Influence of Dietary Palm Oils, Palm Kernel Oil and Soybean Oil in Laying Hens on Production Performance, Egg Quality, Serum Biochemicals and Hepatic Expression of Beta-Carotene, Retinol and Alpha-Tocopherol Genes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
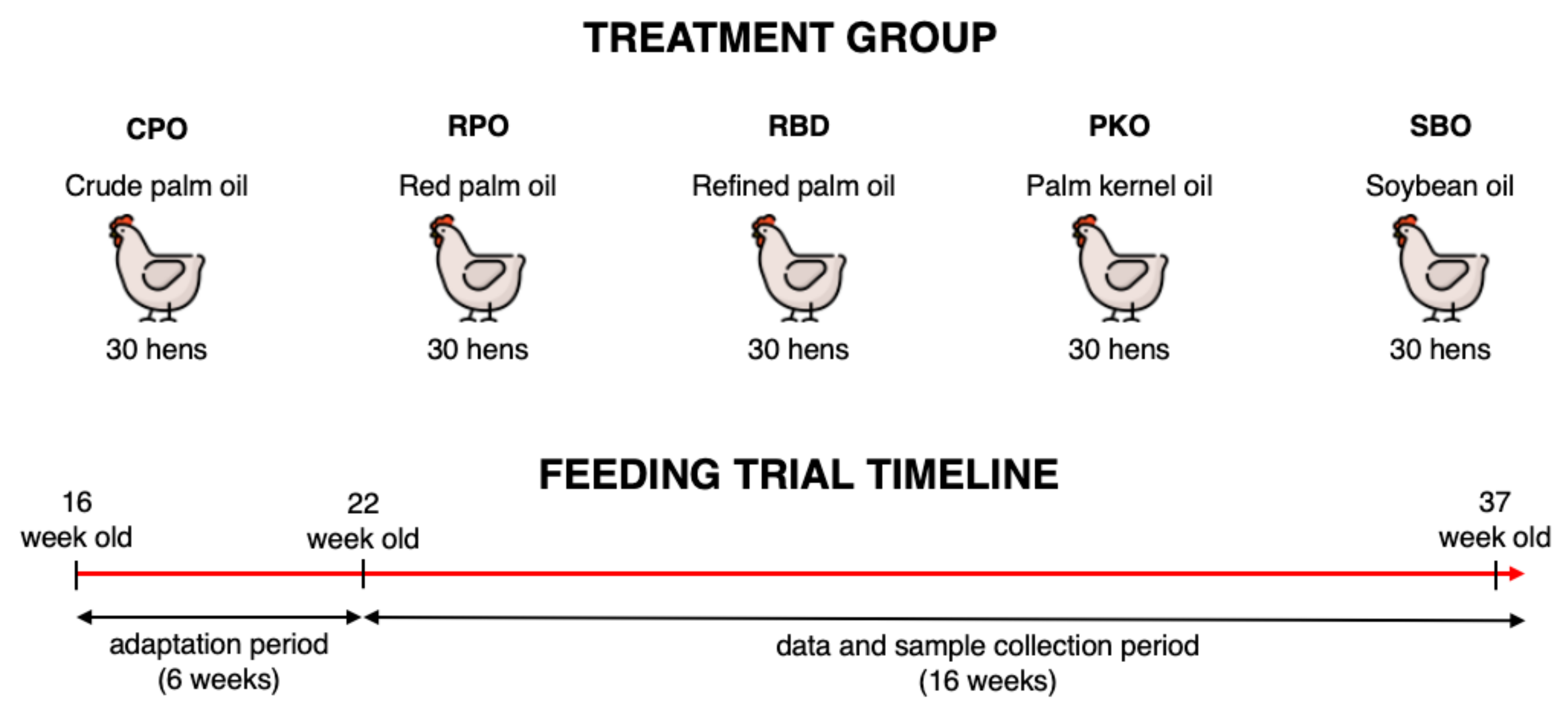
2.1. Ethical Approval, Animals and Management
2.2. Dietary Treatments
2.3. Field Data Collection
2.4. Egg Quality
2.5. Sacrifice and Sample Collection
2.6. Serum Biochemistry
2.7. β-Carotene Determination
2.8. Retinol and α-Tocopherol Concentration by HPLC
2.9. Liver mRNA Expression of β-Carotene, Retinol and Tocopherol
2.10. Experimental Design and Statistical Analysis
3. Results
3.1. Production Performance
3.2. Egg Quality
3.3. Serum Biochemical
3.4. Vitamin A and β-Carotene Concentration
3.5. α-Tocopherol Concentration
3.6. Liver Retinol, β-Carotene and Tocopherol Gene Expression
4. Discussion
4.1. Production Performance
4.2. Egg Quality
4.3. Serum Biochemical
4.4. Retinol, α-Tocopherol and β-Carotene of Feed, Serum, Liver and Yolk
4.5. Liver Retinol, β-Carotene and α-Tocopherol Gene Expression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayed, H.B.; Attia, H.; Ennouri, M. Effect of oil supplemented diet on growth performance and meat quality of broiler chickens. Adv. Tech. Biol. Med. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Khatun, J.; Loh, T.; Akit, H.; Foo, H.; Mohamad, R. Influence of different sources of oil on performance, meat quality, gut morphology, ileal digestibility and serum lipid profile in broilers. J. Appl. Anim. Res. 2018, 46, 479–485. [Google Scholar] [CrossRef]
- Baião, N.C.; Lara, L. Oil and fat in broiler nutrition. Braz. J. Poult. Sci. 2005, 7, 129–141. [Google Scholar] [CrossRef]
- Murugesan, G.R. Understanding the effectiveness of blended fats and oils in poultry diets. Anim. Indian Reprod. 2013, 659, 55. [Google Scholar]
- Kamal-Eldin, A.; Budilarto, E. Tocopherols and tocotrienols as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 141–159. [Google Scholar]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Zimborán, A.; Erdélyi, M.; Szabó, R.; Weber, M. Effects of Medium Chain Fatty Acid Supplementation in Broiler Diet on Microbiological Quality of Litter. Braz. J. Poult. Sci. 2022, 24. [Google Scholar] [CrossRef]
- Jansman, A.; Wagenaars, C.; Schonewille, A.; Snel, H. Bestrijding van Clostridium en Campylobacter infecties in pluimvee via natuurlijk antimicrobiële voedingscomponenten. In Proceedings of the Animal Sciences Group, Wageningen, The Netherlands, 27 June 2006; p. 50. [Google Scholar]
- Kolani, A.; Adjrah, Y.; Eklou-Lawson, M.; Teteh, A.; Tona, K. Effects of dietary palm oil on production performance and serum parameters of laying hens. Int. J. Poult. Sci. 2018, 18, 1–6. [Google Scholar] [CrossRef][Green Version]
- Hosseini-Vashan, S.; Afzali, N. Effect of different levels of palm olein oil in laying hen’s performance and yolk cholesterol. Int. J. Poult. Sci. 2008, 7, 908–912. [Google Scholar] [CrossRef][Green Version]
- Areerob, P.; Dahlan, W.; Angkanaporn, K. Dietary crude palm oil supplementation improves egg quality and modulates tissue and yolk vitamin E concentrations of laying hen. Anim. Prod. Sci. 2018, 59, 1491–1500. [Google Scholar] [CrossRef]
- Punita, A.; Chaturvedi, A. Effect of feeding crude red palm oil (Elaeis guineensis) and grain amaranth (Amaranthus paniculatus) to hens on total lipids, cholesterol, PUFA levels and acceptability of eggs. Plant Foods Hum. