization with chitosan/pCAGGS-flaA nanoparticles inhibits Campylobacter jejuni in a White Leghorn model. J. Biomed. Biotechnol. 2010, 2010, 589476. [Google Scholar] [CrossRef]
- Annamalai, T.; Pina-Mimbela, R.; Kumar, A.; Binjawadagi, B.; Liu, Z.; Renukaradhya, G.J.; Rajashekara, G. Evaluation of nanoparticle-encapsulated outer membrane proteins for the control of Campylobacter jejuni colonization in chickens. Poult. Sci. 2013, 92, 2201–2211. [Google Scholar] [CrossRef]
- Theoret, J.R.; Cooper, K.K.; Zekarias, B.; Roland, K.L.; Law, B.F.; Curtiss, R., 3rd; Joens, L.A. The Campylobacter jejuni Dps homologue is important for in vitro biofilm formation and cecal colonization of poultry and may serve as a protective antigen for vaccination. Clin. Vaccine Immunol. 2012, 19, 1426–1431. [Google Scholar] [CrossRef]
- Ushanov, L.; Lasareishvili, B.; Janashia, I.; Zautner, A.E. Application of Campylobacter jejuni phages: Challenges and perspectives. Animals 2020, 10, 279. [Google Scholar] [CrossRef]
- Chinivasagam, H.N.; Estella, W.; Maddock, L.; Mayer, D.G.; Weyand, C.; Connerton, P.L.; Connerton, I.F. Bacteriophages to control Campylobacter in commercially farmed broiler chickens, in Australia. Front. Microbiol. 2020, 11, 632. [Google Scholar] [CrossRef]
- Vaz CS, L.; Voss-Rech, D.; Alves, L.; Coldebella, A.; Brentano, L.; Trevisol, I.M. Effect of time of therapy with wild-type lytic bacteriophages on the reduction of Salmonella Enteritidis in broiler chickens. Vet. Microbiol. 2020, 240, 108527. [Google Scholar] [CrossRef]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the human gut: The “known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage biocontrol applications in food production and processing. Curr. Issues Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.B.; Kroger, E.C.; Watkins, S.E. Evaluation of disinfectant efficacy when applied to the floor of poultry grow-out facilities. J. Appl. Poult. Res. 2005, 14, 322–329. [Google Scholar] [CrossRef]
- Jackson, T.; Leonard, M. Seasonal Adjustment Using the X12 Procedure; SAS Institute: Cary, NC, USA, 2000. [Google Scholar]
- Bloomfield, S.F.; Arthur, M.; Looney, E.; Begun, K.; Patel, H. Comparative testing of disinfectant and antiseptic products using proposed European suspension testing methods. Lett. Appl. Microbiol. 1991, 13, 233–237. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Center for Food Safety and Applied Nutrition, Bacteriological Analytical Manual Online, Rockville, MD.; 2001. Available online: www.cfsan.fda.gov/~ebam/bam-ri.html (accessed on 11 July 2022).
- Garcia, K.C.O.D.; Corrêa, I.M.O.; Pereira, L.Q.; Silva, T.M.; Mioni, M.S.R.; Izidoro, A.C.M.; Bastos, I.H.V.; Gonçalves, G.A.M.; Okamoto, A.S.; Andreatti Filho, R.L. Bacteriophage use to control Salmonella biofilm on surfaces present in chicken slaughterhouses. Poult. Sci. 2017, 96, 3392–3398. [Google Scholar] [CrossRef]
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.J. Don’t Shut the Stable Door after the Phage Has Bolted-The Importance of Bacteriophage Inactivation in Food Environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef]
- Hungaro, H.M.; Mendonça, R.C.S.; Gouvêa, D.M.; Vanetti, M.C.D.; Pinto, C.L.D. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res. Int. 2013, 52, 75–81. [Google Scholar] [CrossRef]
- El-Gohary, F.A.; Huff, W.E.; Huff, G.R.; Rath, N.C.; Zhou, Z.Y.; Donoghue, A.M. Environmental augmentation with bacteriophage prevents colibacillosis in broiler chickens. Poult. Sci. 2014, 93, 2788–2792. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed (2009 update). EFSA J. 2009, 7, 1431. [Google Scholar] [CrossRef]
- Garcia, P.; Martinez, B.; Obeso, J.M.; Rodriguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Bruttin, A.; Brüssow, H. Human volunteers receiving Escherichia coli phage T4 orally: A safety test of phage therapy. Antimicrob. Agents Chemother. 2005, 49, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Zaczek, M.; Łusiak-Szelachowska, M.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Owczarek, B.; Kopciuch, A.; Fortuna, W.; Rogóż, P.; Górski, A. Antibody Production in Response to Staphylococcal MS-1 Phage Cocktail in Patients Undergoing Phage Therapy. Front. Microbiol. 2016, 7, 1681. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, M.; Karimi Torshizi, M.A.; Rahimi, S.; Engberg, R.M.; Sørensen Dalgaard, T. Effect of serum anti-phage activity on colibacillosis control by repeated phage therapy in broilers. Vet. Microbiol. 2019, 234, 61–71. [Google Scholar] [CrossRef]
- Alban, L.; Pozio, E.; Boes, J.; Boireau, P.; Boué, F.; Claes, M.; Cook, A.J.; Dorny, P.; Enemark, H.L.; Van der Giessen, J.; et al. Towards a standardised surveillance forTrichinellain the European Union. Prev. Vet. Med. 2011, 99, 148–160. [Google Scholar] [CrossRef]
- Perera, M.N.; Abuladze, T.; Li, M.; Woolston, J.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food Microbiol. 2015, 52, 42–48. [Google Scholar] [CrossRef]
- Piniero, M.; Asp, N.-G.; Reid, G. FAO technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42 (Suppl. S3), S156–S162. [Google Scholar] [CrossRef]
- Mills, S.; Shanahan, F.; Stanton, C.; Hill, C.; Coffrey, A.; Ross, R.P. Influence of bacteriophages in shaping the mam-malian gut microbiota. Gut Microbes 2013, 4, 4–16. [Google Scholar] [CrossRef]
- Zhang, A.W.; Lee, B.D.; Lee, S.K.; Lee, K.W.; An, G.H.; Song, K.B.; Lee, C.H. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult. Sci. 2005, 84, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- FAO. Conditions for the Evaluation of Probiotics in Food. 2002. Available online: http://who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 19 May 2022).
