Developing Strategies to Help Bee Colony Resilience in Changing Environments
Abstract
Simple Summary
Abstract
1. Introduction
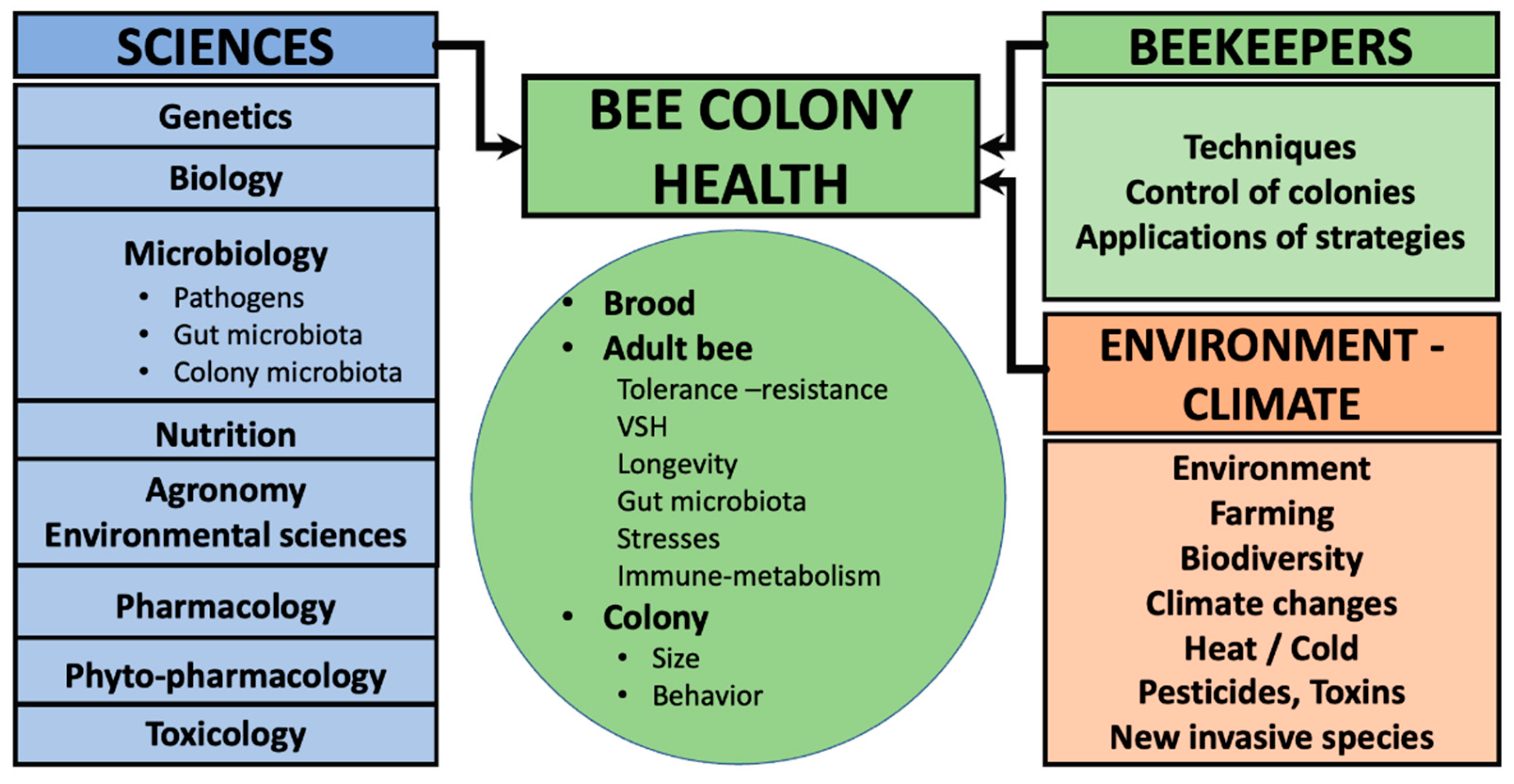
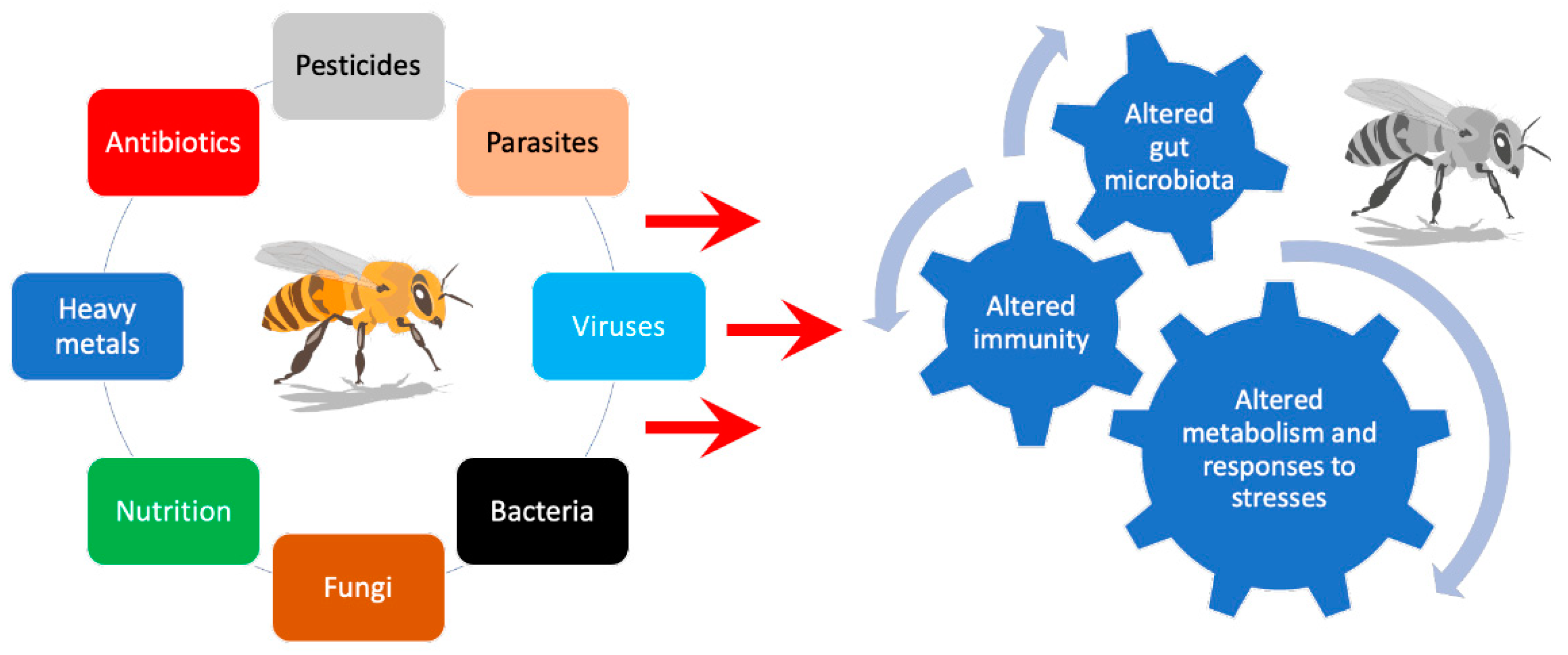
2. Bees Are Superorganisms: Challenges and Strategies to Optimize Their Health
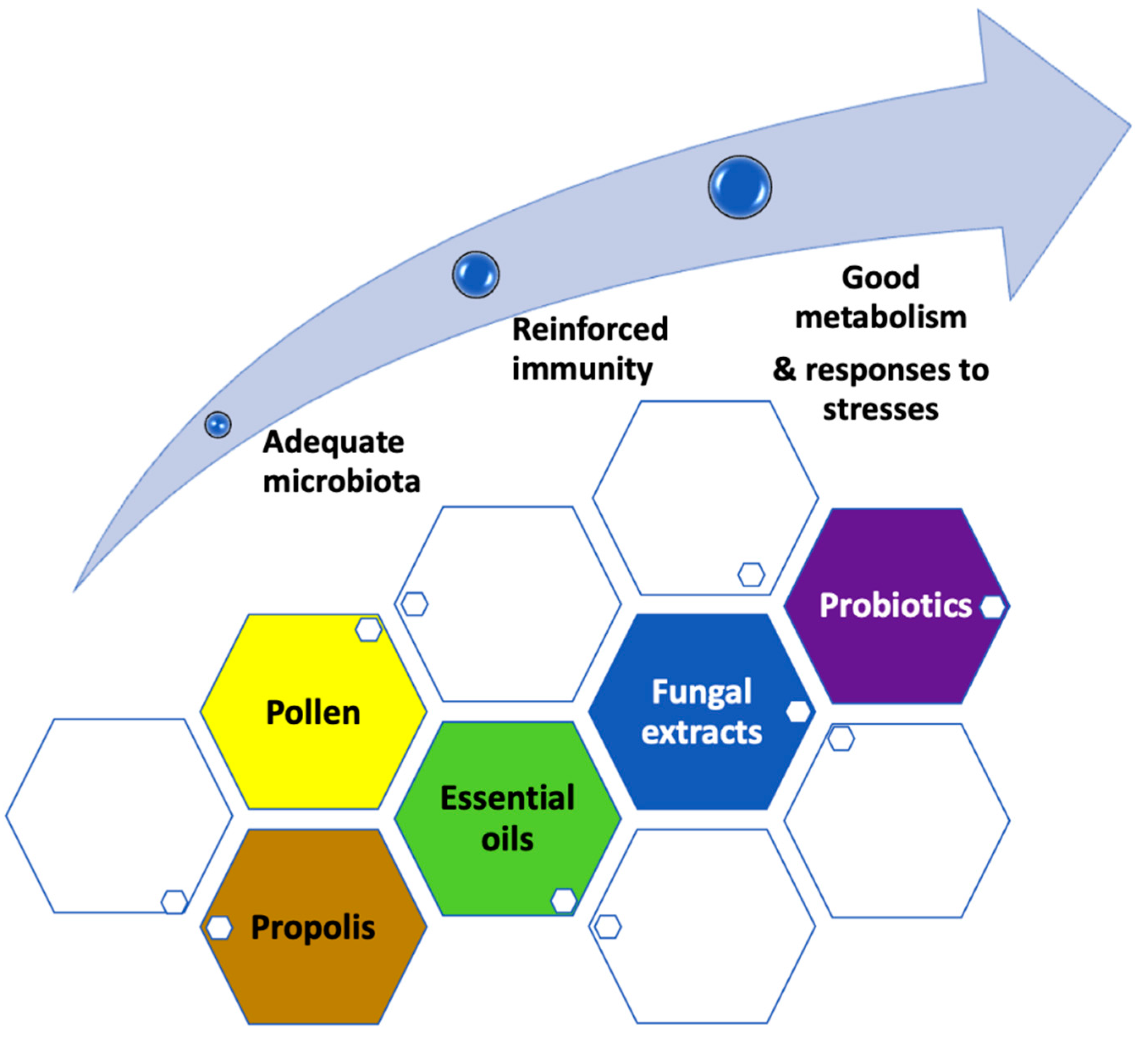
3. The Role of Nutrition
3.1. What Would Be the Interest of Feeding Bees with Pollen?
3.2. Should We Add Essential Oils and Specific Phytonutrients?
3.3. Should We Add Propolis?
3.4. Should We Add Fungal Extracts?
3.5. Therapeutic or Preventive Purpose
4. Bee Microbiota Part of the Strategy for Bee Colony Survival
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amuasi, J.H.; Lucas, T.; Horton, R.; Winkler, A.S. Reconnecting for our future: The Lancet One Health Commission. Lancet 2020, 395, 1469–1471. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G. Bee and Beekeeping Research in a Rapidly Changing World: Advancements and Challenges. Molecules 2021, 26, 3066. [Google Scholar] [CrossRef]
- Destoumieux-Garzon, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Scheffer, M.; Bolhuis, J.E.; Borsboom, D.; Buchman, T.G.; Gijzel, S.M.W.; Goulson, D.; Kammenga, J.E.; Kemp, B.; van de Leemput, I.A.; Levin, S.; et al. Quantifying resilience of humans and other animals. Proc. Natl. Acad. Sci. USA 2018, 115, 11883–11890. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M.; van Nes, E.H.; Vergnon, R. Toward a unifying theory of biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 639–641. [Google Scholar] [CrossRef]
- Zinsstag, J.; Schelling, E.; Wyss, K.; Mahamat, M.B. Potential of cooperation between human and animal health to strengthen health systems. Lancet 2005, 366, 2142–2145. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Wahed, A.A.; Naggar, Y.A.; Saeed, A.; Xiao, J.; Ullah, H.; Musharraf, S.G.; Boskabady, M.H.; Cao, W.; Guo, Z.; et al. Khalifa Insights into the Role of Natural Products in the Control of the Honey Bee Gut Parasite (Nosema spp.). Animals 2022, 12, 3062. [Google Scholar] [CrossRef]
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef]
- Alaux, C.; Soubeyrand, S.; Prado, A.; Peruzzi, M.; Maisonnasse, A.; Vallon, J.; Hernandez, J.; Jourdan, P.; Le Conte, Y. Measuring biological age to assess colony demographics in honeybees. PLoS ONE 2018, 13, e0209192. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Kwong, W.K.; McFrederick, Q.; Anderson, K.E.; Barribeau, S.M.; Chandler, J.A.; Cornman, R.S.; Dainat, J.; de Miranda, J.R.; Doublet, V.; et al. The Bee Microbiome: Impact on Bee Health and Model for Evolution and Ecology of Host-Microbe Interactions. mBio 2016, 7, e02164-15. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2010, 103 (Suppl. S1), S62–S72. [Google Scholar] [CrossRef]
- Even, N.; Devaud, J.M.; Barron, A.B. General Stress Responses in the Honey Bee. Insects 2012, 3, 1271–1298. [Google Scholar] [CrossRef]
- Li, G.; Zhao, H.; Liu, Z.; Wang, H.; Xu, B.; Guo, X. The Wisdom of Honeybee Defenses Against Environmental Stresses. Front. Microbiol. 2018, 9, 722. [Google Scholar] [CrossRef]
- Parreno, M.A.; Alaux, C.; Brunet, J.L.; Buydens, L.; Filipiak, M.; Henry, M.; Keller, A.; Klein, A.M.; Kuhlmann, M.; Leroy, C.; et al. Critical links between biodiversity and health in wild bee conservation. Trends Ecol. Evol. 2022, 37, 309–321. [Google Scholar] [CrossRef]
- Knoll, S.; Pinna, W.; Varcasia, A.; Scala, A.; Cappai, M.G. The honey bee (Apis mellifera L., 1758) and the seasonal adaptation of productions. Highlights on summer to winter transition and back to summer metabolic activity. A review. Livest. Sci. 2020, 235, 104011. [Google Scholar] [CrossRef]
- Almasri, H.; Liberti, J.; Brunet, J.L.; Engel, P.; Belzunces, L.P. Mild chronic exposure to pesticides alters physiological markers of honey bee health without perturbing the core gut microbiota. Sci. Rep. 2022, 12, 4281. [Google Scholar] [CrossRef]
- Arien, Y.; Dag, A.; Yona, S.; Tietel, Z.; Lapidot Cohen, T.; Shafir, S. Effect of diet lipids and omega-6:3 ratio on honey bee brood development, adult survival and body composition. J. Insect Physiol. 2020, 124, 104074. [Google Scholar] [CrossRef]
