Simple Summary
Plastic waste in the marine environment can be degraded into microplastic particles that are smaller than 5 mm in diameter. Such microplastic particles can interfere with the effectors of the nonspecific immune system in crustaceans, effectively debilitating a major defense mechanism against infectious diseases. Pacific white shrimp (Litopenaeus vannamei) is one of the most important marine crustacean species with global presence in the aquaculture industry that is at high risk of being contaminated with microplastics due to its feeding habits and benthic food sources. Such contamination may increase the risk of adverse effects on shrimp health. Thus, this study aimed to investigate the effects of oral intake of microplastic in food on the nonspecific immune system gene expression of Pacific white shrimp. We demonstrated immunosuppression at the gene expression level and histological damage of the hepatopancreas in shrimp fed with microplastic-contaminated food, leading to higher mortality. Information from L. vannamei can be used as a model system for other marine decapods. The increased presence of microplastic pollution in the environment and food has the potential to affect shrimp fisheries and aquaculture in the future, as well as further destabilize marine ecosystems already exposed to the combined anthropogenic and climate changes.
Abstract
Microplastic pollution can interfere with aquatic animal health and nonspecific immunity, increasing the potential for pathogen infection in crustaceans. However, the long-term effects of microplastics on crustacean immunity are less understood, especially regarding their toxicity in Pacific white shrimp (Litopenaeus vannamei). Effects of high-density polyethylene microplastics (HDPE-MPs) in feed on the mortality rate, hepatopancreas, and nonspecific immune system gene expression of Pacific white shrimp are presented. The LC50 at day 28 of HDPE-MP exposure was determined as 3.074% HDPE-MP in feed. A significant upregulation of the superoxide dismutase (SOD) and glutathione peroxidase (GPx) genes was observed in shrimp that were fed with 0.1 and 0.5% of HDPE-MP; then, they were downregulated significantly, except for the SOD gene expression of shrimp fed with 0.1% of HDPE-MP. The lysozyme (LYZ) gene was upregulated significantly in shrimp that were fed with 0.5, 1, and 3% HDPE-MP for 7 days and downregulated significantly in HDPE-receiving groups for at least 14 days. Significant histopathological changes in the hepatopancreas were observed in the treatment groups. The histopathological score of each lesion was correlated with the increase in HDPE-MP concentration. This study shows that the ingestion of HDPE microplastics can alter the expression of nonspecific immune system genes and damage the hepatopancreas in Pacific white shrimp.
1. Introduction
The ubiquitous presence of plastic waste in marine environments is becoming a high-priority concern due to its persistence and adverse effects on marine and coastal species directly and indirectly [1]. Weathering effects on plastic debris at the interface of coastal and offshore marine environments produce particles between 1 µm to 5 mm in diameter, commonly referred to as “microplastic” [2]. Future projections for the accumulated mass of buoyant degraded microplastic materials from the ocean surface layer indicate that over one million tons of these particles will likely be polluting the oceans in the period from 2020 to 2030 [3]. This situation presents multiple new or undefined risks including the potential ingestion of microplastics by aquatic animals [4,5,6], possibly leading to microplastic particles contaminating the marine food chain, including seafood intended for human consumption [7].
One of the most abundant components of plastic waste, including degraded microplastics, is polyethylene (PE), which is a common plastic polymer used in various items such as plastic bags, plastic bottles, food packaging, clothing, ropes, nets, etc. PE plastic items are usually produced as single-use items, with a short useful life and quick transition to plastic waste, and are considered a significant source of microplastics in the environment. PE represents almost two-fifths of all plastic waste (38%) in the marine environment, out of which 21% is low-density polyethylene (LDPE) and 17% is high-density polyethylene (HDPE) [8]. PE microplastics can accumulate in the surface layers of sediments [9], increasing the likelihood of benthic species such as shrimps ingesting them accidentally due to their feeding behavior. PE plastic items are widely used in aquaculture production processes. Shrimp and other crustaceans are frequently raised in HDPE-lined ponds [10], and various plastic items such as anti-predator nets or mesh screens are made from polymers including PE and polypropylene (PP) and used to prevent undesirable organisms from entering the ponds [7]. Moreover, paddle-wheel aerators (usually made from HDPE) are intensively used in shrimp culture to increase dissolved oxygen in the ponds [11,12]. Therefore, plastic equipment routinely used in shrimp culture can act as a significant source of PE (including MP) in the aquaculture ponds, increasing the risk of PE contamination of seafood produced in such conditions.
Microplastics are increasingly being detected in the gastrointestinal tract and gills of various marine fauna, including crustaceans. A study on the langoustine (Nephrops norvegicus) found that microplastic fibers could accumulate in the stomach and result in false satiation and reduced feed consumption and body mass [13]. A study on shore crabs (Carcinus maenas) showed that polystyrene microspheres were retained in the foregut and on the external surface of the gills [14]. Moreover, the translocation of microplastics from the gastrointestinal tract to the internal organs was presented in decapods. Studies on two crab species, the fiddler crab (Uca rapax) and shore crab, demonstrated the translocation of microplastic to hemolymph and internal organs such as hepatopancreas, ovaries, and gills [15,16]. However, the effects of microplastics accumulated in the tissues were not mentioned in these studies. The study on Chinese mitten crabs (Eriocheir sinensis) by Yu et al. [17] demonstrated the accumulation of microplastics in hepatopancreas and noted changes in the enzyme activity and gene expression of superoxide dismutase (SOD), aspartate transaminase (GOT), glutathione (GSH), glutathione peroxidase (GPx), acetylcholinesterase, catalase (CAT), and alanine aminotransferase.
Pacific white shrimp (Litopenaeus vannamei) is one of the most important crustacean species in the global aquaculture industry and a significant source of seafood for people worldwide. In 2021, the world’s shrimp production is estimated to have reached over 4.5 million tons [18,19]. Considering that microplastic contamination in the fish meal showed 19% of PE microplastic [20] and that fish meal is an important ingredient of shrimp feed [21], it is likely that PE-contaminated shrimp feed may result in the ingestion and possibly bioaccumulation of microplastic particles and increased risk of adverse effects on the shrimp health in aquaculture.