- Joerger, R.D.; Ganguly, A. Current state of the preharvest application of pro- and prebiotics to farm animals to enhance the microbial safety of animal products. Microbiolspec 2017, 5, PFS-0012-2016. [Google Scholar] [CrossRef]
- Yang, Y.; Iji, P.A.; Choct, M. Dietary modulation of gut microflora in broiler chickens: A review of the role of six kinds of alternatives to in-feed antibiotics. World’s Poult. Sci. J. 2009, 65, 97–114. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Kłak, M.; Fortuna, W.; Letkiewicz, S.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014, 27, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Choińska-Pulita, A.; Mituła, P.; Sliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- Knezevic, P.; Hoyle, N.S.; Matsuzaki, S.; Gorski, A. Advances in phage therapy: Present challenges and future perspectives. Front. Microbiol. 2021, 12, 701898. [Google Scholar] [CrossRef]
- Regulski, K.; Champion-Arnaud, P.; Gabard, J. Bacteriophage manufacturing: From early twentieth-century processes to current GMP. In Bacteriophages: Biology, Technology, Therapy; Springer: Cham, Switzerland, 2021; pp. 699–729. [Google Scholar]
- Mutti, M.; Corsini, L. Robust Approaches for the Production of Active Ingredient and Drug Product for Human Phage Therapy. Front. Microbiol. 2019, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Blasdel, B.G.; Bretaudeau, L.; Buckling, A.; Chanishvili, N.; Clark, J.R.; Corte-Real, S.; Debarbieux, L.; Dublanchet, A.; De Vos, D.; et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar] [CrossRef]
- Hargreaves, K.R.; Clokie, M.R. Clostridium difficile phages: Still difficult? Front. Microbiol. 2014, 5, 184. [Google Scholar] [CrossRef]
- Hietala, V.; Horsma-Heikkinen, J.; Carron, A.; Skurnik, M.; Kiljunen, S. The Removal of Endo- and Enterotoxins from Bacteriophage Preparations. Front. Microbiol. 2019, 10, 1674. [Google Scholar] [CrossRef]
- Torres-Acosta, M.A.; Castaneda-Aponte, H.M.; Mora-Galvez, L.M.; Gil-Garzon, M.R.; Banda-Magaña, M.P.; Marcellin, E.; Mayolo-Deloisa, K.; Licona-Cassani, C. Comparative economic analysis between endogenous and recombinant production of hyaluronic acid. Front. Bioeng. Biotechnol. 2021, 9, 680278. [Google Scholar] [CrossRef]
- Cazares, A.; García-Contreras, R.; Pérez-Velázquez, J. Eco-Evolutionary Effects of Bacterial Cooperation on Phage Therapy: An Unknown Risk? Front. Microbiol. 2020, 11, 590294. [Google Scholar] [CrossRef]
- Khalid, A.; Lin RC, Y.; Iredell, J.R. A Phage Therapy Guide for Clinicians and Basic Scientists: Background and Highlighting Applications for Developing Countries. Front. Microbiol. 2021, 11, 599906. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, W.N.; Concepcion-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and order effects of antibiotics and phages in killing pseudomonas aeruginosa biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef] [PubMed]
- Martel, B.; Moineau, S. CRISPR-Cas: An efficient tool for genome engineering of virulent bacteriophages. Nucleic Acids Res. 2014, 42, 9504–9513. [Google Scholar] [CrossRef]
- Ando, H.; Lemire, S.; Pires, D.P.; Lu, T.K. Engineering modular viral scaffolds for targeted bacterial population editing. Cell Syst. 2015, 1, 187–196. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, R.Z.; Alsayeqh, A.F.; Aqib, A.I. Role of Bacteriophages for Optimized Health and Production of Poultry. Animals 2022, 12, 3378. https://doi.org/10.3390/ani12233378
Abbas RZ, Alsayeqh AF, Aqib AI. Role of Bacteriophages for Optimized Health and Production of Poultry. Animals. 2022; 12(23):3378. https://doi.org/10.3390/ani12233378
Chicago/Turabian StyleAbbas, Rao Zahid, Abdullah F Alsayeqh, and Amjad Islam Aqib. 2022. "Role of Bacteriophages for Optimized Health and Production of Poultry" Animals 12, no. 23: 3378. https://doi.org/10.3390/ani12233378
APA StyleAbbas, R. Z., Alsayeqh, A. F., & Aqib, A. I. (2022). Role of Bacteriophages for Optimized Health and Production of Poultry. Animals, 12(23), 3378. https://doi.org/10.3390/ani12233378