- Bordier, C.; Dechatre, H.; Suchail, S.; Peruzzi, M.; Soubeyrand, S.; Pioz, M.; Pelissier, M.; Crauser, D.; Conte, Y.L.; Alaux, C. Colony adaptive response to simulated heat waves and consequences at the individual level in honeybees (Apis mellifera). Sci. Rep. 2017, 7, 3760. [Google Scholar] [CrossRef]
- Bordier, C.; Klein, S.; Le Conte, Y.; Barron, A.B.; Alaux, C. Stress decreases pollen foraging performance in honeybees. J. Exp. Biol. 2018, 221, jeb17147. [Google Scholar] [CrossRef]
- Branchiccela, B.; Castelli, L.; Corona, M.; Diaz-Cetti, S.; Invernizzi, C.; Martinez de la Escalera, G.; Mendoza, Y.; Santos, E.; Silva, C.; Zunino, P.; et al. Impact of nutritional stress on the honeybee colony health. Sci. Rep. 2019, 9, 10156. [Google Scholar] [CrossRef]
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antunez, K. Impact of Nutritional Stress on Honeybee Gut Microbiota, Immunity, and Nosema ceranae Infection. Microb. Ecol. 2020, 80, 908–919. [Google Scholar] [CrossRef]
- Daisley, B.A.; Koenig, D.; Engelbrecht, K.; Doney, L.; Hards, K.; Al, K.F.; Reid, G.; Burton, J.P. Emerging connections between gut microbiome bioenergetics and chronic metabolic diseases. Cell Rep. 2021, 37, 110087. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Chmiel, J.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Missing Microbes in Bees: How Systematic Depletion of Key Symbionts Erodes Immunity. Trends Microbiol. 2020, 28, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Corby-Harris, V.; Carroll, M.; Toth, A.L.; Gage, S.; Watkins deJong, E.; Graham, H.; Chambers, M.; Meador, C.; Obernesser, B. The Importance of Time and Place: Nutrient Composition and Utilization of Seasonal Pollens by European Honey Bees (Apis mellifera L.). Insects 2021, 12, 235. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Corby-Harris, V.; Chen, Y.; Graham, H.; Chambers, M.; Watkins deJong, E.; Ziolkowski, N.; Kang, Y.; Gage, S.; Deeter, M.; et al. Can supplementary pollen feeding reduce varroa mite and virus levels and improve honey bee colony survival? Exp. Appl. Acarol. 2020, 82, 455–473. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Gage, S.L.; Corby-Harris, V.; Carroll, M.; Chambers, M.; Graham, H.; Watkins deJong, E.; Hidalgo, G.; Calle, S.; Azzouz-Olden, F.; et al. Connecting the nutrient composition of seasonal pollens with changing nutritional needs of honey bee (Apis mellifera L.) colonies. J. Insect Physiol. 2018, 109, 114–124. [Google Scholar] [CrossRef]
- Dosch, C.; Manigk, A.; Streicher, T.; Tehel, A.; Paxton, R.J.; Tragust, S. The Gut Microbiota Can Provide Viral Tolerance in the Honey Bee. Microorganisms 2021, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Negri, P.; Ramirez, L.; Quintana, S.; Szawarski, N.; Maggi, M.; Le Conte, Y.; Lamattina, L.; Eguaras, M. Dietary Supplementation of Honey Bee Larvae with Arginine and Abscisic Acid Enhances Nitric Oxide and Granulocyte Immune Responses after Trauma. Insects 2017, 8, 85. [Google Scholar] [CrossRef]
- Negri, P.; Villalobos, E.; Szawarski, N.; Damiani, N.; Gende, L.; Garrido, M.; Maggi, M.; Quintana, S.; Lamattina, L.; Eguaras, M. Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health. Insects 2019, 10, 401. [Google Scholar] [CrossRef]
- Nowak, A.; Szczuka, D.; Gorczynska, A.; Motyl, I.; Kregiel, D. Characterization of Apis mellifera Gastrointestinal Microbiota and Lactic Acid Bacteria for Honeybee Protection-A Review. Cells 2021, 10, 701. [Google Scholar] [CrossRef]
- Raymann, K.; Bobay, L.M.; Moran, N.A. Antibiotics reduce genetic diversity of core species in the honeybee gut microbiome. Mol. Ecol. 2018, 27, 2057–2066. [Google Scholar] [CrossRef]
- Raymann, K.; Moran, N.A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 2018, 26, 97–104. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Mott, B.M.; Maes, P.W.; Floyd, A.S.; Fitz, W.; Copeland, D.C.; Meikle, W.G.; Anderson, K.E. Honey bee colony performance and health are enhanced by apiary proximity to US Conservation Reserve Program (CRP) lands. Sci. Rep. 2019, 9, 4894. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Anderson, K.E. Probing the Honey Bee Diet-Microbiota-Host Axis Using Pollen Restriction and Organic Acid Feeding. Insects 2020, 11, 291. [Google Scholar] [CrossRef]
