Embryonic Development and Survival of Siberian Sturgeon × Russian Sturgeon (Acipenser baerii × Acipenser gueldenstaedtii) Hybrids Cultured in a RAS System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Controlled Breeding and Embryo Incubation
2.3. Embryo Sampling
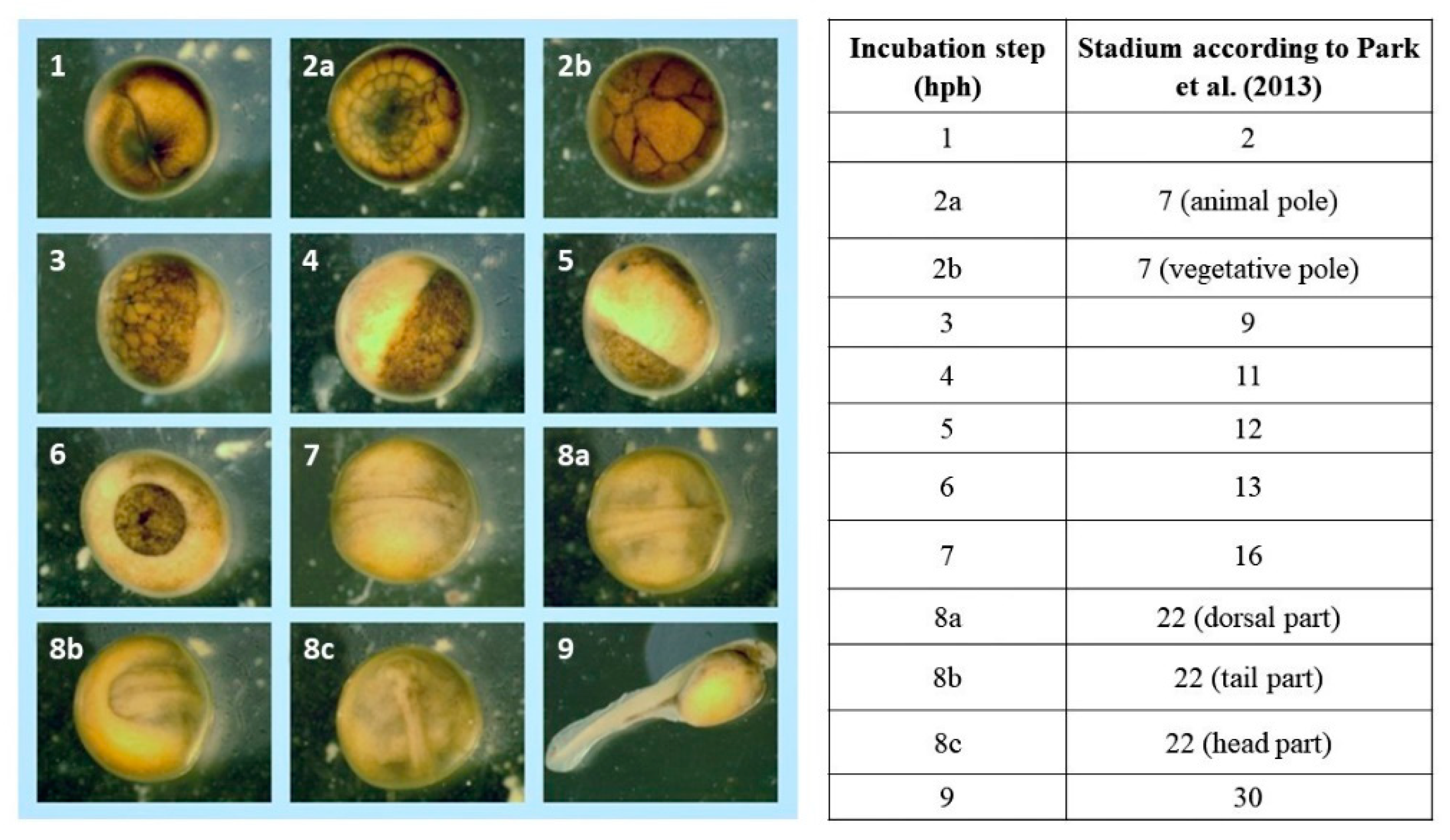
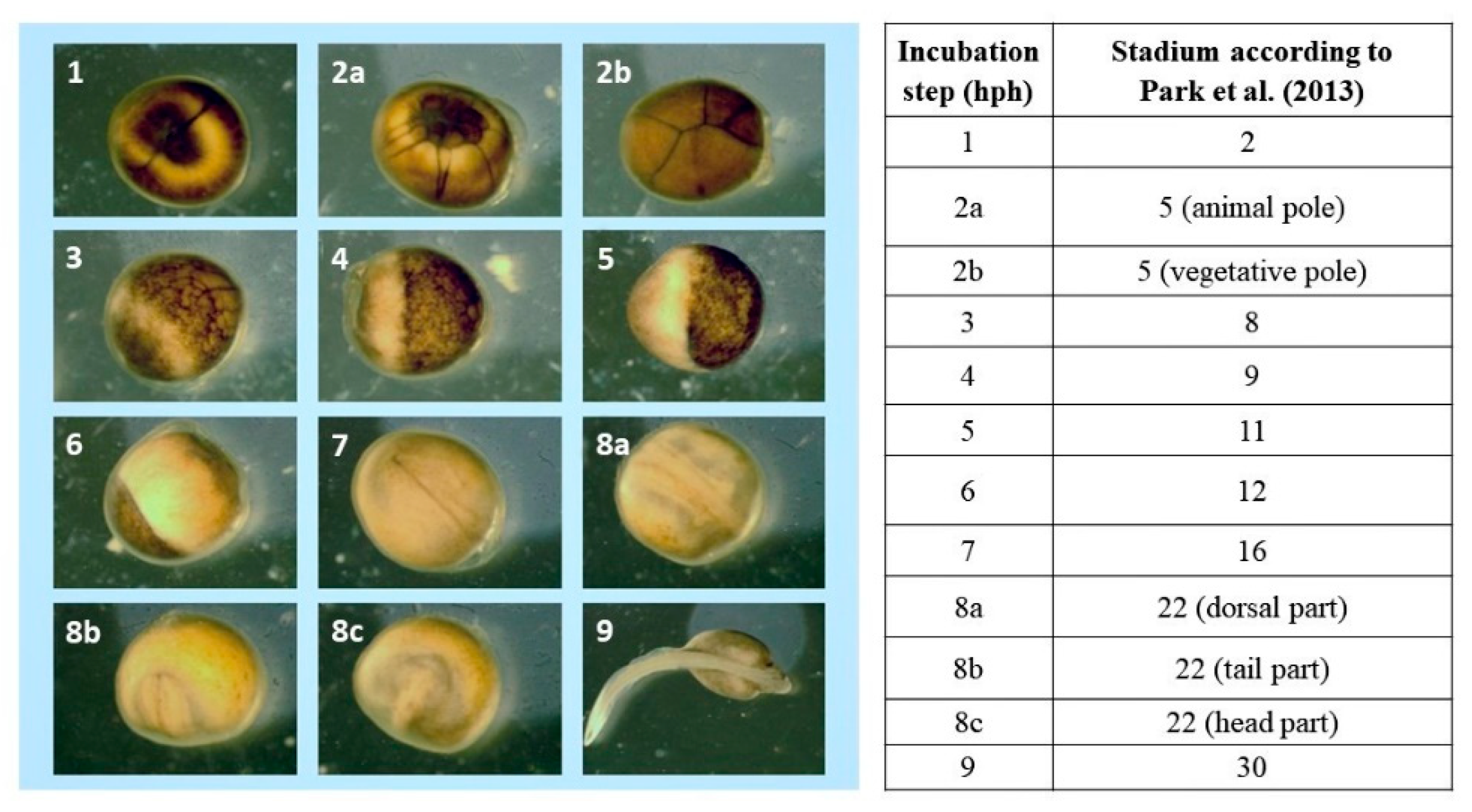
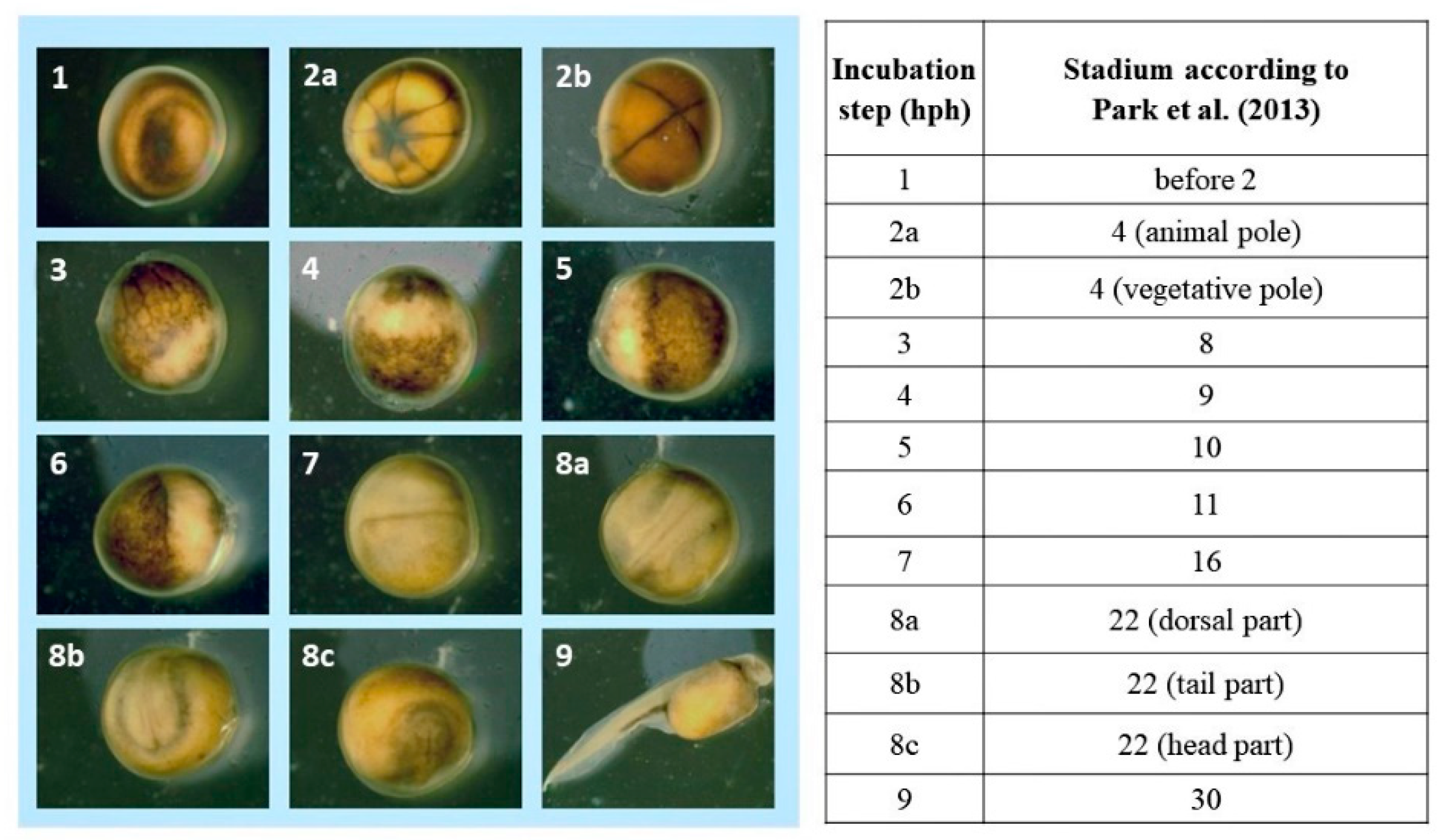
2.4. Observations of Embryonic Development and Acquisition of Microscopic Images
2.5. Identification of Embryonic Development Stages
2.6. Statistical Analysis
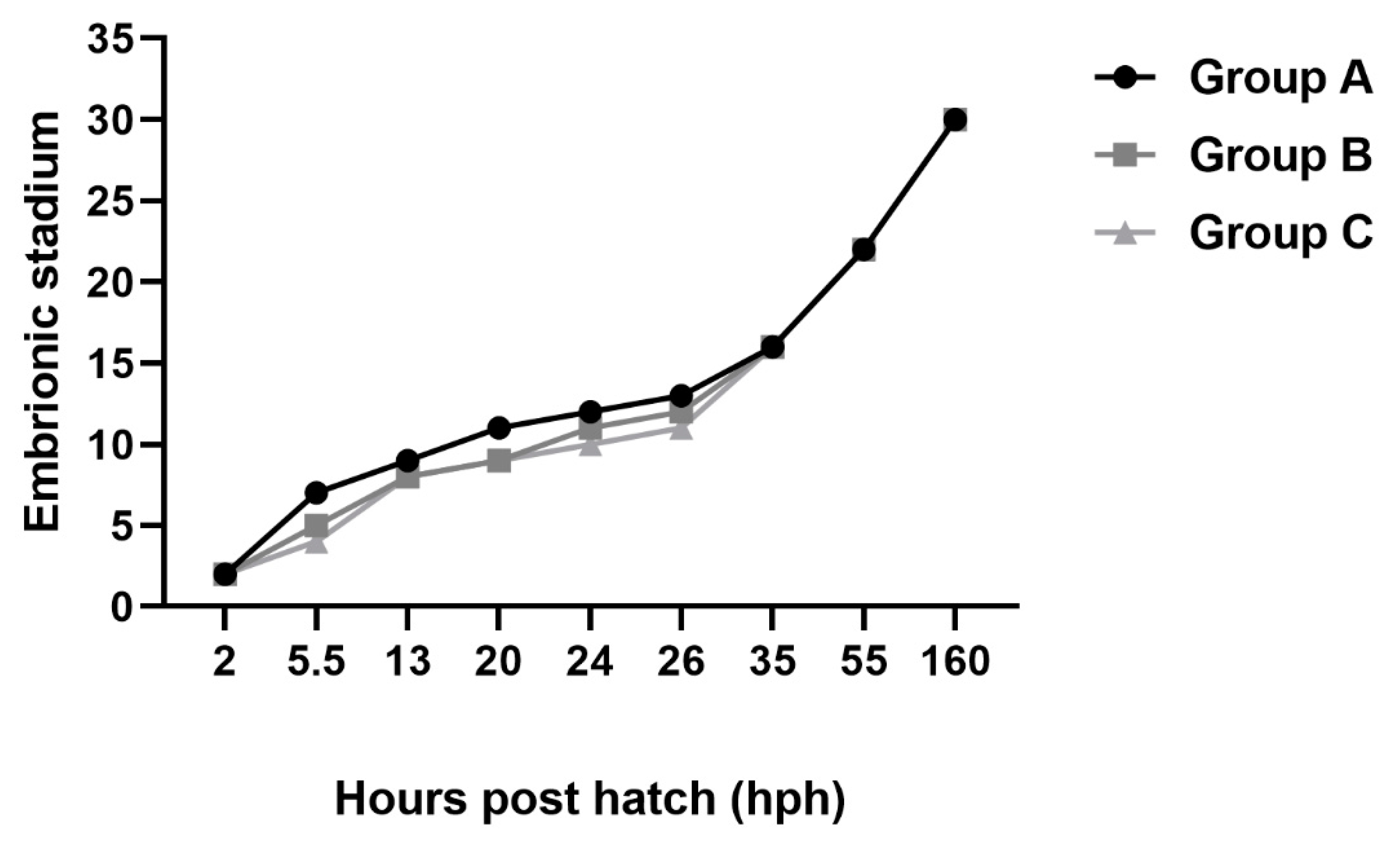
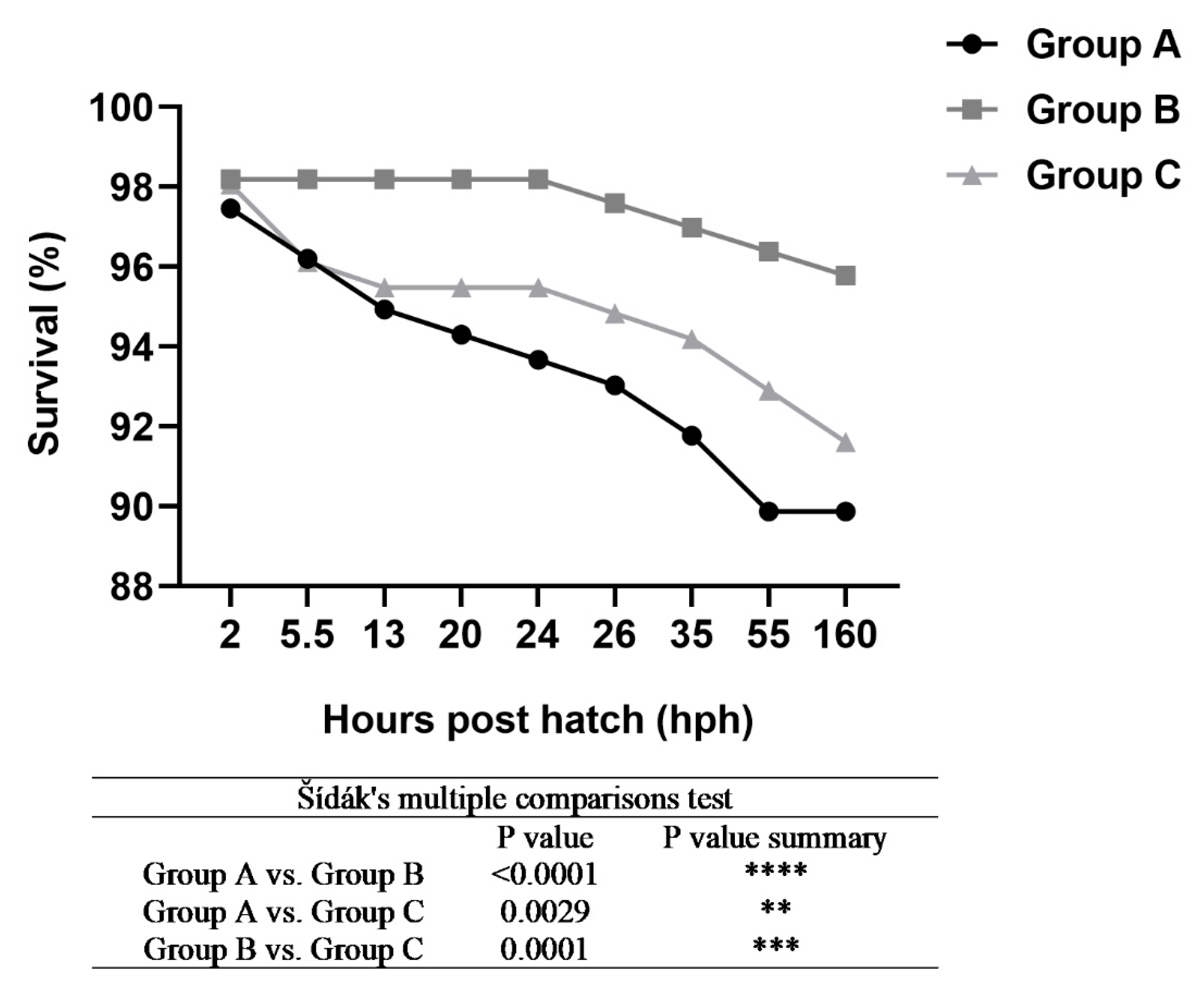
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pikitch, E.K.; Doukakis, P.; Lauck, L.; Chakrabarty, P.; Erickson, D.L. Status, trends and management of sturgeon and paddlefish fisheries. Fish Fish 2005, 6, 233–265. [Google Scholar] [CrossRef]
- Karpinsky, M.G. Review: The Caspian Sea benthos: Unique fauna and community formed under strong grazing pressure. Mar. Pollut. Bull. 2010, 61, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.A.H.; Doroshov, S.I. Importance of environmental endocrinology in fisheries management and aquaculture of sturgeons. Gen. Comp. Endocrionol. 2011, 170, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Burtsev, I.A.; Nikolaev, A.I.; Slizchenko, A.G. Novyy Obekt Tovarnogo Osetrovodstva—Gibrid Mezhdu Russkim i Sibirskim Osetrami (A. gueldenstaedtii Br. × A. baerii Br.). In Kul’tivirivanie Morskikg Organizmov; VNIRO: Moscow, Russia, 1985; pp. 112–116. [Google Scholar]
- Chebanov, M.; Podushka, S.; Rachek, E.; Amvrosov, D.; Merkulov, Y. Hybrids of the Siberian Sturgeon. In The Siberian Sturgeon (Acipenser baerii, Brandt, 1869), Volume 2—Farming; Williot, P., Nonnotte, G., Vizziano-Cantonnet, D., Chebanov, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 289–326. [Google Scholar]
- Kolman, R. Sturgeon Rearing and Breeding. A Breeder’s Guide; IRS: Olsztyn, Poland, 2006; p. 134. [Google Scholar]
- Kolman, R.; Szczepkowski, M. Comparison of behavior and breeding indices of return hybrids of Siberian sturgeon (Acipenser baerii Br.) with Russian sturgeon (Acipenser gueldenstaedtii Br.) at early stages of rearing. In Proceedings of the Research and Practice Conference: Aquaculture of Sturgeon Fishes: Achievements and Prospects of Development, Astrakhan, Russia, 21–22 November 2001; pp. 97–99. [Google Scholar]
- Dettlaff, T.A.; Ginsburg, A.C.; Shmalhausen, O.I. Rozwitije Osetrovykh Ryb; Izd. Nauka: Moscow, Russia, 1981; pp. 77–123. [Google Scholar]
- Dettlaff, T.A.; Vassetzky, S.G. Animal Species for Developmental Studies, Volume 2 Vertebrates; Plenum Publishing: New York, NY, USA, 1991; p. 466. [Google Scholar]
- Park, C.; Lee, S.Y.; Kim, D.S.; Nam, J.K. Embryonic Development of Siberian Sturgeon Acipenser baerii under Hatchery Conditions: An Image Guide with Embryological Descriptions. J. Fish. Aquat. Sci. 2013, 16, 15–23. [Google Scholar] [CrossRef]
- Burtsev, I.A. Hybridization and Selection of Sturgeon Fish at Full Cycle Rearing and Domestication. In Biological Bases of Fish Farming: Problems of Genetics and Selection; Nauka: Moscow, Russia, 1983; pp. 102–113. [Google Scholar]
