Management of Genetic Variation in the Gamete Bank of the Endangered Lake Minnow Eupallasella percnurus, Using Genassemblage 2.2 Software
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Genassemblage Setup
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabrita, E.; Sarasquete, C.; Martinez-Páramo, S.; Robles, V.; Beirao, J.; Pérez-Cerezales, S.; Herráez, M.P. Cryopreservation of fish sperm: Applications and perspectives. J. Appl. Ichthyol. 2010, 26, 623–635. [Google Scholar] [CrossRef]
- Dietrich, G.; Wolnicki, J.; Słowińska, M.; Sikorska, J.; Hliwa, P.; Kamiński, R.; Ciereszko, A. Preliminary characteristics of lake minnow Eupallasella percnurus (Pall.), semen. Arch. Pol. Fish. 2011, 19, 133–136. [Google Scholar] [CrossRef]
- Dietrich, G.; Wolnicki, J.; Słowińska, M.; Sikorska, J.; Hliwa, P.; Kamiński, R.; Liszewska, E.; Ciereszko, A. Short-term storage and cryopreservation of lake minnow (Eupallasella percnurus (Pallas, 1814)) sperm. J. Appl. Ichthyol. 2015, 31, 75–78. [Google Scholar] [CrossRef]
- Fraser, D.J. How well can captive breeding programs conserve biodiversity? A review of salmonids. Evol. Appl. 2008, 1, 535–586. [Google Scholar] [PubMed]
- Hulak, M.; Kaspar, V.; Kohlmann, K.; Coward, K.; Tešitel, J.; Rodina, M.; Gela, D.; Kocour, M.; Linhart, M. Microsatellite-based genetic diversity and differentiation of foreign common carp (Cyprinus carpio) strains farmed in the Czech Republic. Aquaculture 2010, 298, 194–201. [Google Scholar] [CrossRef]
- FAO. Cryoconservation of animal genetic resources. In FAO Animal Production and Health Guidelines; FAO: Rome, Italy, 2012; No. 12. [Google Scholar]
- Napora-Rutkowski, Ł.; Rakus, K.; Nowak, Z.; Szczygieł, J.; Pilarczyk, A.; Ostaszewska, T.; Irnazarow, I. Genetic diversity of common carp (Cyprinus carpio L.) strains breed in Poland based on microsatellite, AFLP, and mtDNA genotype data. Aquaculture 2017, 473, 433–442. [Google Scholar] [CrossRef]
- Yang, H.; Tiersch, T. Current status of sperm cryopreservation in biomedical research fish models: Zebrafish, medaka, and xiphophorus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 224–232. [Google Scholar] [CrossRef]