- Anderson, K.E.; Ricigliano, V.A. Honey bee gut dysbiosis: A novel context of disease ecology. Curr. Opin. Insect Sci. 2017, 22, 125–132. [Google Scholar] [CrossRef]
- Vanengelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony Collapse Disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Ahmed, H.R.; El-Wahed, A.A.A.; Saeed, A.; Algethami, A.F.; Attia, N.F.; Guo, Z.; Musharraf, S.G.; Khatib, A.; Alsharif, S.M.; et al. Bee Stressors from an Immunological Perspective and Strategies to Improve Bee Health. Vet. Sci. 2022, 9, 199. [Google Scholar] [CrossRef]
- Alaux, C.; Maisonnasse, A.; Le Conte, Y. Pheromones in a superorganism: From gene to social regulation. Vitam. Horm. 2010, 83, 401–423. [Google Scholar]
- Corby-Harris, V.; Meador, C.A.; Snyder, L.A.; Schwan, M.R.; Maes, P.; Jones, B.M.; Walton, A.; Anderson, K.E. Transcriptional, translational, and physiological signatures of undernourished honey bees (Apis mellifera) suggest a role for hormonal factors in hypopharyngeal gland degradation. J. Insect Physiol. 2016, 85, 65–75. [Google Scholar] [CrossRef]
- Chowanski, S.; Walkowiak-Nowicka, K.; Winkiel, M.; Marciniak, P.; Urbanski, A.; Pacholska-Bogalska, J. Insulin-Like Peptides and Cross-Talk With Other Factors in the Regulation of Insect Metabolism. Front. Physiol. 2021, 12, 701203. [Google Scholar] [CrossRef]
- Copeland, D.C.; Anderson, K.E.; Mott, B.M. Early Queen Development in Honey Bees: Social Context and Queen Breeder Source Affect Gut Microbiota and Associated Metabolism. Microbiol. Spectr. 2022, 10, e00383-22. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Deeter, M.E.; Snyder, L.; Meador, C.; Welchert, A.C.; Hoffman, A.; Obernesser, B.T. Octopamine mobilizes lipids from honey bee (Apis mellifera) hypopharyngeal glands. J. Exp. Biol. 2020, 223, jeb216135. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Snyder, L.; Meador, C. Fat body lipolysis connects poor nutrition to hypopharyngeal gland degradation in Apis mellifera. J. Insect Physiol. 2019, 116, 1–9. [Google Scholar] [CrossRef]
- Coulon, M.; Dalmon, A.; Di Prisco, G.; Prado, A.; Arban, F.; Dubois, E.; Ribiere-Chabert, M.; Alaux, C.; Thiery, R.; Le Conte, Y. Interactions Between Thiamethoxam and Deformed Wing Virus Can Drastically Impair Flight Behavior of Honey Bees. Front. Microbiol. 2020, 11, 766. [Google Scholar] [CrossRef]
- Coulon, M.; Schurr, F.; Martel, A.C.; Cougoule, N.; Begaud, A.; Mangoni, P.; Dalmon, A.; Alaux, C.; Le Conte, Y.; Thiery, R.; et al. Metabolisation of thiamethoxam (a neonicotinoid pesticide) and interaction with the Chronic bee paralysis virus in honeybees. Pestic. Biochem. Physiol. 2018, 144, 10–18. [Google Scholar] [CrossRef]
- Cuesta-Mate, A.; Renelies-Hamilton, J.; Kryger, P.; Jensen, A.B.; Sinotte, V.M.; Poulsen, M. Resistance and Vulnerability of Honeybee (Apis mellifera) Gut Bacteria to Commonly Used Pesticides. Front. Microbiol. 2021, 12, 717990. [Google Scholar] [CrossRef]
- Daisley, B.A.; Trinder, M.; McDowell, T.W.; Welle, H.; Dube, J.S.; Ali, S.N.; Leong, H.S.; Sumarah, M.W.; Reid, G. Neonicotinoid-induced pathogen susceptibility is mitigated by Lactobacillus plantarum immune stimulation in a Drosophila melanogaster model. Sci. Rep. 2017, 7, 2703. [Google Scholar] [CrossRef]
- Floyd, A.S.; Mott, B.M.; Maes, P.; Copeland, D.C.; McFrederick, Q.S.; Anderson, K.E. Microbial Ecology of European Foul Brood Disease in the Honey Bee (Apis mellifera): Towards a Microbiome Understanding of Disease Susceptibility. Insects 2020, 11, 555. [Google Scholar] [CrossRef]
- Maes, P.W.; Floyd, A.S.; Mott, B.M.; Anderson, K.E. Overwintering Honey Bee Colonies: Effect of Worker Age and Climate on the Hindgut Microbiota. Insects 2021, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Janashia, I.; Alaux, C. Specific Immune Stimulation by Endogenous Bacteria in Honey Bees (Hymenoptera: Apidae). J. Econ. Entomol. 2016, 109, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Janashia, I.; Choiset, Y.; Jozefiak, D.; Deniel, F.; Coton, E.; Moosavi-Movahedi, A.A.; Chanishvili, N.; Haertle, T. Beneficial Protective Role of Endogenous Lactic Acid Bacteria Against Mycotic Contamination of Honeybee Beebread. Probiotics Antimicrob. Proteins 2018, 10, 638–646. [Google Scholar] [CrossRef]
- Rand, E.E.D.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.W.W.; Nicolson, S.W. Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci. Rep. 2015, 5, 11779. [Google Scholar] [CrossRef]
- Vanderplanck, M.; Martinet, B.; Carvalheiro, L.G.; Rasmont, P.; Barraud, A.; Renaudeau, C.; Michez, D. Ensuring access to high-quality resources reduces the impacts of heat stress on bees. Sci. Rep. 2019, 9, 12596. [Google Scholar] [CrossRef]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional Physiology and Ecology of Honey Bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef]
- Shanahan, M. Honey Bees and Industrial Agriculture: What Researchers are Missing, and Why it’s a Problem. J. Insect Sci. 2022, 22, 14. [Google Scholar] [CrossRef]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef]
- Anderson, K.E.; Maes, P. Social microbiota and social gland gene expression of worker honey bees by age and climate. Sci. Rep. 2022, 12, 10690. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Rodrigues, P.A.; Mott, B.M.; Maes, P.; Corby-Harris, V. Ecological Succession in the Honey Bee Gut: Shift in Lactobacillus Strain Dominance During Early Adult Development. Microb. Ecol. 2016, 71, 1008–1019. [Google Scholar] [CrossRef]
- Bonilla-Rosso, G.; Engel, P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 2018, 43, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Brochet, S.; Quinn, A.; Mars, R.A.T.; Neuschwander, N.; Sauer, U.; Engel, P. Niche partitioning facilitates coexistence of closely related honey bee gut bacteria. eLife 2021, 10, e68583. [Google Scholar] [CrossRef]
- Cornet, L.; Cleenwerck, I.; Praet, J.; Leonard, R.R.; Vereecken, N.J.; Michez, D.; Smagghe, G.; Baurain, D.; Vandamme, P. Phylogenomic Analyses of Snodgrassella Isolates from Honeybees and Bumblebees Reveal Taxonomic and Functional Diversity. mSystems 2022, 7, e0150021. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Pitek, A.P.; Chmiel, J.A.; Al, K.F.; Chernyshova, A.M.; Faragalla, K.M.; Burton, J.P.; Thompson, G.J.; Reid, G. Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. ISME J. 2020, 14, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Pitek, A.P.; Chmiel, J.A.; Gibbons, S.; Chernyshova, A.M.; Al, K.F.; Faragalla, K.M.; Burton, J.P.; Thompson, G.J.; Reid, G. Lactobacillus spp. attenuate antibiotic-induced immune and microbiota dysregulation in honey bees. Commun. Biol. 2020, 3, 534. [Google Scholar] [CrossRef]
- Daisley, B.A.; Reid, G. BEExact: A Metataxonomic Database Tool for High-Resolution Inference of Bee-Associated Microbial Communities. mSystems 2021, 6, e00082-21. [Google Scholar] [CrossRef] [PubMed]
- Dalenberg, H.; Maes, P.; Mott, B.; Anderson, K.E.; Spivak, M. Propolis Envelope Promotes Beneficial Bacteria in the Honey Bee (Apis mellifera) Mouthpart Microbiome. Insects 2020, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. Functional and evolutionary insights into the simple yet specific gut microbiota of the honey bee from metagenomic analysis. Gut Microbes 2013, 4, 60–65. [Google Scholar] [CrossRef]
- Engel, P.; Bartlett, K.D.; Moran, N.A. The Bacterium Frischella perrara Causes Scab Formation in the Gut of its Honeybee Host. mBio 2015, 6, e00193-15. [Google Scholar] [CrossRef]
- Kesnerova, L.; Mars, R.A.T.; Ellegaard, K.M.; Troilo, M.; Sauer, U.; Engel, P. Disentangling metabolic functions of bacteria in the honey bee gut. PLoS Biol. 2017, 15, e2003467. [Google Scholar] [CrossRef]
- Kesnerova, L.; Emery, O.; Troilo, M.; Liberti, J.; Erkosar, B.; Engel, P. Gut microbiota structure differs between honeybees in winter and summer. ISME J. 2020, 14, 801–814. [Google Scholar] [CrossRef]
- Jones, J.C.; Fruciano, C.; Marchant, J.; Hildebrand, F.; Forslund, S.; Bork, P.; Engel, P.; Hughes, W.O.H. The gut microbiome is associated with behavioural task in honey bees. Insectes Sociaux 2018, 65, 419–429. [Google Scholar] [CrossRef]
- Keller, A.; McFrederick, Q.S.; Dharampal, P.; Steffan, S.; Danforth, B.N.; Leonhardt, S.D. (More than) Hitchhikers through the network: The shared microbiome of bees and flowers. Curr. Opin. Insect Sci. 2021, 44, 8–15. [Google Scholar] [CrossRef]
- Liberti, J.; Kay, T.; Quinn, A.; Kesner, L.; Frank, E.T.; Cabirol, A.; Richardson, T.O.; Engel, P.; Keller, L. The gut microbiota affects the social network of honeybees. Nat. Ecol. Evol. 2022, 6, 1471–1479. [Google Scholar] [CrossRef]
- Khan, K.A.; Al-Ghamdi, A.A.; Ghramh, H.A.; Ansari, M.J.; Ali, H.; Alamri, S.A.; Al-Kahtani, S.N.; Adgaba, N.; Qasim, M.; Hafeez, M. Structural diversity and functional variability of gut microbial communities associated with honey bees. Microb. Pathog. 2020, 138, 103793. [Google Scholar] [CrossRef]
- Kwong, W.K.; Moran, N.A. Evolution of host specialization in gut microbes: The bee gut as a model. Gut Microbes 2015, 6, 214–220. [Google Scholar] [CrossRef]
- Leonard, S.P.; Powell, J.E.; Perutka, J.; Geng, P.; Heckmann, L.C.; Horak, R.D.; Davies, B.W.; Ellington, A.D.; Barrick, J.E.; Moran, N.A. Engineered symbionts activate honey bee immunity and limit pathogens. Science 2020, 367, 573–576. [Google Scholar] [CrossRef]
- Moran, N.A.; Sloan, D.B. The Hologenome Concept: Helpful or Hollow? PLoS Biol. 2015, 13, e1002311. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.A.; Yun, Y. Experimental replacement of an obligate insect symbiont. Proc. Natl. Acad. Sci. USA 2015, 112, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Motta, E.V.S.; Raymann, K.; Moran, N.A. Glyphosate perturbs the gut microbiota of honey bees. Proc. Natl. Acad. Sci. USA 2018, 115, 10305–10310. [Google Scholar] [CrossRef]
- Powell, J.E.; Leonard, S.P.; Kwong, W.K.; Engel, P.; Moran, N.A. Genome-wide screen identifies host colonization determinants in a bacterial gut symbiont. Proc. Natl. Acad. Sci. USA 2016, 113, 13887–13892. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.E.; Martinson, V.G.; Urban-Mead, K.; Moran, N.A. Routes of Acquisition of the Gut Microbiota of the Honey Bee Apis mellifera. Appl. Environ. Microbiol. 2014, 80, 7378–7387. [Google Scholar] [CrossRef] [PubMed]
- Rozas, M.; Brillet, F.; Callewaert, C.; Paetzold, B. MinION Nanopore Sequencing of Skin Microbiome 16S and 16S-23S rRNA Gene Amplicons. Front. Cell. Infect. Microbiol. 2021, 11, 806476. [Google Scholar] [CrossRef]
- Steele, M.I.; Moran, N.A. Evolution of Interbacterial Antagonism in Bee Gut Microbiota Reflects Host and Symbiont Diversification. mSystems 2021, 6, e00063-21. [Google Scholar] [CrossRef]
- Steele, M.I.; Motta, E.V.S.; Gattu, T.; Martinez, D.; Moran, N.A. The Gut Microbiota Protects Bees from Invasion by a Bacterial Pathogen. Microbiol. Spectr. 2021, 9, e0039421. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Steele, M.I.; Leonard, S.P.; Motta, E.V.S.; Moran, N.A. Honey bees as models for gut microbiota research. Lab. Anim. 2018, 47, 317–325. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Decourtye, A.; Alaux, C.; Le Conte, Y.; Henry, M. Toward the protection of bees and pollination under global change: Present and future perspectives in a challenging applied science. Curr. Opin. Insect Sci. 2019, 35, 123–131. [Google Scholar] [CrossRef]
- Jack, C.J.; Ellis, J.D. Integrated Pest Management Control of Varroa destructor (Acari: Varroidae), the Most Damaging Pest of (Apis mellifera L. (Hymenoptera: Apidae)) Colonies. J. Insect Sci. 2021, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Hybl, M.; Mraz, P.; Sipos, J.; Hostickova, I.; Bohata, A.; Curn, V.; Kopec, T. Polyphenols as Food Supplement Improved Food Consumption and Longevity of Honey Bees (Apis mellifera) Intoxicated by Pesticide Thiacloprid. Insects 2021, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Arien, Y.; Dag, A.; Shafir, S. Omega-6:3 Ratio More Than Absolute Lipid Level in Diet Affects Associative Learning in Honey Bees. Front. Psychol. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.M.; Welchert, A.C.; Carroll, M.; Shafir, S.; Smith, B.H.; Corby-Harris, V. Unbalanced fatty acid diets impair discrimination ability of honey bee workers to damaged and healthy brood odors. J. Exp. Biol. 2022, 225, jeb244103. [Google Scholar] [CrossRef]