- Szczepkowska, B.; Kolman, R.; Szczepkowski, M. Morphological characters of artificially induced hybrids of Siberian sturgeon, Acipenser baerii baerii Brandt, and green sturgeon, Acipenser medirostris Ayres. Arch. Pol. Fish. 2011, 19, 77–85. [Google Scholar] [CrossRef]
- Szczepkowski, M.; Kolman, R. Development and behavior of two reciprocal back cross hybrids of Siberian sturgeon (Acipenser baerii Brandt) and Russian sturgeon (Acipenser gueldenstaedtii Brandt) during early ontogenesis sturgeon. Czech J. Anim. Sci. 2002, 47, 289–296. [Google Scholar]
- Dettlaff, T.A.; Ginsburg, A.S.; Schmalhausen, O. Sturgeon Fishes. Developmental Biology and Aquaculture; Springer: Berlin, Germany, 1993; p. 300. [Google Scholar]
- Fopp-Bayat, D. Spontaneous gynogenesis in Siberian sturgeon Acipenser baeri Brandt. Aquacult. Res. 2007, 38, 776–779. [Google Scholar] [CrossRef]
- Fopp-Bayat, D.; Woznicki, P. Spontaneous and induced gynogenesis in sterlet Acipenser ruthenus Brandt. Caryologia 2007, 6, 315–318. [Google Scholar]