- Zhang, T. Cryopreservation of gametes and embryos of aquatic species. In Life in the Frozen State; Fuller, B., Lane, N., Benson, E., Eds.; CRC Press: London, UK, 2004; pp. 415–435. [Google Scholar]
- Kaczmarczyk, D. Techniques based on the polymorphism of microsatellite DNA as tools for conservation of endangered populations. Appl. Ecol. Environ. Res. 2019, 17, 1599–1615. [Google Scholar] [CrossRef]
- Weeks, A.R.; Stoklosa, J.; Hoffmann, A.A. Conservation of genetic uniqueness of populations may increase extinction likelihood of endangered species: The case of Australian mammals. Front. Zool. 2016, 13, 31. [Google Scholar] [CrossRef]
- Howard, J.T.; Pryce, J.E.; Baes, C.; Maltecca, C. Invited review: Inbreeding in the genomics era: Inbreeding, inbreeding depression, and management of genomic variability. J. Dairy Sci. 2017, 100, 6009–6024. [Google Scholar] [CrossRef]
- Wolnicki, J.; Kamiński, R.; Sikorska, J.; Kusznierz, J. Assessment of the size and structure of lake minnow Eupallasella percnurus (Pallas, 1814) population inhabiting small water body in Central Poland. Teka. Kom. Ochr. Kształt. Środ. Przyr.—OL PAN 2008, 5, 181–189. [Google Scholar]
- Kamiński, R.; Kusznierz, J.; Myszkowski, L.; Wolnicki, J. The first attempt to artificially reproduce the endangered cyprinid lake minnow Eupallasella perenurus (Pallas). Aquacult. Int. 2004, 12, 3–10. [Google Scholar] [CrossRef]
- Kaczmarczyk, D.; Wolnicki, J. Conservation of genetic variation of captively propagated species using Genassemblage 2.0 software: An example involving the lake minnow (Eupallasella percnurus), an endangered cyprinid fish. Sci. Rep. 2020, 10, 17871. [Google Scholar] [CrossRef]
- Singh, U.; Deb, R.; Alyethodi, R.R.; Alex, R.; Kumar, S.; Chakraborty, S.; Dhama, K.; Sharma, A. Molecular markers and their application in cattle genetic research—A review. Biomark. Genom. Med. 2014, 6, 49–58. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.; Wang, W.; Gui, J.-F.; Mei, J. Parentage determination of yellow catfish (Pelteobagrus fulvidraco) based on microsatellite DNA markers. Aquacult. Int. 2016, 24, 567–576. [Google Scholar] [CrossRef]
- Macdonald, H.C.; Ormerod, S.J.; Bruford, M.W. Enhancing capacity for freshwater conservation at the genetic level: A demonstration using three stream macroinvertebrates. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 27, 452–461. [Google Scholar] [CrossRef]
- Kaczmarczyk, D. Genassemblage software, a tool for management of genetic diversity in human dependent population. Conserv. Genet. Resour. 2015, 7, 49–51. [Google Scholar] [CrossRef][Green Version]
- Kaczmarczyk, D.; Wolnicki, J. Genetic diversity of the endangered cyprinid fish lake minnow Eupallasella percnurus in Poland and its implications for conservation. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Dimsoski, P.; Toth, G.P.; Bagley, M.J. Microsatellite characterization in central stoneroller Campostoma anomalum (Pisces: Cyprinidae). Mol. Ecol. 2000, 9, 2187–2189. [Google Scholar] [CrossRef]
- Holmen, J.; Vøllestad, L.A.; Jakobsen, K.S.; Primmer, C.R. Cross-species amplification of zebrafish and central stoneroller microsatellite loci in six other cyprinids. J. Fish Biol. 2005, 66, 851–859. [Google Scholar] [CrossRef]
- Molecular Ecology Resources Primer Development Consortium; Aksoy, S.; Almeida-Val, V.M.; Azevedo, V.C.; Baucom, R.; Bazaga, P.; Beheregaray, L.B.; Bennetzen, J.L.; Brassaloti, R.A.; Burgess, T.I.; et al. Permanent Genetic Resources added to Molecular Ecology Resources Database 1 October–November 2012. Mol. Ecol. Resour. 2012, 13, 341–343. [Google Scholar]
- Ruzzante, D.E. A comparison of several measures of genetic distance and population structure with microsatellite data: Bias and sampling variance. Can. J. Fish. Aquat. Sci. 1998, 55, 1–14. [Google Scholar] [CrossRef]
- Danchin-Burge, C.; Palhière, I.; François, D.; Bibé, B.; Leroy, G.; Verrier, E. Pedigree analysis of seven small French sheep populations and implications for the management of rare breeds. J. Anim. Sci. 2010, 88, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Fopp-Bayat, D.; Kaczmarczyk, D.; Szczepkowski, M. Genetic characteristics of Polish whitefish (Coregonus lavaretus maraena) broodstocks—Recommendations for the conservation management. Czech. J. Anim. Sci. 2015, 60, 171–177. [Google Scholar] [CrossRef]