- Barascou, L.; Sene, D.; Barraud, A.; Michez, D.; Lefebvre, V.; Medrzycki, P.; Di Prisco, G.; Strobl, V.; Yanez, O.; Neumann, P.; et al. Pollen nutrition fosters honeybee tolerance to pesticides. R. Soc. Open Sci. 2021, 8, 210818. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Carroll, M.J.; Sheehan, T.; Lanan, M.C.; Mott, B.M.; Maes, P.; Corby-Harris, V. Hive-stored pollen of honey bees: Many lines of evidence are consistent with pollen preservation, not nutrient conversion. Mol. Ecol. 2014, 23, 5904–5917. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Disruption of quercetin metabolism by fungicide affects energy production in honey bees (Apis mellifera). Proc. Natl. Acad. Sci. USA 2017, 114, 2538–2543. [Google Scholar] [CrossRef]
- Smart, M.D.; Otto, C.R.V.; Lundgren, J.G. Nutritional status of honey bee (Apis mellifera L.) workers across an agricultural land-use gradient. Sci. Rep. 2019, 9, 16252. [Google Scholar] [CrossRef]
- Vanderplanck, M.; Moerman, R.; Rasmont, P.; Lognay, G.; Wathelet, B.; Wattiez, R.; Michez, D. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS ONE 2014, 9, e86209. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, A.D.; Tooker, J.F.; Patch, H.M.; Biddinger, D.J.; Coccia, M.; Crone, M.K.; Fiely, M.; Francis, J.S.; Hines, H.M.; Hodges, M.; et al. Pollen Protein: Lipid Macronutrient Ratios May Guide Broad Patterns of Bee Species Floral Preferences. Insects 2020, 11, 132. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Snyder, L.A. Measuring Hypopharyngeal Gland Acinus Size in Honey Bee (Apis mellifera) Workers. J. Vis. Exp. 2018, 139, e58261. [Google Scholar] [CrossRef]
- Malone, L.A.; Gatehouse, H.S. Effects of Nosema apis in Infection on honey bee (Apis mellifera) Digestive Proteolytic Enzyme Activity. J. Invertebr. Pathol. 1998, 71, 169–174. [Google Scholar] [CrossRef]
- Ahn, K.; Xie, X.; Riddle, J.; Pettis, J.; Huang, Z.Y. Effects of Lond Distance Transportation on Honey bee physiology. Psyche: A J. Entomol. 2012, 2012, 193029. [Google Scholar]
- Schulz, D.J.; Sullivan, J.P.; Robinson, G.E. Juvenile hormone and octopamine in the regulation of division of labor in honey bee colonies. Horm. Behav. 2002, 42, 222–231. [Google Scholar] [CrossRef] [PubMed]
- van der Steen, J.J.M.; Martel, A.-C.; Hendrickx, P. The fraction haemolymph vitellogenin of a honey bee colony, derived from a pooled haemolymph sample, a colony vitality parameter. J. Apic. Res. 2015, 54, 55–58. [Google Scholar] [CrossRef]
- Mondet, F.; Rau, A.; Klopp, C.; Rohmer, M.; Severac, D.; Le Conte, Y.; Alaux, C. Transcriptome profiling of the honeybee parasite Varroa destructor provides new biological insights into the mite adult life cycle. BMC Genom. 2018, 19, 328. [Google Scholar] [CrossRef]
- Doublet, V.; Poeschl, Y.; Gogol-Doring, A.; Alaux, C.; Annoscia, D.; Aurori, C.; Barribeau, S.M.; Bedoya-Reina, O.C.; Brown, M.J.; Bull, J.C.; et al. Unity in defence: Honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genom. 2017, 18, 207. [Google Scholar] [CrossRef]
- Ebeling, J.; Funfhaus, A.; Gisder, S. Special Issue: Honey Bee Pathogens and Parasites. Vet. Sci. 2022, 9, 515. [Google Scholar] [CrossRef]
- Belasli, A.; Ben Miri, Y.; Aboudaou, M.; Ait Ouahioune, L.; Montanes, L.; Arino, A.; Djenane, D. Antifungal, antitoxigenic, and antioxidant activities of the essential oil from laurel (Laurus nobilis L.): Potential use as wheat preservative. Food Sci. Nutr. 2020, 8, 4717–4729. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepulveda, B.; Delporte, C.; Valdovinos, C.E.; Martin-Hernandez, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Damiani, N.; Fernandez, N.J.; Porrini, M.P.; Gende, L.B.; Alvarez, E.; Buffa, F.; Brasesco, C.; Maggi, M.D.; Marcangeli, J.A.; Eguaras, M.J. Laurel leaf extracts for honeybee pest and disease management: Antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 2014, 113, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Hybl, M.; Bohata, A.; Radsetoulalova, I.; Kopecky, M.; Hostickova, I.; Vanickova, A.; Mraz, P. Evaluating the Efficacy of 30 Different Essential Oils against Varroa destructor and Honey Bee Workers (Apis mellifera). Insects 2021, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Kacaniova, M.; Terentjeva, M.; Ziarovska, J.; Kowalczewski, P.L. In Vitro Antagonistic Effect of Gut Bacteriota Isolated from Indigenous Honey Bees and Essential Oils against Paenibacillus Larvae. Int. J. Mol. Sci. 2020, 21, 6736. [Google Scholar] [CrossRef] [PubMed]
- Noudogbessi, J.P.; Gary-Bobo, M.; Adomou, A.; Adjalian, E.; Alitonou, G.A.; Avlessi, F.; Garcia, M.; Sohounhloue, D.C.; Menut, C. Comparative chemical study and cytotoxic activity of Uvariodendron angustifolium essential oils from Benin. Nat. Prod. Commun. 2014, 9, 261–264. [Google Scholar] [CrossRef]
- Damiani, N.; Fernandez, N.J.; Maldonado, L.M.; Alvarez, A.R.; Eguaras, M.J.; Marcangeli, J.A. Bioactivity of propolis from different geographical origins on Varroa destructor (Acari: Varroidae). Parasitol. Res. 2010, 107, 31–37. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Pasca, C.; Moise, A.R.; Bobis, O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants 2020, 10, 22. [Google Scholar] [CrossRef]
- Lesmana, R.; Zulhendri, F.; Fearnley, J.; Irsyam, I.A.; Rasyid, R.; Abidin, T.; Abdulah, R.; Suwantika, A.; Paradkar, A.; Budiman, A.S.; et al. The Suitability of Propolis as a Bioactive Component of Biomaterials. Front. Pharmacol. 2022, 13, 930515. [Google Scholar] [CrossRef]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis Consumption Reduces Nosema ceranae Infection of European Honey Bees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef]
- Wilson, M.B.; Brinkman, D.; Spivak, M.; Gardner, G.; Cohen, J.D. Regional variation in composition and antimicrobial activity of US propolis against Paenibacillus larvae and Ascosphaera apis. J. Invertebr. Pathol. 2015, 124, 44–50. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Wilson, M.B.; Pawlus, A.D.; Brinkman, D.; Gardner, G.; Hegeman, A.D.; Spivak, M.; Cohen, J.D. 3-Acyl dihydroflavonols from poplar resins collected by honey bees are active against the bee pathogens Paenibacillus larvae and Ascosphaera apis. Phytochemistry 2017, 138, 83–92. [Google Scholar] [CrossRef]
- Stamets, P.E.; Naeger, N.L.; Evans, J.D.; Han, J.O.; Hopkins, B.K.; Lopez, D.; Moershel, H.M.; Nally, R.; Sumerlin, D.; Taylor, A.W.; et al. Extracts of Polypore Mushroom Mycelia Reduce Viruses in Honey Bees. Sci. Rep. 2018, 8, 13936. [Google Scholar] [CrossRef]
- Wiese, N.; Fischer, J.; Heidler, J.; Lewkowski, O.; Degenhardt, J.; Erler, S. The terpenes of leaves, pollen, and nectar of thyme (Thymus vulgaris) inhibit growth of bee disease-associated microbes. Sci. Rep. 2018, 8, 14634. [Google Scholar] [CrossRef]
- Damiani, N.; Gende, L.B.; Bailac, P.; Marcangeli, J.A.; Eguaras, M.J. Acaricidal and insecticidal activity of essential oils on Varroa destructor (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae). Parasitol. Res. 2009, 106, 145–152. [Google Scholar] [CrossRef]
- Dhinaut, J.; Balourdet, A.; Teixeira, M.; Chogne, M.; Moret, Y. A dietary carotenoid reduces immunopathology and enhances longevity through an immune depressive effect in an insect model. Sci. Rep. 2017, 7, 12429. [Google Scholar] [CrossRef]
- Khan, S.; Shaheen, H.; Mehmood, A.; Nasar, S.; Khan, T. Ethnobotanical and antibacterial study of Primula plants traditionally used in the indigenous communities of Western Himalaya, Pakistan. Saudi J. Biol. Sci. 2022, 29, 3244–3254. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Ansari, M.J.; Khan, M.H.U.; Kamal, S.; Rahman, K.; Shoaib, M.; Man, S.; Khan, A.J.; Khan, S.U.; et al. Antimicrobial potentials of medicinal plant’s extract and their derived silver nanoparticles: A focus on honey bee pathogen. Saudi J. Biol. Sci. 2019, 26, 1815–1834. [Google Scholar] [CrossRef]