Since crustaceans do not have effectors of an acquired immune system similar to vertebrates, their defense mechanisms against pathogens rely almost exclusively on innate (nonspecific) immune responses such as anti-bacterial and antioxidant enzyme activity [22]. An anti-bacterial enzyme such as lysozyme (LYZ) responds against the infection of Vibrio harveyi, V. campbellii, V. penaecida, V. parahaemolyticus, and white spot syndrome virus (WSSV) [23,24,25,26]. Antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx) act against reactive oxygen species (ROS) produced by V. parahaemolyticus and WSSV [23,27,28].
Microplastics can interfere with nonspecific immune function in crustaceans: studies on Chinese mitten crabs [17,29] and penaeid shrimp [30] found that microplastics alter the expression of enzymes and genes related to immune responses. Microplastics can also cause histopathological lesions of the hepatopancreas of Pacific white shrimp [31]. Therefore, L. vannamei can be used as a reliable model for ecotoxicological investigations in marine decapods. However, our understanding of the connection between immunosuppression and tissue damage in penaeid shrimp is not complete. The aim of the presented studies was to determine the effects of dietary HDPE microplastics (HDPE-MPs) on white-legged Pacific shrimp health, histopathology, antioxidant, and innate immune gene responses.
2. Materials and Methods
2.1. Animal Use Protocol
Pacific white shrimp (Litopenaeus vannamei) were obtained from a shrimp farm in Phetchaburi province (Phetchaburi, Thailand). The average weight and total length of shrimp used in this study were approximately 4 g and 7 cm, respectively. The shrimp’s general health and presence of external parasites were checked, and the shrimp were quarantined in a 500 L tank with aerated seawater of 5 g L−1 salinity for 2 weeks. The stock density was set at 70 shrimp per m2 of water surface, 20% water change was performed twice a week, and the water quality parameters were monitored daily. During the experiment, ten shrimp were reared in a glass tank filled with 45 L of seawater at a salinity of 5 g L−1. Tanks were installed with an undergravel filtration system and aerated for 24 h. The water was changed by 20% twice a week, and water quality was monitored every day The shrimp were fed at the rate of 4% of their total body weight per day, divided into 3 feedings/day with commercial shrimp feed (Charoen Pokphand Foods, CP). The IACUC protocol number of this study is 2031077.
2.2. Phase 1: Toxicity Test
2.2.1. Feed Preparation
Shrimp feed pellet was prepared from commercial shrimp feed powder (Charoen Pokphand Foods, CP; Supplementary Table S1) mixed with test concentrations of pure HDPE-MP with a diameter of 50 µm (CosphericTM, Santa Barbara, CA, USA) and 3% wheat gluten, using the extruder machine with a 2 mm sieve. Pellets were dried in a hot air oven at 60 °C for 6 h. The feed pellets were divided into rations and stored at 4 °C until use. HDPE-MP was added as a percentage of feed (g kg−1) [32]. Five feeds were produced for the toxicity test: (A) control feed without HDPE-MP/0%; (B) feed with 0.5% HDPE-MP; (C) feed with 5% HDPE-MP; (D) feed with 10% HDPE-MP; (E) feed with 20% HDPE-MP.
2.2.2. HDPE-MP Exposure Scheme and Determination of LD50
Several factors can influence the toxicity of microplastic (shape, size, polymer type, presence of chemical additives, etc.) [33,34,35,36,37]. Therefore, a toxicity test was performed to determine the LD50 of HDPE-MP used in this study. Shrimp were divided into 5 groups of 10 shrimp each in triplicate tanks (30 shrimp/group). Each group was randomly assigned a different dose (0, 0.5, 5, 10, and 20%) of HDPE-MP and fed for 28 days. Shrimp were reared in a 60 L glass tank with a filtration system and continuous aeration [38]. Probit analysis of recorded mortality rates was used to calculate LD50 at day 28 after exposure with SPSS version 22 (IBM®).
2.3. Phase 2: Determination of Gene Expression and Histopathology
2.3.1. Feed Preparation
A shrimp feed pellet was prepared with the same method as in the toxicity test but with a different percentage of HDPE-MP. Five feeds were produced for determination of gene expression and histopathology: (A) control feed without HDPE-MP/0%; (B) feed with 0.1% HDPE-MP; (C) feed with 0.5% HDPE-MP; (D) feed with 1% HDPE-MP; (E) feed with 3% HDPE-MP. The highest concentration of HDPE-MP in feed (3%) was selected based on the calculated LD50 on the 28th day, and lower concentrations (0.1, 0.5, and 1% HDPE-MP) were designed based on the previous toxicity reports of microplastic in finfish diets [32,39,40]. Therefore, the highest concentration of HDPE-MP in this study is not representative of the actual level of microplastic that can be found in aquafeed.
2.3.2. HDPE-MP Exposure Scheme
The LD50 of HDPE-MP was used to determine the sublethal doses fed to shrimp in this experiment. Shrimp were randomly selected from a 500 L stock tank and divided into 5 groups (negative control and 4 treatments) of 10 individuals in each of the three replicate aquaria (60 L, with husbandry conditions as described above). Different amount of HDPE-MP was added to feed for each group (0/control, 0.1, 0.5, 1, and 3%), and shrimp were fed for 28 days.
2.3.3. Gene Expression Assay
The hepatopancreas was used to determine the gene expression in this study, since the microplastic was previously reported to interfere with various gene expression and enzyme activities of hepatopancreas [17,31,41]. Moreover, the hepatopancreas is sensitive to oxidative stress [42,43], one of the known toxic effects of microplastic [31,44,45,46]. Three shrimp from each group were randomly sacrificed for hepatopancreas collection on days 7, 14, 21, and 28. Hepatopancreas was preserved in QIAzol lysis reagent (Qiagen®, Düsseldorf, Germany) and stored at −80 °C, before homogenization with a vortex mixer and RNA extraction with RNeasy Mini kit (Qiagen® Germany) and QIAzol lysis reagent. The reverse transcription was performed with QuantiNova reverse transcription kit (Qiagen® Germany). The real-time polymerase chain reaction was performed with the QuantiNova SYBR Green PCR kit (Qiagen® Germany). The expression of superoxide dismutase (SOD), glutathione peroxidase (GPx), and lysozyme (LYZ) genes were measured in this study. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a reference gene [47]. Specific primers were used for each gene (Table 1). All samples were analyzed in triplicate, using Rotor-Gene Q (Qiagen®) with the following thermocycling profile: 95 °C for 2 min of initial activation, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s and with extension at 72 °C for 30 s. Relative gene expression was calculated by using the 2−ΔΔCt method [48].

