Simple Summary
Dietary fiber has been long been established as a nutritionally important, health-promoting food ingredient. Our aim was to explore differences in physiological and metabolic adaptations to DF in two pig breeds, and the mechanisms behind DF-regulated intestinal health have also been investigated. Our results showed a difference in dietary fiber utilization by the two pig breeds (Taoyuan and Duroc), which might be connected with the integrity of the intestinal epithelium and the microbial activity of the gastrointestinal tract, and the results may also suggest a beneficial role of dietary fiber in regulating intestinal health.
Abstract
To explore the effect of dietary fiber on growth performance and intestinal health in different pig breeds, forty Taoyuan and Duroc pigs (pure breeds) of 60 days of age were randomly divided into a 2 (diet) × 2 (breed) factorial experiment (n = 10), and fed with a basal diet (BD) or high-fiber diet (HFD). The trial lasted for 28 d, and results showed that the Taoyuan pigs had a higher average daily feed intake (ADFI) than the Duroc pigs (p < 0.05). The average daily gain (ADG) and digestibilities of gross energy (GE) and crude protein (CP) were higher in Taoyuan pigs than in the Duroc pigs under HFD feeding (p < 0.05). The HFD increased the superoxide dismutase (SOD) and catalase (CAT) activity in Taoyuan pigs (p < 0.05). Interestingly, Taoyuan pigs had a higher jejunal villus height and ratio of villus height to crypt depth (V/C) than the Duroc pigs. The HFD significantly improved the villus height and V/C ratio in duodenum and jejunum (p < 0.05). The HFD also increased the jejunal maltase and ileal sucrase activities in Duroc and Taoyuan pigs, respectively (p < 0.05). Taoyuan pigs had a higher expression level of duodenal fatty acid transport protein-1 (FATP-1) than the Duroc pigs (p < 0.05). Furthermore, the HFD acutely improved the expression levels of ileal SGLT-1 and GLUT-2, and the expression levels of jejunal occludin and claudin-1 in Taoyuan pigs (p < 0.05). Importantly, Taoyuan pigs had a higher colonic Bifidobacterium abundance than the Duroc pigs (p < 0.05). The HFD not only elevated the colonic Lactobacillus abundance and butyrate acid content in Taoyuan pigs, but also increased the acetic and propionic acid contents in both the pig breeds (p < 0.05). These results indicated a difference in dietary fiber (DF) utilization by the two pig breeds, and results may also suggest a beneficial character of DF in regulating intestinal health.
1. Introduction
Dietary fiber (DF), usually defined as the indigestible portion of plant-derived foods [1], has been long been established as a nutritionally important, health-promoting food ingredient [1,2]. However, a dominant concern for mono-gastric animals (e.g., pigs) is that a high-fiber diet (HFD) is associated with reduced nutrient utilization and low net energy values, as the DF cannot be digested by endogenous digestive enzymes [3,4]. Currently, a number of studies have indicated a positive role of DF in maintaining regular physiological functions of the digestive tract, and a significant reduction in DF consumption is always linked to an increased prevalence of gut diseases such as inflammatory bowel disease [5,6]. It is a well-known fact that the influence of DFs on animals depends on their levels, types, and physicochemical properties [7].
DF components such as cellulose, hemicellulose, and pectin are the main components of plant cell walls and cannot be broken down by mammalian enzymes, but can be fermented through a wide variety of microorganisms in the hindgut [8]. In contrast, lignin, a high molecular weight polymer, cannot be broken down in the digestive tract [9,10]. Previous studies indicated that various microorganisms in the hindgut can ferment DF to produce a number of short-chain fatty acids (SCFAs), which not only promote the growth of beneficial microorganisms, but also improve the intestinal integrity and functions [11,12]. A diet rich in DF has also been found to reduce the risk of many dietary problems such as cardiovascular disease, type 2 diabetes, and Crohn’s disease in humans [13,14,15]. Moreover, a recent study indicated that DF supplementation has no effect on the growth of growing-finishing pigs, but can obviously improve their meat quality [16].
China is the world’s largest consumer of pork, not only accounting for more than half of the global pork consumption each year, but also having a variety of pig breeds [17]. The meat produced by Chinese local pigs has been characterized by tender flesh, high intramuscular fat (IMF), homogeneous marbling, and better flavor juiciness [18,19,20]. Importantly, the Chinese local pigs can digest roughage more efficiently than other commercial pig breeds [21]. Taoyuan pigs are one of the famous local breeds in China [22]. The breed possesses all the merits of Chinese local pigs, and has been looked upon as the representative breed of the local pigs [23,24]. Duroc pigs are one of the most utilized commercial breeds because of their fast growth and high feed utilization [25,26]. This study investigated the effect of DF on the growth performance, nutrient digestibility, and intestinal health in the two pig breeds. Our aim was to explore their differences in physiological and metabolic adaptations to DF, and the mechanisms behind DF-regulated intestinal health have also been investigated.
2. Materials and Methods
The animal experiment in this study was carried out after approval by the Animal Care and Use Committee of Sichuan Agricultural University (Chengdu, China). The wheat bran fiber (WBF) used in this study was bought from Chengdu Tubaite Technology Co. Ltd. The total dietary fiber content in the raw material is more than 95%, and the IDF content of the wheat bran fiber raw material is 98%.
2.1. Experimental Design, Diet, and Animal Housing
Forty Taoyuan (average weight 13.87 ± 0.58 kg) and Duroc (average weight 18.50 ± 1.09 kg) pigs (pure breeds) of 60 days of age were randomly divided into a 2 (diet) × 2 (breed) factorial experiment (n = 10), exposed to a normal basal diet (BD, 3.14% dietary fiber) or high-fiber diet (HFD, 6.86% dietary fiber). The trial lasted for 28 days. The diets (Table 1) were formulated based on National Research Council 2012 (NRC, 2012) [27]. The WBF was utilized to modify the DF level in the diets. Pigs were raised individually in metabolic cages (0.7 m × 1.5 m) under an appropriate temperature (24–29 °C) and humidity (55–65%) with ad libitum access to water and food.

Table 1.
Ingredients and nutrient composition of the basal and high-fiber diet *.
2.2. Sample Collection
The fecal samples were collected on days 26–28 of the trial. Immediately after defecation, fresh feces of each pen were collected into their own valve bags, and 10 mL of 10% H2SO4 solution was evenly added to 0.1 kg of feces to fix fecal nitrogen. On the morning of day 29, blood samples were collected through vein puncture and inject into 20 mL plain tubes. Then, these blood samples were centrifuged at 3500× g at 4 °C (15 min). After subsequent centrifugation, the serums were stored at −20 °C until the serum index analysis. After the blood sampling, pigs were slaughtered by electrical stunning to collect the remaining samples. The segments of the small intestine (approximately 4 cm each) were immediately separated, slowly rinsed with cold phosphate buffer, and fixed in paraformaldehyde solution for further intestinal morphological analysis. Moreover, the mucosa samples were preserved at −80 °C for convenience of examination, which were collected by scraping fragments from the small intestine by a scalpel blade.
2.3. Growth Performance Evaluation
The initial body weight, final body weight, and the feed intake of each pig were measured. The feed efficiency (F:G) of every pig was computed based on the average daily gain (ADG) and average daily feed intake (ADFI).
2.4. Apparent Total Tract Nutrient Digestibility Analysis
Diet and fecal samples were thawed, homogenized, and freeze-dried for the nutrient digestibility analysis, and the Cr2O3 served as an external indicator. The DM, CP, EE, CF, and ash contents were measured by an AOAC standard [28]. An adiabatic bomb calorimeter was utilized to measure GE. The apparent digestibility of nutrients was computed by the equation below:
2.5. Serum Parameter Analysis
The D-lactate (Porcine D-lactate ELISA Kit MM33732O1) and diamine oxidase (Porcine DAO ELISA Kit MM-0438O1) were measured by using enzyme-linked immunosorbent assay kits (Jiangsu Enzyme-linked Biotechnology Co., Ltd. Nanjing, China). The commercial kits purchased from Nanjing Jiancheng Biotechnology Co., Ltd (Nanjing, China) were utilized for measurement of serum parameters such as the catalase (CAT), malondialdehyde (MDA), glutathione (GSH), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), urea nitrogen (BUN), glucose (GLU), triglyceride (TG), and total cholesterol (TC). All procedures were carried out in strict accordance with the instructions. The specific information of these kits is as follows:
CAT (Cat. No. A007-1-1), MDA (Cat. No. A003-1-2), GSH (Cat. No. A005-1-2), T-SOD (Cat. No. A001-1-2), T-AOC (Cat. No. A015-1-2), BUN (Cat. No. C013-2-1), GLU (Cat. No. F006-1-1), TG (Cat. No. A110-1-1), TC (Cat. No. A111-1-1).
2.6. Intestinal Morphological Analysis
Intestinal segments fixed with 4% paraformaldehyde were dewaxed by graded anhydrous ethanol. A cross-section of every sample was prepared and dyed with hematoxylin and eosin (H&E), followed by being sealed by a neutral resin size. ImageJ software was used to measure the crypt depth and villus height of the intestine, and the ratio of villus height to crypt depth (V/C) was calculated. Ten crypt depths and villus heights were calculated and the average value was calculated [29].
2.7. Enzyme Activity
The intestinal mucosa was homogenized with cold saline, and then the supernatants were isolated (centrifugation at 3500× g for 15 min) and utilized to determine the enzyme activities of lactase, sucrase, and maltase. The measurements were carried out by using specific assay kits: sucrase (A082-2-1), lactase (A082-1-1), and maltase (A082-3-1), purchased from Nanjing Jiancheng Biotechnology Co., Ltd. (Nanjing, China).
2.8. Colonic Microbiological Analysis
An estimated 200 mg colon digesta was processed to obtain total DNA with the Omega Bio-Tek Stool DNA Kits for quantification real-time PCR, which was performed through the Quant Studio 6 Flex real-time PCR system (Bio-Rad). Total bacteria number was detected by the reaction which runs in a total volume of 25 μL, including SYBR Premix Ex Taq (2 × concentrated), forward and reverse primers (100 nM), DNA, RNase-free ddH2O, and 50 × ROX Reference Dye*3. All procedures of microbial real-time quantitative PCR were based on the methods reported by Wu et al. (2018) [7]. The SuperReal PreMix (Probe) kits obtained from Tiangen Biotech Co., Ltd. (Beijing, China) were utilized to determine E. coli, Bifidobacterium, Lactobacillus, and Bacillus. Each reaction was run in a total volume of 25 μL, including DNA, forward and reverse primers (100 nmol/L), 2 × Super Real PreMix (Probe), probe (100 nmol/L), RNase-free ddH2O, and 50 × ROX Reference Dye*3. Standard curves were generated by 10-fold serial dilutions (1 × 101 to 1 × 109 copies/μL), and the target group copy number of each reaction was calculated from the standard curves.
2.9. Metabolite Concentrations of Colonic Contents
The concentrations of SCFAs (propionic, butyric, and acetic acid) were determined by a gas chromatograph (VARIAN CP-3800, Walnut Creek, CA, USA) with capillary column (30 m × 0.32 mm × 0.25 μm) [29]. The supernatant was mixed with a certain volume of 210 mmol/L crotonic acid and metaphosphoric acid in a new tube after centrifuging (12,000× g for 10 min), then those mixtures were centrifuged for 30 min with incubation with identical conditions again at 4 °C. The gas chromatograph was used to analyze 1 μL of the supernatant. The polyethylene glycol column used high-purity N2 as carrier gas at a flow rate of 1.8 mL/min.
2.10. RNA Isolation, Reverse Transcription and Real-Time Quantitative PCR
Intestinal mucosa (about 100 mg) was homogenized in 1 mL TRIzol reagent, and the total RNA was isolated based on the instructions. Before the RNA samples were reverse transcribed into cDNA with a PrimeScript™ RT reagent kit (Dalian, China), the concentration and fineness of total RNA were analyzed with a spectrophotometer. The qPCR was executed with the SYBR® Green PCR I PCR reagents using the aforesaid uniform PCR Linux, the oligonucleotide primers sequences are shown in Table S1. The reaction mixture (10 μL) includes the SYBR Premix Ex Taq II, forward and reverse primers, cDNA, and RNase-free ddH2O. Cycling in qPCR was as follows: first 95 °C (30 s), then repeat 95 °C (5 s) and 60 °C (30 s) 40 times. β-actin was used as a housekeeping gene to calibrate the mRNA relative expression level of target genes, according to the 2−ΔΔCt method. All the reagents were obtained from Takara Biotechnology Co., Ltd. (Dalian, China).
2.11. Statistical Analysis
The data were analyzed by two-way ANOVA with the general linear model (GLM) procedure of SPSS as a 2 (diet) × 2 (breed) factorial design. Differences among the treatments were estimated using a Student–Newman–Keuls multiple comparisons test, and values were indicated as means with their standard errors. The differences were considered significant at p-values < 0.05.
3. Results
3.1. Influence of DF on Growth Performance and Nutrient Digestibility
The Taoyuan pigs had a higher ADFI than the Duroc pigs (Table 2, p < 0.05). The ADG was higher in the Taoyuan pigs than in the Duroc pigs under HFD feeding (p < 0.05). The F:G ratio between the two pig breeds showed no difference (p > 0.05). HFD feeding significantly decreased the apparent digestibilities of DM, GE, and CP in the two pig breeds (p < 0.05). The digestibilities of DM, EE, GE, and CP were higher in the Taoyuan pigs than in the Duroc pigs (p < 0.05). Moreover, the digestibility of CF was higher in the Taoyuan pigs than in the Duroc under BD feeding (p < 0.05). No difference was found in digestibility of ash between the two pig breeds (p > 0.05).

Table 2.
Effects of DF on the performance and nutrient digestibility in different pig breeds.
3.2. Influence of DF on Serum Biochemical Parameters
As shown in Table 3, the serum GSH and T-AOC concentrations were higher in the Taoyuan pigs than in the Duroc pigs (p < 0.05). As compared to the Taoyuan pigs, the Duroc pigs had a higher concentration of D-lactate, BUN, and TC in the serum (p < 0.05). HFD feeding remarkably reduced the serum MDA concentration, but increased the serum BUN concentration (p < 0.05). HFD feeding also improved the serum CAT and T-SOD concentrations in the Taoyuan pigs (p < 0.05).

Table 3.
Effects of DF on serum biochemical parameters.
3.3. Influence of DF on Intestinal Morphology and Mucosal Enzyme Activity
As shown in Table 4 and Figure 1, the Taoyuan pigs had a higher villus height and ratio of V/C than the Duroc pigs in the jejunum and ileum (p < 0.05). HFD feeding remarkably improved the villus height and the V/C ratio in the duodenum and jejunum (p < 0.05). The Taoyuan pigs had a lower maltase activity in the jejunal and ileal mucosa than the Duroc pigs (p < 0.05). The Duroc pigs also had a higher ileal sucrase activity than the Taoyuan pigs (Table 5). Interestingly, HFD feeding elevated the activities of jejunal maltase and ileal sucrase in the Duroc pigs, and improved activity of ileal sucrase in the Taoyuan pigs (p < 0.05).

Table 4.
Effects of DF on intestinal morphology.
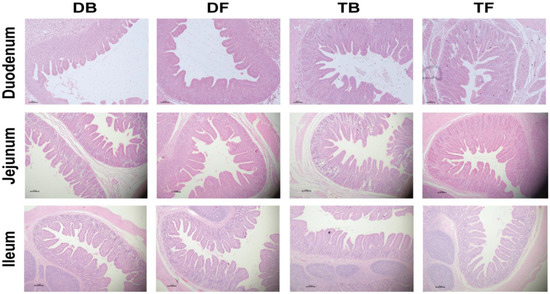
Figure 1.
Effect of DF on intestinal morphology in different pig breeds (H&E; × 40). DB, Durocs were exposed to basal diet; DF, Durocs were exposed to high-fiber diet; TB, Taoyuan pigs were exposed to a basal diet; TF, Taoyuan pigs were exposed to a high-fiber diet.

Table 5.
Effects of DF on mucosal enzyme activity.
3.4. Influence of DF on Expression Levels of Genes Related to Intestinal Epithelial Functions
As shown in Figure 2, the Taoyuan pigs had a higher expression level of FATP-1 than the Duroc pigs in the duodenum (p < 0.05). HFD feeding not only elevated the expression levels of jejunal and ileal SGLT-1, but also increased the ileal GLUT-2 expression level in the Taoyuan pigs (p < 0.05). Additionally, HFD feeding acutely increased the expression levels of jejunal occludin and claudin-1 in both the Taoyuan and Duroc pigs (p < 0.05). The duodenal claudin-1 and ileal ZO-1 expression levels were also elevated in the two pig breeds upon HFD feeding (p < 0.05). In addition, HFD feeding increased the jejunal ZO-1 expression level in the Duroc pigs (p < 0.05).
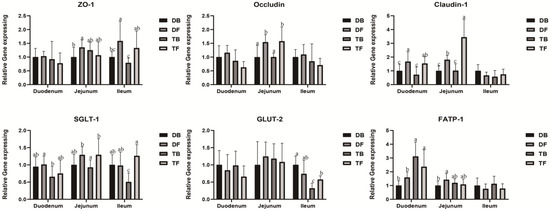
Figure 2.
Effect of DF on expressions of critical genes involved in intestinal epithelium functions. ZO-1, zonula occludens-1; SGLT-1, sodium/glucose cotransporter-1; GLUT-2, glucose transporter-2; FATP, fatty acid transport protein-1; a, b, c, mean values within a row with different superscript letters were significantly different (p < 0.05). DB, Durocs were exposed to a basal diet; DF, Durocs were exposed to a high-fiber diet; TB, Taoyuan pigs were exposed to a basal diet; TF, Taoyuan pigs were exposed to a high-fiber diet.
3.5. Influence of DF on Intestinal Microbiota and Microbial Metabolites
As compared to the Duroc pigs, the Taoyuan pigs had a lower E. coli abundance and a higher Bifidobacterium abundance in the colon (Table 6). However, HFD feeding reduced the colonic E. coli abundance in the Duroc pigs (p < 0.05). Additionally, HFD feeding remarkably increased the Lactobacillus abundance in the Taoyuan pigs (p < 0.05). Interestingly, HFD feeding increased the concentrations of acetic acid and propionic acid in the colon (p < 0.05). The concentrations of butyrate acid and total acid in the colon were also elevated in the Taoyuan pigs upon HFD feeding (p < 0.05).

Table 6.
Effect of DF on colon microbiota and microbial metabolites in different pig breeds.
4. Discussion
In this study, we explored the effects of DF on growth performance, nutrient digestibility, and intestinal functions in Taoyuan and Duroc pigs. Pigs were selected based on their ages (60 days of age), as it is difficult to obtain pigs with a similar age and body weight for different pig breeds. We found that the Taoyuan pigs were more resistant to HFD than the Duroc pigs, as indicated by higher ADG and digestibilities of DM, EE, GE, and CP under HFD feeding. As with other local pig breeds, Taoyuan pigs have been reported to digest high-fiber diets more efficiently than commercial pig breeds such as the Duroc and Large White pigs [30,31]. The difference in high-fiber digestion might be associated with the size and microbial activity of the gastrointestinal tract, as the local pig breeds have a greater size (as a percentage of body weight) than that of commercial pig breeds (e.g., Duroc and Large White pigs) [32]. Moreover, adding appropriate DF in the diet increased the abundance and metabolic capacity of distal gut microbiota without altering CF digestibility and growth rate of native pigs [33], which is also consistent with our study. A previous study indicated that HFD feeding may decrease the nutrient digestibility in pigs [34]. In our study, the digestibilities of DM, GE, and CP in the two pig breeds were decreased upon HFD feeding. The decreased nutrient digestibility may be closely associated with the increase in evacuation rate and decrease in the transit time of nutrients due to HFD feeding [26].
Reactive oxygen species (ROS) generated in the body have been implicated in a variety of biological functions such as signal transduction, gene expression, and receptor activation [35]. For instance, ROS signaling was found to regulate cell proliferation, differentiation, migration, immune response, cell senescence and death, and numerous inherited or acquired pathologies such as atherosclerosis, malignant transformation, diabetes mellitus, and aging [36,37,38]. However, overproduction of ROS may conduce disruption of protein conformation and generation of lipid peroxides, leading to damage of their structure and function in cells and tissues [39]. Antioxidative enzymes generated in the body such as T-SOD, GSH, and CAT are responsible for eliminating ROS and play a critical role in maintaining redox homeostasis [40]. A previous study on rats indicated that ingestion of HFD can elevate their antioxidative capacity through modulating the activity of antioxidant enzymes [41]. In the present study, we detect that HFD feeding not just decreased the serum concentration of MDA in the two pig breeds, but also significantly elevated the serum concentrations of CAT and T-SOD in the Taoyuan pigs, which indicated an elevated antioxidative capacity in pigs after HFD ingestion. A previous study indicated that the metabolic differences among pigs with distinct genotypes can be monitored by blood parameters such as the concentration of metabolites and hormones [42]. Urea is produced proportionally to dietary protein levels and has been looked at as an indicator of protein metabolism [43]. In this study, the serum concentrations of BUN were increased in the two pig breeds upon HFD feeding, indicating an increase in protein breakdown (or decrease in protein deposition) [44]. As compared to the Taoyuan pigs, the Duroc pigs had a higher concentration of serum BUN, which suggested that the body protein metabolism may be more sensitive to DF in the Duroc pigs than in the Taoyuan pigs. Moreover, the serum concentration of TG was lower in the Taoyuan pigs than in the Duroc pigs. The result is consistent with a previous report that a local pig breed (e.g., Heigai pig) had lower expression levels of lipolysis-related genes such as the adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) than commercial pigs (e.g., DLY pig) in the muscle, resulting in a decrease in serum TG concentration [45,46]. Moreover, HFD feeding significantly decreased the serum concentration of glucose in Taoyuan pigs. This is probably due to the reduced substrate (e.g., starch) in the diet that can be enzymatically digested to produce glucose in the small intestine [47,48].
The intestine is the primary location of nutrient digestion and absorption for piglets [49]. Disruption of the villus–crypt integrity (e.g., villus shedding, villus atrophy, and crypt hyperplasia) may lead to invasion of pathogenic bacteria, which subsequently induces various bowel inflammatory diseases [50]. In this study, HFD feeding remarkably increased the villus height and the V/C ratio in the duodenum and jejunum, which suggested an increased absorption area on the surface of the intestinal epithelium [51], and an improved rate of epithelial turnover [52]. The serum D-lactate acid is a critical indicator of intestinal permeability, which is a particular end-product of bacterial fermentation and is released into the blood during disruption of the intestinal mucosa [53]. We found that the serum D-lactate concentration was lower in the Taoyuan pigs than in the Duroc pigs; however, HFD feeding significantly decreased the D-lactate concentration in the Duroc pigs. The result agrees with the intestinal morphology, and both results suggested that local breeds such as the Taoyuan pig may have a better absorption capacity than the Duroc pig. A previous study indicated that changes of the intestinal morphology are followed by alterations in the brush-border enzyme activities [54]. In this study, HFD feeding not only increased the jejunal maltase and ileal sucrase activities in the Duroc pigs, but also increased the ileal sucrase activity in the Taoyuan pigs. Sucrase and maltase are two important disaccharide enzymes that participate in carbohydrate digestion [55]. Moreover, the activities of maltase and sucrase are critical markers to evaluate the development or maturation of the intestinal epithelium [56]. The result is consistent with previous reports that dietary fiber could increase sucrase and maltase in the small intestinal mucosa [57].
The intestinal epithelium protects the host against pathogenic microorganisms through tight junction (TJ) structures [58]. TJ proteins (e.g., claudin-1 and ZO-1) can bind to cytoskeletons, which not only act as staple constituents of the intestinal epithelial epithelium but also act as pivotal regulators of paracellular permeability [59]. A deficit of ZO-1 in mice (specific ZO-1 knockout) showed a disrupted intestinal epithelium, as indicated by abnormal microvillus length and diameter [60]. Moreover, claudin-1 knockdown in mice caused epithelial barrier dysfunction and morphological features of atopic dermatitis in the skin, including hyperkeratosis, acanthosis, and neutrophil infiltration [61]. In the present study, HFD feeding remarkably elevated the expression levels of claudin-1 and occludin in jejunum, and elevated the expression level of ZO-1 in the ileum, which suggested an improved integrity of the intestinal epithelium. Moreover, HFD feeding remarkably improved the expression levels of SGLT-1 and GLUT-2 in the jejunum and ileum of the Taoyuan pigs, and elevated the expression level of jejunal FATP-1 in Duroc pigs. GLUT-2 and SGLT-1 are two of the dominating glucose uptake transporters, and FATP-4 is crucial for long-chain fatty acid absorption in enterocytes [62,63].
DF can be fermented by various microorganisms in the hindgut, and the produced microbial metabolites such as acetic acid, propionic acid, and butyric acid can decrease the lumen pH and inhibit the growth of pathogenic bacteria [64,65,66]. Moreover, the butyric acid can act as a critical energy source of the intestinal epithelial cells [67]. Previous studies also indicated that butyric acid can act as a histone deacetylase (HDAC) inhibitor, which facilitates differentiation of the regulatory T (Treg) cells by up-regulating Foxp3 expression [68,69]. In the present study, HFD feeding dramatically elevated the concentrations of acetic acid, propionic acid, and butyrate acid in the colon. Propionic acid has also been found to improve the integrity of the intestinal epithelium through promoting the proliferation and differentiation of intestinal epithelial cells and enhancing the expression of tight proteins [70]. Importantly, HFD feeding reduced the abundance of E. coli in Duroc pigs and increased the abundance of Lactobacillus in Taoyuan pigs. Previous studies indicated that DF can promote the growth of beneficial bacteria such as Lactobacilli, and inhibit the growth of several pathogenic bacterial species such as Escherichia coli [71,72]. Interestingly, the abundances of Lactobacilli and Bacillus were higher in Taoyuan pigs than in the Duroc pigs. The result is consistent with previous reports that the intestinal microbial composition is closely related to pig breeds, and local pigs may have a higher abundance of DF-degrading bacteria than commercial pigs (e.g., landrace and Yorkshire pig) [31,73].
5. Conclusions
In summary, our results showed a difference in dietary fiber utilization by the two pig breeds (Taoyuan and Duroc), and the results may also suggest a beneficial role of dietary fiber in regulating intestinal health. The Taoyuan pig can digest high-fiber diets more efficiently than commercial pig breeds such as the Duroc pig, and the difference in high-fiber digestion might be connected with the integrity of the intestinal epithelium and the microbial activity of the gastrointestinal tract.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12233298/s1, Table S1. Primers sequences used for quantitative RT-PCR.
Author Contributions
J.H. conceived and designed the experiments. J.L. (Jiahao Liu) performed the experiments and wrote the manuscript. J.L. (Junqiu Luo), X.K., B.Y., X.M., P.Z., J.Y., Z.H., Y.L. and H.Y. gave constructive comments for the results and discussion. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (no. U20A2056) and the Key Research and Development Program of Sichuan Province (no. 2020YFN0147).
Institutional Review Board Statement
The animal experiment in this study was carried out after approval by the Animal Care and Use Committee of Sichuan Agricultural University (Chengdu, China, No.20211105).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
We thank Huifen Wang, Fali Wu, En Yu, and SuJuan Ding for their help during the animal trial and sample collections.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Jarrett, S.; Ashworth, C.J. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.Y.F.; Smeele, R.J.M.; Harington, K.D. The effects of functional fiber on postprandial glycemia, energy intake, satiety, palatability and gastrointestinal wellbeing: A randomized crossover trial. Nutr. J. 2014, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, T.; Tatemoto, P.; de Moraes, J.E. High fiber diet reduces stereotypic behavior of gilts but does not affect offspring performance. Appl. Anim. Behav. Sci. 2021, 243, 105433. [Google Scholar] [CrossRef]
- Ratanpaul, V.; Zhang, D.; Williams, B.A. Interplay between grain digestion and fibre in relation to gastro-small-intestinal passage rate and feed intake in pigs. Eur. J. Nutr. 2021, 60, 4001–4017. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, D.; Yu, B. Effect of different dietary non-starch fiber fractions on growth performance, nutrient digestibility, and intestinal development in weaned pigs. Nutrition 2018, 51, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, J.; Tan, B.; Chen, J.; Zhang, H.; Li, Z.; Ma, X. Physiological function and application of dietary fiber in pig nutrition: A review. Anim. Nutr. 2021, 7, 259–267. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, S.; Yang, Q. Effect of high fibre diets formulated with different fibrous ingredients on performance, nutrient digestibility and faecal microbiota of weaned piglets. Arch. Anim. Nutr. 2016, 70, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Salazar, N.; Ruas-Madiedo, P. Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly. Nutr. Res. 2013, 33, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Pu, G.; Hou, L.; Du, T. Effects of short-term feeding with high fiber diets on growth, utilization of dietary fiber, and microbiota in pigs. Front. Microbiol. 2022, 13, 963917. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Berrocoso, J.D. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Anim. Int. J. Anim. Biosci. 2015, 9, 1441–1452. [Google Scholar] [CrossRef]
- Vuksan, V.; Sievenpiper, J.L.; Jovanovski, E. Effect of soluble-viscous dietary fibre on coronary heart disease risk score across 3 population health categories: Data from randomized, double-blind, placebo-controlled trials. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2020, 45, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Budhwar, S.; Chakraborty, M.; Sethi, K. Antidiabetic properties of rice and wheat bran—A review. J. Food Biochem. 2020, 44, e13424. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.L. Dietary fibre and cardiovascular health: A review of current evidence and policy. Proc. Nutr. Soc. 2020, 79, 61–67. [Google Scholar] [CrossRef]
- Han, P.; Li, P.; Zhou, W. Effects of various levels of dietary fiber on carcass traits, meat quality and myosin heavy chain I, IIa, IIx and IIb expression in muscles in Erhualian and Large White pigs. Meat Sci. 2020, 169, 108160. [Google Scholar] [CrossRef]
- Yang, S.L.; Wang, Z.G.; Liu, B. Genetic variation and relationships of eighteen Chinese indigenous pig breeds. Genet. Sel. Evol. GSE 2003, 35, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, Y.; Chen, J. Growth, carcass characteristics and meat quality of Chinese indigenous Yanan pig crossbred with Duroc and Berkshire genotypes. Anim. Prod. Sci. 2019, 59, 1147. [Google Scholar] [CrossRef]
- Zhang, X.D.; Zhang, S.J.; Ding, Y.Y. Association between ADSL, GARS-AIRS-GART, DGAT1, and DECR1 expression levels and pork meat quality traits. Genet. Mol. Res. GMR 2015, 14, 14823–14830. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, Q.; Xu, H. Comparison and relationship between meat colour and antioxidantcapacity of different pig breeds. Anim. Prod. Sci. 2018, 58, 2152. [Google Scholar] [CrossRef]
- Diao, S.; Xu, Z.; Ye, S. Exploring the genetic features and signatures of selection in South China indigenous pigs. J. Integr. Agric. 2021, 20, 1359–1371. [Google Scholar] [CrossRef]
- Tao, J.; Qin, Z.-Q.; Tao, Y. Genetic relationships among Chinese pigs and other pig populations from Hunan Province, China. Anim. Genet. 2007, 38, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chai, Y.L.; Jiang, J. The complete mitochondrial genome of the Taoyuan Black pig. Mitochondrial DNA 2015, 26, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Chu, H.P.; Jiang, Y.N. Empirical Selection of Informative Microsatellite Markers within Co-ancestry Pig Populations Is Required for Improving the Individual Assignment Efficiency. Asian-Australas. J. Anim. Sci. 2014, 27, 616–627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, D.; Huang, M.; Zhuang, Z. Genomic Analyses Revealed the Genetic Difference and Potential Selection Genes of Growth Traits in Two Duroc Lines. Front. Vet. Sci. 2021, 8, 725367. [Google Scholar] [CrossRef] [PubMed]
- Giuffra, E.; Kijas, J.M.; Amarger, V. The origin of the domestic pig: Independent domestication and subsequent introgression. Genetics 2000, 154, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.). Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Latimer, G.W., Jr. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Wan, J.; Zhang, J.; Chen, D. Alginate oligosaccharide-induced intestinal morphology, barrier function and epithelium apoptosis modifications have beneficial effects on the growth performance of weaned pigs. J. Anim. Sci. Biotechnol. 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.M.; Fialho, R.; Albuquerque, A. Growth, blood, carcass and meat quality traits from local pig breeds and their crosses. Anim. Int. J. Anim. Biosci. 2020, 14, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Borin, K.; Lindberg, J.E.; Ogle, R.B. Effect of variety and preservation method of cassava leaves on diet digestibility by indigenous and improved pigs. Anim. Sci. 2005, 80, 319–324. [Google Scholar] [CrossRef]
- Yang, L.; Bian, G.; Su, Y. Comparison of faecal microbial community of lantang, bama, erhualian, meishan, xiaomeishan, duroc, landrace, and yorkshire sows. Asian-Australas. J. Anim. Sci. 2014, 27, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Pu, G.; Li, P.; Du, T.; Niu, Q. Adding Appropriate Fiber in Diet Increases Diversity and Metabolic Capacity of Distal Gut Microbiota without Altering Fiber Digestibility and Growth Rate of Finishing Pig. Front. Microbiol. 2020, 11, 533. [Google Scholar] [CrossRef]
- Heyer, C.M.E.; Wang, L.F.; Beltranena, E. Nutrient digestibility of extruded canola meal in ileal-cannulated growing pigs and effects of its feeding on diet nutrient digestibility and growth performance in weaned pigs. J. Anim. Sci. 2021, 99, skab135. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, D.; Yu, B. Influences of Selenium-Enriched Yeast on Growth Performance, Immune Function, and Antioxidant Capacity in Weaned Pigs Exposure to Oxidative Stress. BioMed Res. Int. 2021, 2021, 5533210. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, D.; Wood, C.D.; Castro-Obregón, S. Reactive oxygen species: A radical role in development? Free. Radic. Biol. Med. 2010, 49, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Smith, G.P. Analysis of lick rate measure the positive and negative feedback effects of carbohydrates on eating. Appetite 1988, 11, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A. Ferulated Arabinoxylans and Their Gels: Functional Properties and Potential Application as Antioxidant and Anticancer Agent. Oxidative Med. Cell. Longev. 2018, 2018, 2314759. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fígares, I.; Lachica, M.; Nieto, R. Serum profile of metabolites and hormones in obese (Iberian) and lean (Landrace) growing gilts fed balanced or lysine deficient diets. Livest. Sci. 2007, 110, 73–81. [Google Scholar] [CrossRef]
- Bressan, M.C.; Belo, A.T.; Amaral, A. The impact of genetic groups (Alentejano and F1 Landrace x Large White pigs) and body weight (90, 120 and 160 kg) on blood metabolites. Livest. Sci. 2022, 255, 104810. [Google Scholar] [CrossRef]
- Segar, M.W.; Patel, R.B.; Patel, K.V. Association of Visit-to-Visit Variability in Kidney Function and Serum Electrolyte Indexes With Risk of Adverse Clinical Outcomes Among Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2021, 6, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Cardenia, V.; Zappaterra, M.; Motta, V.; Bosi, P. Evaluation of Breed and Parity Order Effects on the Lipid Composition of Porcine Colostrum. J. Agric. Food. Chem. 2018, 66, 12911–12920. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nong, Q.; Wang, J.; Chen, W. Breed difference and regulatory role of CRTC3 in porcine intramuscular adipocyte. Anim. Genet. 2020, 51, 521–530. [Google Scholar] [CrossRef]
- Ellis, P.R.; Roberts, F.G.; Low, A.G.; Morgan, L.M. The effect of high-molecular-weight guar gum on net apparent glucose absorption and net apparent insulin and gastric inhibitory polypeptide production in the growing pig: Relationship to rheological changes in jejunal digesta. Br. J. Nutr. 1995, 74, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.R.; van Kempen, T.A.; Matte, J.J.; Zijlstra, R.T. Starch with high amylose and low in vitro digestibility increases short-chain fatty acid absorption, reduces peak insulin secretion, and modulates incretin secretion in pigs. J. Nutr. 2011, 141, 398–405. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best practice & research. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar]
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 193–206. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.P.; Nelson, L.A.; Huang, F.S. Intestinal adaptation: Structure, function, and regulation. Semin. Pediatr. Surg. 2001, 10, 56–64. [Google Scholar] [CrossRef]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, B.; Chen, D. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Yu, B. Effects of dietary inulin supplementation on growth performance, intestinal barrier integrity and microbial populations in weaned pigs. Br. J. Nutr. 2020, 124, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Pyner, A.; Nyambe-Silavwe, H.; Williamson, G. Inhibition of Human and Rat Sucrase and Maltase Activities To Assess Antiglycemic Potential: Optimization of the Assay Using Acarbose and Polyphenols. J. Agric. Food Chem. 2017, 65, 8643–8651. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. 2017, 7, 9669–9679. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; Michiels, J.; De Smet, S. Dietary fiber affects intestinal mucosal barrier function by regulating intestinal bacteria in weaning piglets. Commun. Agric. Appl. Biol. Sci. 2013, 78, 71–78. [Google Scholar] [PubMed]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Choi, W.; Kuo, W.-T.; Singh, G.; Sailer, A.; Wang, Y.; Shen, L.; Fanning, A.S.; Turner, J.R. The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J. Biol. Chem. 2018, 293, 17317–17335. [Google Scholar] [CrossRef] [PubMed]
- Tokumasu, R.; Yamaga, K.; Yamazaki, Y. Dose-Dependent Role of Claudin-1 In Vivo in Orchestrating Features of Atopic Dermatitis. Proc. Natl. Acad. Sci. USA 2016, 113, E4061–E4068. [Google Scholar] [CrossRef] [PubMed]
- Sangild, P.T.; Tappenden, K.A.; Malo, C. Glucagon-like peptide 2 stimulates intestinal nutrient absorption in parenterally fed newborn pigs. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 160–167. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- He, J.; Xie, H.; Chen, D. Synergetic responses of intestinal microbiota and epithelium to dietary inulin supplementation in pigs. Eur. J. Nutr. 2021, 60, 715–727. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Y.; Yu, B. Effect of β-Glucan Supplementation on Growth Performance and Intestinal Epithelium Functions in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli. Antibiotics 2022, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Grant, L.J.; Gidley, M.J. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.; Zhang, M.; Wu, T.; Liu, R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate AMPK levels and intestinal homeostasis. Food Funct. 2019, 10, 7174–7187. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Lackraj, T.; Kim, J.; Tran, S.-L. Differential modulation of flagella expression in enterohaemorrhagic Escherichia coli O157: H7 by intestinal short-chain fatty acid mixes. Microbiology 2016, 162, 1761–1772. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, L.; Ma, S. High-Dietary Fiber Intake Alleviates Antenatal Obesity-Induced Postpartum Depression: Roles of Gut Microbiota and Microbial Metabolite Short-chain Fatty Acid Involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, C.; Li, F. Characteristics of faecal bacterial flora and volatile fatty acids in Min pig, Landrace pig, and Yorkshire pig. Electron. J. Biotechnol. 2021, 53, 33–43. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).