- Fopp-Bayat, D.; Hliwa, P.; Ocalewicz, K. Presence of gynogenetic males suggests a female heterogamety in sterlet Acipenser ruthenus L. Anim. Reprod. Sci. 2018, 189, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Van Eenennaam, J.P.; Linares-Casenave, J.; Deng, X.; Doroshov, S.I. Effect of incubation temperature on green sturgeon embryos, Acipenser medirostris. Environ. Biol. Fish. 2005, 72, 145–154. [Google Scholar] [CrossRef]
- Gisbert, E.; Nam, Y.K. Early Ontogeny in the Siberian Sturgeon. In The Siberian Sturgeon (Acipenser baerii, Brandt, 1869); Williot, P., Nonnotte, G., Vizziano-Cantonnet, D., Chebanov, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; Volume 1—Biology, pp. 131–157. [Google Scholar]
- Fopp-Bayat, D.; Ciereszko, A. Microsatellite genotyping of cryopreserved spermatozoa for the improvement of whitefish semen cryobanking. Cryobiology 2012, 65, 196–201. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fopp-Bayat, D.; Ciemniewski, T.; Cejko, B.I. Embryonic Development and Survival of Siberian Sturgeon × Russian Sturgeon (Acipenser baerii × Acipenser gueldenstaedtii) Hybrids Cultured in a RAS System. Animals 2023, 13, 42. https://doi.org/10.3390/ani13010042
Fopp-Bayat D, Ciemniewski T, Cejko BI. Embryonic Development and Survival of Siberian Sturgeon × Russian Sturgeon (Acipenser baerii × Acipenser gueldenstaedtii) Hybrids Cultured in a RAS System. Animals. 2023; 13(1):42. https://doi.org/10.3390/ani13010042
Chicago/Turabian StyleFopp-Bayat, Dorota, Tomasz Ciemniewski, and Beata Irena Cejko. 2023. "Embryonic Development and Survival of Siberian Sturgeon × Russian Sturgeon (Acipenser baerii × Acipenser gueldenstaedtii) Hybrids Cultured in a RAS System" Animals 13, no. 1: 42. https://doi.org/10.3390/ani13010042
APA StyleFopp-Bayat, D., Ciemniewski, T., & Cejko, B. I. (2023). Embryonic Development and Survival of Siberian Sturgeon × Russian Sturgeon (Acipenser baerii × Acipenser gueldenstaedtii) Hybrids Cultured in a RAS System. Animals, 13(1), 42. https://doi.org/10.3390/ani13010042