Table 1.
Genes and primers for each gene used in RT-qPCR.
2.3.4. Histopathology
Two shrimp from each group were sacrificed on day 28 of microplastic exposure. The shrimp were individually preserved in Davidson’s solution before histology processing. The samples were prepared with a thickness of 5 µm and stained with H&E stain as in Gonçalves et al.’s [50] method. Histopathology lesions were observed through a light microscope (Olympus, CX21F21, 40×). Histopathological scoring was based on the amount of area that histopathological lesion occupied in the medium power field (40x) as in Littik’s [51] method. The scale indication of the criteria of the histopathological score is shown in Table 2.

Table 2.
Criteria of histopathological scoring.
2.3.5. Statistical Analysis
Gene expression and mean histopathology score of each lesion were considered dependent values. Data between groups were evaluated by one-way analysis of variance (ANOVA) with Duncan’s MRT post hoc test. Results were considered significant if p < 0.05, and statistical analysis was performed using SPSS® version 22 (IBM®).
3. Results
3.1. Mortality Rate and HDPE-MP LD50
LD50 at day 28 of HDPE-MP ingestion was determined to be 3.074% HDPE-MP. Shrimp provided with 20% HDPE-MP started dying on day 3, with 100% mortality with 10 and 20% HDPE-MP on days 19 and 13, respectively (Figure 1A). The mortality rate was positively correlated with increases in HDPE microplastic concentration.
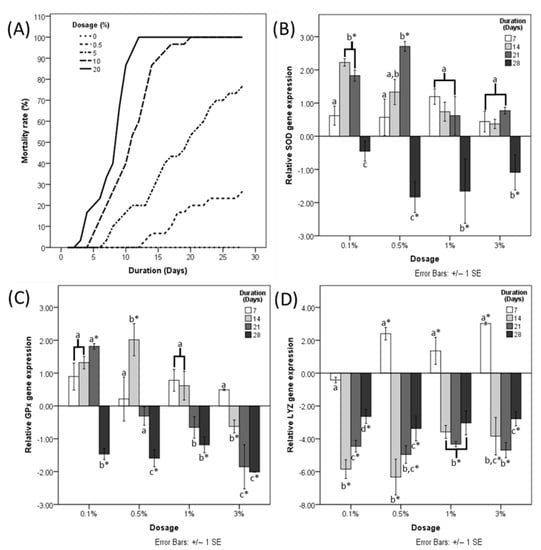
Figure 1.
(A) Mortality rate of shrimp fed with different doses of HDPE microplastic. Fold change of (B) SOD, (C) GPx, and (D) LYZ gene expression normalized to a negative control at four different time points. Different letters (a, b, and c) above bars belonging to the same data series indicate significant differences between sampling times for each treatment. Asterisk indicates a significant difference from the negative control. p < 0.05.
3.2. Nonspecific Immunity Gene Expression
Significant upregulation of the SOD gene was observed in shrimp provided with 0.1% HDPE MP for 14 days and shrimp provided with 0.5% HDPE-MP for 14 and 21 days (Figure 1B). SOD gene expression of 0.5, 1, and 3% HDPE-MP was downregulated significantly at day 28 (Figure 1B). GPx gene expression was upregulated significantly in shrimp provided with 0.1 and 0.5% HDPE-MP for 21 and 14 days, respectively (Figure 1C). GPx gene expression of shrimp provided with 0.1, 0.5, and 1% HDPE-MP for 28 days was downregulated significantly, while shrimp provided with 3% HDPE-MP were downregulated significantly at days 14, 21, and 28 (Figure 1C). LYZ gene was upregulated significantly in shrimp provided with 0.5, 1, and 3% HDPE-MP for 7 days and downregulated significantly in all groups provided with HDPE-MP for at least 14 days (Figure 1D).
3.3. Histopathology Lesions
Histopathological lesions were not observed in the hepatopancreas of the negative control group (Figure 2A). In contrast, the hepatopancreas of treatment groups presented with histopathological lesions, including interstitial hemocyte infiltration (Figure 2B), epithelium hyperplasia (Figure 2B,C), tubular deformity (Figure 2C,D), nodule formation (Figure 2E), and melanization (Figure 2E). Shrimp fed with 3% had a significantly higher histopathological score for interstitial hemocyte infiltration. Scores for shrimp fed with other concentrations of HDPE-MP were significantly different from the negative control group but not significantly different from each other (Figure 3). Shrimp provided with the lowest concentration of HDPE-MP (0.1% HDPE-MP) had the highest histopathological score of tubular hyperplasia. Shrimp provided with higher concentrations were not significantly different from the negative control group. There was no significant difference in the histopathological score of tubular deformity among the groups provided with HDPE-MP at different concentrations. Shrimp provided with at least 0.5% HDPE-MP had a significantly higher histopathological score of nodule formation than the negative control group. Only the shrimp provided with the highest HDPE-MP concentration (3% of HDPE-MP) had a significantly higher histopathological score of melanization than the negative control groups. All histopathological scores were increased corresponding to increased HDPE concentration, except tubular hyperplasia, which had a lesser histopathological score in shrimp provided with higher concentration (Figure 3).
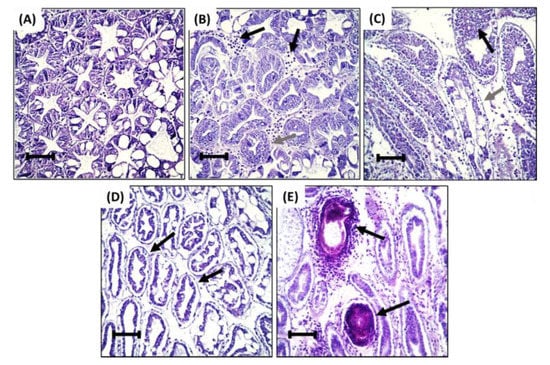
Figure 2.