- Charistos, L.; Parashos, N.; Hatjina, F. Long term effects of a food supplement HiveAlive™ on honey bee colony strength and Nosema ceranae spore counts. J. Apic. Res. 2015, 54, 420–426. [Google Scholar] [CrossRef]
- Zhang, G.; St Clair, A.L.; Dolezal, A.; Toth, A.L.; O’Neal, M. Limited Diet Diversity and its relationship to virus resistance. J. Econ. Entomol. 2020, 113, 1062–1072. [Google Scholar] [CrossRef]
- Ellegaard, K.M.; Engel, P. Beyond 16S rRNA Community Profiling: Intra-Species Diversity in the Gut Microbiota. Front. Microbiol. 2016, 7, 1475. [Google Scholar] [CrossRef]
- Moran, N.A. Genomics of the honey bee microbiome. Curr. Opin. Insect Sci. 2015, 10, 22–28. [Google Scholar] [CrossRef]
- Bennett, G.M.; Moran, N.A. Heritable symbiosis: The advantages and perils of an evolutionary rabbit hole. Proc. Natl. Acad. Sci. USA 2015, 112, 10169–10176. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, S.; Kleinman, D.L.; Gratton, C.; Toth, A.; Guedot, C.; Groves, R.; Piechowski, J.; Moore, B.; Hagedorn, D.; Kauth, D.; et al. Collaboration Matters: Honey Bee Health as a Transdisciplinary Model for Understanding Real-World Complexity. BioScience 2018, 68, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Berthe, F.C.J.; Bouley, T.A.; Karesh, W.B.; Legall, I.C.; Machalaba, C.; Plante, C.A.; Seifman, R. One Health: Operational Framework for Strengthening Human, Animal, and Environmental Public Health Systems at Their Interface; World Bank Group: Washington, DC, USA, 2018. [Google Scholar]
- Mondet, F.; Beaurepaire, A.; McAfee, A.; Locke, B.; Alaux, C.; Blanchard, S.; Danka, B.; Le Conte, Y. Honey bee survival mechanisms against the parasite Varroa destructor: A systematic review of phenotypic and genomic research efforts. Int. J. Parasitol. 2020, 50, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhou, P.; Zhu, Y.J.; Zou, S.; Zhao, Q.; Yu, J.; Hu, Y.; Li, J. Gut Microbiota Dysbiosis and Altered Bile Acid Catabolism Lead to Metabolic Disorder in Psoriasis Mice. Front. Microbiol. 2022, 13, 853566. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Alaux, C.; Martin, J.F.; Harbo, J.R.; Harris, J.W.; Dantec, C.; Severac, D.; Cros-Arteil, S.; Navajas, M. Social immunity in honeybees (Apis mellifera): Transcriptome analysis of varroa-hygienic behaviour. Insect Mol. Biol. 2011, 20, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.; Le Conte, Y.; Mondet, F. Varroa destructor: How does it harm Apis mellifera honey bees and what can be done about it? Emerg. Top. Life Sci. 2020, 4, 45–57. [Google Scholar]
- Vilarem, C.; Piou, V.; Vogelweith, F.; Vetillard, A. Varroa destructor from the Laboratory to the Field: Control, Biocontrol and IPM Perspectives—A Review. Insects 2021, 12, 800. [Google Scholar] [CrossRef]
| Composition of the Solution | Quantities |
|---|---|
| A basic commercial product of fruit juice | For example: HAPPYFLOR Z (10 L) made from fruit juice, beet extract, chicory in variable proportions |
| Pollen | Ideally from your bees (250 g) |
| Honey | Ideal source from Our bees (2 kg) |
| Propolis | in alcoholic solution (5 mL) |
| Essential oils #: Rosmarinus officinalis Thymus vulgaris Laurel nobilis Eucalyptus globulus | 5 drops of each |
| Apple vinegar | 5 tablespoons |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dequenne, I.; Philippart de Foy, J.-M.; Cani, P.D. Developing Strategies to Help Bee Colony Resilience in Changing Environments. Animals 2022, 12, 3396. https://doi.org/10.3390/ani12233396
Dequenne I, Philippart de Foy J-M, Cani PD. Developing Strategies to Help Bee Colony Resilience in Changing Environments. Animals. 2022; 12(23):3396. https://doi.org/10.3390/ani12233396
Chicago/Turabian StyleDequenne, Isabelle, Jean-Michel Philippart de Foy, and Patrice D. Cani. 2022. "Developing Strategies to Help Bee Colony Resilience in Changing Environments" Animals 12, no. 23: 3396. https://doi.org/10.3390/ani12233396
APA StyleDequenne, I., Philippart de Foy, J.-M., & Cani, P. D. (2022). Developing Strategies to Help Bee Colony Resilience in Changing Environments. Animals, 12(23), 3396. https://doi.org/10.3390/ani12233396