- Wolnicki, J.; Kamiński, R.; Sikorska, J.; Kaczmarczyk, D. Occurrence and active protection of the endangered cyprinid fish species, lake minnow Eupallasella percnurus (Pall.), in Poland. In Proceedings of the XVI European Congress of Ichthyology, Lausanne, Switzerland, 2–6 September 2019. [Google Scholar]
- Nynca, J.; Kuźmiński, H.; Dietrich, G.J.; Hliwa, P.; Dobosz, S.; Liszewska, E.; Karol, H.; Ciereszko, A. Biochemical and physiological characteristics of semen of sex-reversed female rainbow trout (Oncorhynchus mykiss, Walbaum). Theriogenology 2012, 77, 174–183. [Google Scholar] [CrossRef]
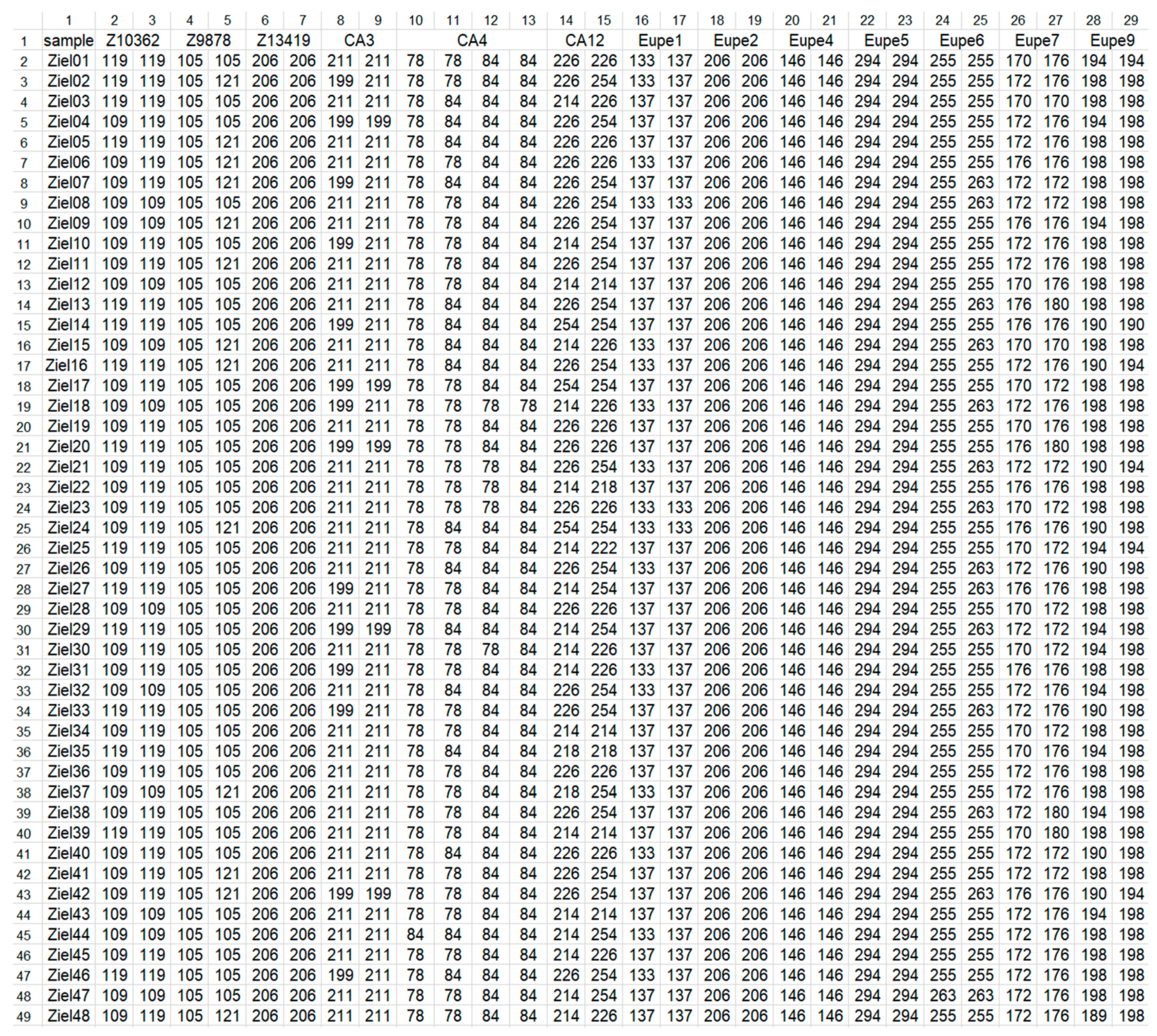
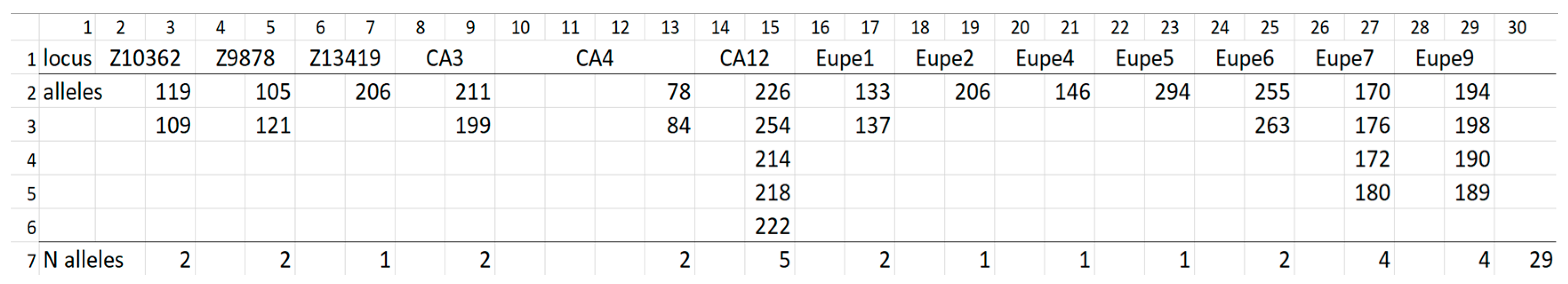
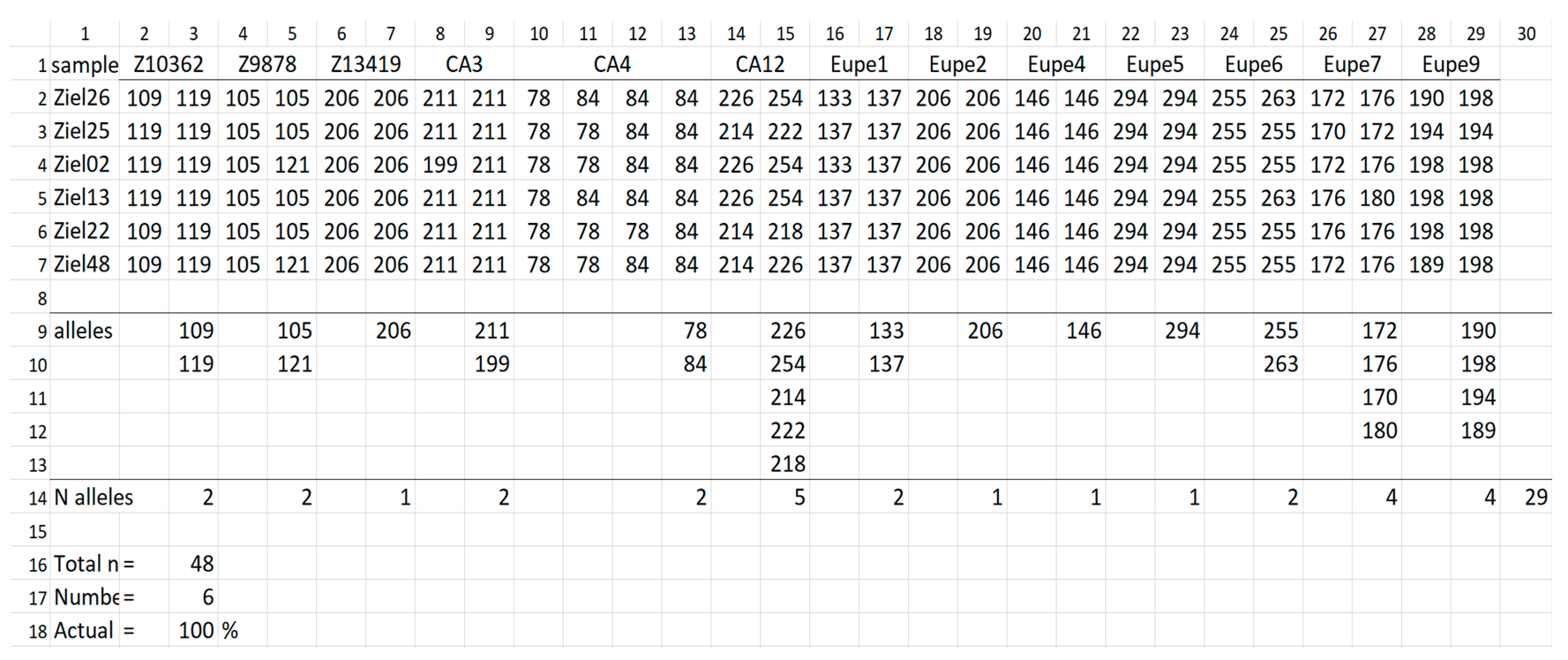
| Indicator | Populations | ||||
|---|---|---|---|---|---|
| Mikołajki Pomorskie (MP) | Drozdowo (DR) | Zielonka (ZI) | Bełcząc (BE) | All Samples (ALL) | |
| N | 48 | 48 | 48 | 48 | 192 |
| nA | 21 | 27 | 29 | 53 | 66 |
| nA/N | 0.44 | 0.56 | 0.60 | 1.10 | 0.34 |
| N’ | 5 | 4 | 6 | 13 | 19 |
| N’/N (%) | 10.42 | 8.33 | 12.50 | 27.08 | 9.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarczyk, D.; Wolnicki, J. Management of Genetic Variation in the Gamete Bank of the Endangered Lake Minnow Eupallasella percnurus, Using Genassemblage 2.2 Software. Animals 2022, 12, 3329. https://doi.org/10.3390/ani12233329
Kaczmarczyk D, Wolnicki J. Management of Genetic Variation in the Gamete Bank of the Endangered Lake Minnow Eupallasella percnurus, Using Genassemblage 2.2 Software. Animals. 2022; 12(23):3329. https://doi.org/10.3390/ani12233329
Chicago/Turabian StyleKaczmarczyk, Dariusz, and Jacek Wolnicki. 2022. "Management of Genetic Variation in the Gamete Bank of the Endangered Lake Minnow Eupallasella percnurus, Using Genassemblage 2.2 Software" Animals 12, no. 23: 3329. https://doi.org/10.3390/ani12233329
APA StyleKaczmarczyk, D., & Wolnicki, J. (2022). Management of Genetic Variation in the Gamete Bank of the Endangered Lake Minnow Eupallasella percnurus, Using Genassemblage 2.2 Software. Animals, 12(23), 3329. https://doi.org/10.3390/ani12233329