Representative histopathological findings from: (A) Normal hepatopancreatic tubule of negative control group. (B) Hepatopancreatic tubule of shrimp fed with 0.1% HDPE-MP with hemocyte between interstitial tissue of hepatopancreas (black arrow) and epithelium hyperplasia (gray arrow). (C) Hepatopancreatic tubule of shrimp fed with 0.5% HDPE-MP with epithelium hyperplasia (black arrow), epithelium detachment, and deformation of hepatopancreatic tubule (gray arrow). (D) Hepatopancreatic tubule of shrimp fed with 1% HDPE-MP with pronounced epithelium detachment and deformation of hepatopancreatic tubule (black arrow). (E) Hepatopancreatic tubule of shrimp fed with 3% HDPE-MP with nodule formation and melanization (black arrow). H&E staining, bar = 100 µm.
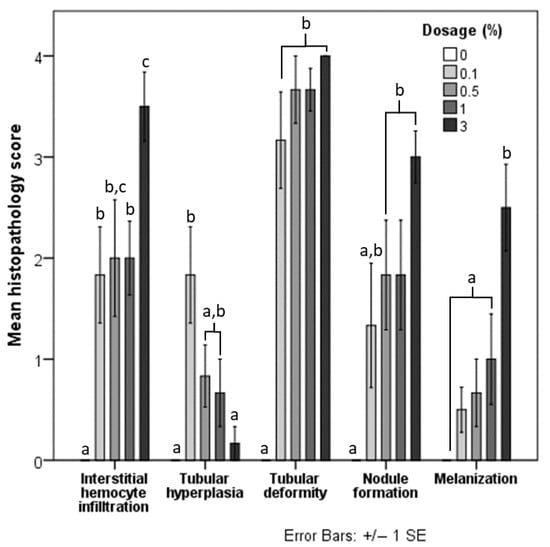
Figure 3.
Mean histopathological score of each lesion in shrimp fed with five different doses of HDPE microplastics. Different letters (a, b, and c) above bars of the same series indicate significant differences in the mean histopathological score between the different doses (p < 0.05).
3.4. Observation Finding Note
During the toxicity test, one of the deceased shrimp that were fed with 20% HDPE had intestine obstruction by a clump of HDPE-MP when observed under a light microscope (Figure 4A). The excreta of the shrimp were also collected and observed under a light microscope, and HDPE-MP was rediscovered in the excreta of shrimp that were fed with HDPE-MP (Figure 4B).
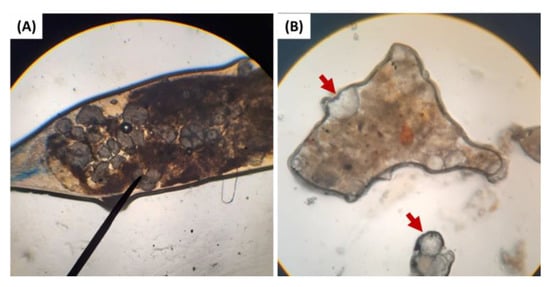
Figure 4.
(A) The obstruction of HDPE-MP in the intestinal tract of shrimp that were fed with 20% HDPE. (B) HDPE-MP in excreta of HDPE-MP-fed shrimp. The red arrow indicates HDPE particles in excreta.
4. Discussion
A direct correlation between mortality rate and concentration of HDPE-MP was demonstrated. However, it is known that many factors can contribute to the toxicity of microplastic (including size, shape, polymerization, and chemical additives), which were previously described to affect ingestion, gut retention, and mortality rate [36,37,52,53,54]. For example, the impact of microplastic-contaminated water on the mortality rate and retention time in the gastrointestinal tract and gills of Daggerblade grass shrimp (Palaemonetes pugio) was influenced by microplastic particle shape and size [55]. Further, microplastics in the marine environment can also absorb toxic contaminants from their surroundings [56,57,58,59,60,61]. Ingestion of such microplastic fomites and their exposure to changes in the digestive tract environment (pH, enzymes) can cause the leaching of attached toxicants in the gut lumen and entering blood circulation [62,63,64,65,66], possibly exercising synergistic effects resulting in increased microplastic toxicity [32]. Thus, the LD50 of the microplastic particulate contaminants in the natural environment could be lower than determined here, indicating that a thorough toxicological risk assessment supported by relevant data is needed to estimate the impact of these novel contaminants.
Expression of the SOD and GPx genes at day 21 followed the gene expression pattern reported in mitten crab studies by Yu et al. [17]. Mitten crab exposure to polystyrene microplastic at low concentration (≤4 mg L−1) for 21 days upregulated SOD and GPx gene expression, while this effect was not noticed when a high dose of microplastic (40 mg L−1) was used. In our study, shrimp that ingested low concentrations of HDPE-MP (1 and 5% HDPE-MP) showed increased SOD and GPx gene expression compared to control, providing evidence of shrimp response to reactive oxygen species (ROS) or other forms of oxidative stress induced by HDPE-MP. This is in accordance with recent studies that demonstrated how the upregulation of antioxidant enzymes (SOD, GPx, catalase (CAT), and glutathione S-transferase (GST)) in animals exposed to microplastic was indicative of responding to the generation of ROS [17,31,67]. ROS increase is cytotoxic and can induce apoptosis in various organs [68], and the capacity of an organism for prevention of ROS-induced damage via antioxidant enzymes has limitations [69,70]. In our study, shrimp fed with a high concentration of HDPE-MP (1 and 3% HDPE-MP) for a longer time could not respond adequately to ROS, as evidenced by suppression of the expression of antioxidant enzymes. Such suppression resulted in excessive ROS damage to the hepatopancreas [71].
The alteration of antioxidant gene expression in this study could be also a consequence of stress response arising from ingestion of HDPE-MP. Stress factors such as abnormal temperature, pH changing, poor water quality, and starvation could induce oxidative stress and change antioxidant gene expression [43,72,73,74]. Dietary microplastic has been shown to induce starvation stress, as microplastic fibers disrupted feed ingestion and nutrient absorption, leading to starvation in the Norwegian lobster (Nephrops norvegicus) [13,75]. In the present study, during the toxicity test, obstructions of HDPE-MP in the intestine of one shrimp fed with 20% HDPE were observed under a light microscope (Figure 4A). This disruption of ingestion and nutrient absorption indicates that starvation stress might play a partial role in the alteration of antioxidant gene expression. It should be noted that in the present experiment, this was a single finding attributed to the nature of microplastic spheres, rarely found to accumulate in the gastrointestinal tracts of exposed animals [34,35,52]. The study by Lin et al. [74] indicated the downregulation of SOD gene expression to the lowest level within 5 days, in contrast with the upregulation (or absence of the downregulation) of the SOD gene expression within 7 days that is presented in this study. Thus, the result of gene expression in this study could be associated with the toxicity of microplastics instead of starvation, which implied the blockage of the intestine. Nevertheless, the HDPE-MP was routinely observed under a light microscope in the excreta of shrimp fed with HDPE-MP (Figure 4B). Fecal HDPE-MPs were not intact, and some were broken into pieces, strongly suggesting that the gastric mill (tooth-like structure in the anterior stomach of shrimp) could break down HDPE spheres into smaller microplastic or even grind them to nano-plastic sizes, increasing the risk of translocation to hepatopancreas. A similar correlation was observed in Antarctic krill (Euphausia superba) by Dawson et al. [76]. The possibility of translocation of microplastic/nano-plastic into hepatopancreas, together with starvation stress occurrence, suggested that alteration of SOD and GPx gene expression was related to HDPE-MP exposure.
The LYZ gene expression in this study was significantly upregulated after ingesting at least 0.5% HDPE-MP for 7 days. The previous studies showed that short-term microplastic exposure could upregulate LYZ gene expression and enzyme activity [29,30]. Interestingly, our study also showed that ingestion of at least 0.1% HDPE-MP for 14 days could significantly downregulate LYZ gene expression. Since lysozyme is the vital defense mechanism against harmful bacteria such as Vibrio spp. [26,77,78], downregulation of LYZ gene expression can increase shrimp vulnerability to this pathogen that causes early mortality syndrome [79]. Current literature presents evidence of both reduction and increase in Lysozyme activity and gene expression when an organism is exposed to plastic micro/nano-particles [30,80,81,82], suggesting that variability or contradiction in reported responses can be attributed to differences in study methods including animal species, type/size of plastic particles, and other confounding factors in non-standardized studies. Therefore, the exact mechanisms and causes of the upregulation or downregulation of LYZ gene expression in shrimp exposed to HDPE-MP currently remain unknown.
The study of the acute effect of microplastics on hepatopancreas histology by Wang et al. [30] showed that fluorescent red polyethylene microspheres (FRPE) could induce deformation in hepatopancreatic tubular. The present study focused on the chronic (>7 days) effects of microplastics in the hepatopancreas, and similar changes to tubular deformity and other histopathology lesions were observed with increased severity. The intensity of histopathology lesions observed in this study behaved in a dose-dependent manner: tubular deformity, interstitial hemocyte infiltration, nodule formation, and melanization increased at higher concentrations of ingestible HDPE-MP. Epithelial hyperplasia in shrimp that consumed a high dose of HDPE-MP advanced into later stages of this lesion (such as nodule formation and melanization), causing a relative reduction in early-stage hyperplastic changes.
Increased interstitial hemocyte infiltration, nodule formation, and melanization that presented in the hepatopancreas of shrimp fed with HDPE-MP in this study might be evidence of chronic inflammation [83,84] and translocation of HDPE-MP from the gastrointestinal tract into hepatopancreas. Although we did not focus on the translocation and accumulation of ingested HDPE-MP, previous studies in fiddler crab (Uca rapax) by Brennecke et al. [15,30] demonstrated that microplastics with a diameter between 180 and 250 µm (larger than HDPE-MP Ø 50 µm used in our study) were observed in hepatopancreas. This information, along with evidence of breaking down HDPE-MP (Figure 4B), supports the possibility of translocation of microplastics into hepatopancreas that occurred in our study. Increased hepatopancreas nodule formation in shrimp exposed to HDPE-MP, together with other pathological responses indicative of chronic inflammation, suggests the possibility of reactive inflammation against HDPE-MP in hepatopancreas tissue, with subsequent severe tissue damage after prolonged exposure [22,85]. Such sustained damage leads to cellular dysfunction and can result in a decrease in nonspecific immunity gene expression observed in this and other studies [86]. Microplastic damage to the hepatopancreas could be the leading cause of mortality in this study, considering its essential role in digestion and metabolism regulation in crustaceans [87]. As mechanisms of microplastic recognition by the immune system in shrimp are not fully understood, further studies are necessary to support this hypothesis [88,89].
Microplastic effects on the antioxidant and lysozyme of L. vannamei with the different routes of administration and duration of exposure were reported. Wang et al. [30] immersed shrimps in seawater contaminated with polystyrene microspheres (Ø 5 µm) for 48 h and observed upregulation of lysozyme gene expression which correlated with the result of this study. Effects of polyethylene microplastics on oxidative stress were reported by Hsieh et al. [31] Shrimp were directly injected with fluorescent red polyethylene microspheres or FRPE (Ø 10–22 µm) intramuscularly. The results show significant upregulation of SOD and GPx gene expression at 7 days post initial injection, except in shrimp injected with the highest dosage of FRPE that showed significant downregulation of GPx gene expression. The results of SOD and GPx gene expression in our study are not significantly different from the negative control on day 7 of the experiment. However, the result of Hsieh et al. [31] indicated a pattern of SOD and GPx gene expression similar to this study, as well as the study in mitten crab by Yu et al. [17]. In all of the above reports, lower concentrations of microplastic were shown to upregulate the antioxidant genes, while the highest concentration of microplastic could downregulate the antioxidant genes, indicating a bi-phasic response possibly caused by depletion of the antioxidant capacity of animals exposed to a high concentration of microplastic.
Considering the MP toxicity presented in this study, estimates of average microplastic contamination in shrimp feed are 39 mg kg−1 of feed. Calculations were based on the estimated weight of microplastic particles of 0.02 mg and using 9% of fish meal [39,90] and the assumption that feed ingredients (fish meal and soy meal) were contaminated with microplastic in the worst-case scenario [91,92]. This estimate was lower than the concentration of HDPE-MP in this study; therefore, the result of this study was not representative of the actual level of microplastic that can be found in aquafeed today. However, as a significant increase in microplastic contamination in the environment is predicted [3], microplastic contamination level in the aquafeed will likely reflect this increase with a negative impact on Pacific white shrimp culture.
5. Conclusions
The presented results strongly suggest that ingestion of HDPE-MP with a diameter of 50 µm is related to significant mortality of Pacific white shrimp, with the determined LD50 at day 28 of 3.074% HDPI-MP. Shrimp receiving a sublethal dose of HDPE-MP for 28 days showed significantly altered expression of antioxidant enzyme genes and lysozyme, considered critical components of shrimp disease resistance and innate immunity. Pathology of hepatopancreas positively correlated with the dose and duration of HDPE-MP exposure. In conclusion, long-term ingestion of microplastics even in amounts below LD50 can cause increases in shrimp mortality. Dietary microplastics interfere with lysozyme gene expression and resilience to oxidative stress, contributing to increased pathogen susceptibility in exposed animals. The increased presence of microplastic pollution in the environment and food has the potential to affect marine shrimp, including increased risks of fishery and aquaculture production losses in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12233308/s1. Table S1: HDPE content in feed used in the toxicity test, Table S2: HDPE content in feed used to determine microplastic effects on gene expression and histopathology, Table S3: Nutrient composition of base shrimp feed, Table S4: Details of temperature recirculation settings for qPCR, Table S5: Number of shrimp with different histopathological scores for each lesion, Table S6: The mortality rate of shrimp during the second phase of the experiment on day 28 (the sacrificed shrimp before day 28 were not included).
Author Contributions
Conceptualization, S.N., T.H. and N.C.; Methodology, S.N.; Validation, S.N. and T.H.; Formal analysis, S.N.; Investigation, S.N. and T.H.; Resources, S.N.; Data curation, S.N.; Writing—original draft preparation, S.N.; Writing—review and editing, D.P.; Visualization, S.N. and D.P.; Supervision, D.P. and N.C.; Project administration, S.N.; Funding acquisition, S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Ratchadapisek Sompoch Endowment Fund (2020) under the Microplastics Cluster of Chulalongkorn University with grant number 763016. This research and innovation activity is funded by National Research Council of Thailand (NRCT) with grant number N41B650173. This research is funded by CU. Graduate School Thesis Grant with grant number GCUGR1225642032M.
Institutional Review Board Statement
The study was conducted according to the guidelines documented in Ethical Principles and Guidelines for the Use of Animals for Scientific Purposes edited by the National Research Council of Thailand and approved by the Institutional Animal Care and Use Committee (IACUC) of Chulalongkorn University (protocol code 2031077 and date of approval 25 August 2021).
Informed Consent Statement
Not applicable. This study did not involve human beings.
Data Availability Statement
Not applicable.
Acknowledgments
This would not be a success without the support of the Veterinary Medical Aquatic Animals Research Center of Excellence (VMARCE) for providing the facility and supporting with hospitality throughout the study. We would also like to thank iScience Technology Co., Ltd., for their great technical support and Chok-Chai shrimp farm for providing high-quality shrimp specimens for this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Verma, R.; Vinoda, K.; Papireddy, M.; Gowda, A. Toxic pollutants from plastic waste-a review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Egger, M.; Slat, B. A global mass budget for positively buoyant macroplastic debris in the ocean. Sci. Rep. 2019, 9, 12922. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.M.; Lavers, J.L.; Figueiredo, B. Plastic ingestion by fish in the Southern Hemisphere: A baseline study and review of methods. Mar. Pollut. Bull. 2016, 107, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in north sea fish. Environ. Pollut. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Naidoo, T.; Smit, A.; Glassom, D. Plastic ingestion by estuarine mullet Mugil cephalus (Mugilidae) in an urban harbour, KwaZulu-Natal, South Africa. Afr. J. Mar. Sci. 2016, 38, 145–149. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Buenaventura, N. Microplastic Pollution in an Urban Norwegian River Sediment—An Investigation of Freshwater Sediment Extraction by Elutriation. Master Thesis, Norwegian Universty of Life Sciences, Oslo, Norway.
- Prawitwilaikul, O.; Limsuwan, C.; Taparhudee, W.; Chuchird, N. A comparison of rearing Pacific white shrimp (Liptopenaeus vannamei Boone, 1931) in earthen ponds and in ponds lined with polyethylene. Agric. Nat. Resour. 2006, 40, 167–171. [Google Scholar]
- Wyban, J.A.; Pruder, G.D.; Leber, K.M.; Burzell, L. Paddlewheel effects on shrimp growth, production and crop value in commercial earthen ponds. J. World Aquac. Soc. 1989, 20, 18–23. [Google Scholar] [CrossRef]
- Ruttanagosrigit, W.; Musig, Y.; Boyd, C.E.; Sukchareon, L. Effect of salinity on oxygen transfer by propeller-aspirator-pump and paddle wheel aerators used in shrimp farming. Aquac. Eng. 1991, 10, 121–131. [Google Scholar] [CrossRef]
- Welden, N.A.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef]
- Watts, A.J.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef]
- Brennecke, D.; Ferreira, E.C.; Costa, T.M.; Appel, D.; da Gama, B.A.; Lenz, M. Ingested microplastics (>100 μm) are translocated to organs of the tropical fiddler crab Uca rapax. Mar. Pollut. Bull. 2015, 96, 491–495. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- FAO. FAO Yearbook. Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2019. [Google Scholar]
- Global shrimp production sees significant growth in 2021. Available online: https://thefishsite.com/articles/global-shrimp-production-sees-significant-growth-in-2021-gorjan-nikolik-rabobank (accessed on 20 March 2022).
- Hanachi, P.; Karbalaei, S.; Walker, T.R.; Cole, M.; Hosseini, S.V. Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ. Sci. Pollut. Res. 2019, 26, 23777–23787. [Google Scholar] [CrossRef]
- Tantikitti, C.; Chookird, D.; Phongdara, A. Effects of fishmeal quality on growth performance, protein digestibility and trypsin gene expression in pacific white shrimp (Litopenaeus vannamei). Warasan Songkla Nakharin 2016, 38, 73–82. [Google Scholar]
- Aguirre-Guzman, G.; Sanchez-Martinez, J.G.; Campa-Cordova, A.I.; Luna-Gonzalez, A.; Ascencio, F. Penaeid shrimp immune system. Thai J. Vet. Med. 2009, 39, 205–215. [Google Scholar]
- Ji, P.-F.; Yao, C.-L.; Wang, Z.-Y. Reactive oxygen system plays an important role in shrimp Litopenaeus vannamei defense against Vibrio parahaemolyticus and WSSV infection. Dis. Aquat. Org. 2011, 96, 9–20. [Google Scholar] [CrossRef]
- Somboonwiwat, K.; Supungul, P.; Rimphanitchayakit, V.; Aoki, T.; Hirono, I.; Tassanakajon, A. Differentially expressed genes in hemocytes of Vibrio harveyi-challenged shrimp Penaeus monodon. BMB Rep. 2006, 39, 26–36. [Google Scholar] [CrossRef]
- De Lorgeril, J.; Gueguen, Y.; Goarant, C.; Goyard, E.; Mugnier, C.; Fievet, J.; Piquemal, D.; Bachère, E. A relationship between antimicrobial peptide gene expression and capacity of a selected shrimp line to survive a Vibrio infection. Mol. Immunol. 2008, 45, 3438–3445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burge, E.J.; Madigan, D.J.; Burnett, L.E.; Burnett, K.G. Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish Shellfish Immunol. 2007, 22, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, K.; Ramasamy, P. White spot syndrome virus infection decreases the activity of antioxidant enzymes in Fenneropenaeus indicus. Virus Res. 2006, 115, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Dai, T.; Zhong, S.; Jin, M.; Sun, P.; Zhou, Q. Vibrio parahaemolyticus infection influenced trace element homeostasis, impaired antioxidant function, and induced inflammation response in Litopenaeus vannamei. Biol. Trace Elem. Res. 2021, 199, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir sinensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, L.; Wang, J.; Zhou, J.; Ye, Q.; Zhang, L.; Xu, G.; Zou, J. Impacts of microplastics on three different juvenile shrimps: Investigating the organism response distinction. Environ. Res. 2021, 198, 110466. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Wu, Y.-C.; Xu, R.-Q.; Chen, Y.-T.; Chen, C.-W.; Singhania, R.R.; Dong, C.-D. Effect of polyethylene microplastics on oxidative stress and histopathology damages in Litopenaeus vannamei. Environ. Pollut. 2021, 288, 117800. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef]
- Deanin, R.D. Additives in plastics. Environ. Health Perspect. 1975, 11, 35–39. [Google Scholar] [CrossRef]
- Au, S.Y.; Bruce, T.F.; Bridges, W.C.; Klaine, S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef]
- Choi, D.; Hwang, J.; Bang, J.; Han, S.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. In vitro toxicity from a physical perspective of polyethylene microplastics based on statistical curvature change analysis. Sci. Total Environ. 2021, 752, 142242. [Google Scholar] [CrossRef]
- Jeong, C.-B.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Hwang, D.-S.; Hwang, U.-K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.-S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Lei, L.; Liu, M.; Song, Y.; Lu, S.; Hu, J.; Cao, C.; Xie, B.; Shi, H.; He, D. Polystyrene (nano) microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci. Nano 2018, 5, 2009–2020. [Google Scholar] [CrossRef]
- Tacon, A.G.; Jory, D.; Nunes, A. Shrimp Feed Management: Issues and Perspectives; FAO: Rome, Italy, 2013. [Google Scholar]
- Jovanović, B.; Gökdağ, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef]
- Peda, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Hua, X.; Dang, Y.; Han, Y.; Yu, Z.; Chen, X.; Ding, P.; Li, H. Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 726, 138679. [Google Scholar] [CrossRef]
- Han, S.-Y.; Wang, M.-Q.; Liu, M.; Wang, B.-J.; Jiang, K.-Y.; Wang, L. Comparative sensitivity of the hepatopancreas and midgut in the white shrimp Litopenaeus vannamei to oxidative stress under cyclic serious/medium hypoxia. Aquaculture 2018, 490, 44–52. [Google Scholar] [CrossRef]
- Han, S.-Y.; Wang, M.-Q.; Wang, B.-J.; Liu, M.; Jiang, K.-Y.; Wang, L. A comparative study on oxidative stress response in the hepatopancreas and midgut of the white shrimp Litopenaeus vannamei under gradual changes to low or high pH environment. Fish Shellfish Immunol. 2018, 76, 27–34. [Google Scholar] [CrossRef]
- Espinosa, C.; Beltrán, J.M.G.; Esteban, M.A.; Cuesta, A. In vitro effects of virgin microplastics on fish head-kidney leucocyte activities. Environ. Pollut. 2018, 235, 30–38. [Google Scholar] [CrossRef]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Bowers, R.M.; Licon, K.S.; Veazey, G.; Read, B. Validation of reference genes for quantitative measurement of immune gene expression in shrimp. Mol. Immunol. 2009, 46, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.-C.; Tseng, C.-W.; Lin, H.-Y.; Chen, I.-T.; Chen, Y.-H.; Chen, Y.-M.; Chen, T.-Y.; Yang, H.-L. RNAi knock-down of the Litopenaeus vannamei Toll gene (LvToll) significantly increases mortality and reduces bacterial clearance after challenge with Vibrio harveyi. Dev. Comp. Immunol. 2010, 34, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Martins, M.; Costa, M.H.; Costa, P.M. Development of a method for the detection of polystyrene microplastics in paraffin-embedded histological sections. Histochem. Cell Biol. 2018, 149, 187–191. [Google Scholar] [CrossRef]
- Littik, S.A.M. Quantitative histopathology and epidemiology of prawn viral diseases. Doctoral dissertation, James Cook University, Douglas, Australia, 2003.
- Frydkjær, C.K.; Iversen, N.; Roslev, P. Ingestion and egestion of microplastics by the cladoceran Daphnia magna: Effects of regular and irregular shaped plastic and sorbed phenanthrene. Bull. Environ. Contam. Toxicol. 2017, 99, 655–661. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.; Koelmans, A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef]
- Zarfl, C.; Matthies, M. Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar. Pollut. Bull. 2010, 60, 1810–1814. [Google Scholar] [CrossRef]
- Gray, A.D.; Weinstein, J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—An emerging contaminant of potential concern? Integr. Environ. Assess. Manag. Int. J. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Endo, S.; Yuyama, M.; Takada, H. Desorption kinetics of hydrophobic organic contaminants from marine plastic pellets. Mar. Pollut. Bull. 2013, 74, 125–131. [Google Scholar] [CrossRef]
- Llorca, M.; Schirinzi, G.; Martínez, M.; Barceló, D.; Farré, M. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ. Pollut. 2018, 235, 680–691. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar. Coast. Shelf Sci. 2014, 140, 14–21. [Google Scholar] [CrossRef]
- Besseling, E.; Wegner, A.; Foekema, E.M.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Tickner, J.; Rossi, M.; Haiama, N.; Lappe, M.; Hunt, P. The use of di-2-ethylhexyl phthalate in PVC medical devices: Exposure, toxicity, and alternatives. In Lowell Center for Sustainable Production; University of Massachusetts: Lowell, MA, USA, 1999; p. 72. [Google Scholar]
- Kang, H.-M.; Byeon, E.; Jeong, H.; Kim, M.-S.; Chen, Q.; Lee, J.-S. Different effects of nano-and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. J. Hazard. Mater. 2021, 405, 124207. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Parrilla-Taylor, D.P.; Zenteno-Savín, T.; Magallón-Barajas, F.J. Antioxidant enzyme activity in pacific whiteleg shrimp (Litopenaeus vannamei) in response to infection with white spot syndrome virus. Aquaculture 2013, 380, 41–46. [Google Scholar] [CrossRef]
- Morel, Y.; Barouki, R. Repression of gene expression by oxidative stress. Biochem. J. 1999, 342, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-N.; Zhou, J.; Wang, P.; Tian, T.-T.; Zheng, Y.; Liu, Y.; Mai, W.-J.; Wang, A.-L. Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp. Biochem. Phys. C 2009, 150, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Xin, Y.; Wang, W.-N.; He, W.-Y.; Wang, A.-L.; Liu, Y. Effect of temperature on antioxidant enzyme gene expression and stress protein response in white shrimp, Litopenaeus vannamei. J. Therm. Biol. 2010, 35, 284–289. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, J.-C.; Man, S.N.C.; Morni, W.Z.W.; Suhaili, A.S.N.; Cheng, S.-Y.; Hsu, C.-H. Modulation of innate immunity and gene expressions in white shrimp Litopenaeus vannamei following long-term starvation and re-feeding. Results Immunol. 2012, 2, 148–156. [Google Scholar] [CrossRef]
- Welden, N.A.; Cowie, P.R. Environment and gut morphology influence microplastic retention in langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 214, 859–865. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- De-La-Re-Vega, E.; García-Galaz, A.; Díaz-Cinco, M.E.; Sotelo-Mundo, R.R. White shrimp (Litopenaeus vannamei) recombinant lysozyme has antibacterial activity against Gram negative bacteria: Vibrio alginolyticus, Vibrio parahemolyticus and Vibrio cholerae. Fish Shellfish Immunol. 2006, 20, 405–408. [Google Scholar] [CrossRef]
- Ji, P.-F.; Yao, C.-L.; Wang, Z.-Y. Immune response and gene expression in shrimp (Litopenaeus vannamei) hemocytes and hepatopancreas against some pathogen-associated molecular patterns. Fish Shellfish Immunol. 2009, 27, 563–570. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, J.-C.; Chen, Y.-Y.; Yeh, S.-T.; Chen, L.-L.; Huang, C.-L.; Hsieh, J.-F.; Li, C.-C. Crowding of white shrimp Litopenaeus vananmei depresses their immunity to and resistance against Vibrio alginolyticus and white spot syndrome virus. Fish Shellfish Immunol. 2015, 45, 104–111. [Google Scholar] [CrossRef]
- Muhammad, A.; Zhou, X.; He, J.; Zhang, N.; Shen, X.; Sun, C.; Yan, B.; Shao, Y. Toxic effects of acute exposure to polystyrene microplastics and nanoplastics on the model insect, silkworm Bombyx mori. Environ. Pollut. 2021, 285, 117255. [Google Scholar] [CrossRef]
- Yang, H.; Lai, H.; Huang, J.; Sun, L.; Mennigen, J.A.; Wang, Q.; Liu, Y.; Jin, Y.; Tu, W. Polystyrene microplastics decrease F–53B bioaccumulation but induce inflammatory stress in larval zebrafish. Chemosphere 2020, 255, 127040. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Liu, Y.-N.; Liu, J.-H.; Feng, X.-S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Liu, C.-H.; Cheng, W.; Hsu, J.-P.; Chen, J.-C. Vibrio alginolyticus infection in the white shrimp Litopenaeus vannamei confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis. Aquat. Org. 2004, 61, 169–174. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano, R.; del Rio-Rodríguez, R.; Diéguez, A.L.; Romalde, J.L. Virulence of Vibrio harveyi responsible for the “Bright-red” Syndrome in the Pacific white shrimp Litopenaeus vannamei. J. Invertebr. Pathol. 2012, 109, 307–317. [Google Scholar] [CrossRef]
- Battistella, S.; Bonivento, P.; Amirante, G.A. Hemocytes and immunological reactions in crustaceans. Ital. J. Zool. 1996, 63, 337–343. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Gorgani-Firuzjaee, S.; Panahi, G.; Meshkani, R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl. Res. 2016, 167, 228–256. [Google Scholar] [CrossRef]
- Vogt, G. Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol. 2019, 280, 1405–1444. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, L.; Wang, J.; Xie, S.; Zhang, C.; Zhou, J.; Zhang, L.; Xu, G.; Zou, J. Insight into the immune and microbial response of the white-leg shrimp Litopenaeus vannamei to microplastics. Mar. Environ. Res. 2021, 169, 105377. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, A.H. Microplastics in decapod crustaceans: Accumulation, toxicity and impacts, a review. Sci. Total Environ. 2022, 832, 154963. [Google Scholar] [CrossRef] [PubMed]
- Amaya, E.; Davis, D.A.; Rouse, D.B. Alternative diets for the Pacific white shrimp Litopenaeus vannamei. Aquaculture 2007, 262, 419–425. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Tolhurst, T.J.; Lindeque, P.K.; Thompson, R.; Cole, M. Detection and characterisation of microplastics and microfibres in fishmeal and soybean meal. Mar. Pollut. Bull. 2022, 185, 114189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Zhu, X.; Sun, C.; Teng, J.; Chen, L.; Shan, E.; Zhao, J. Microplastics in fish meals: An exposure route for aquaculture animals. Sci. Total Environ. 2022, 807, 151049. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).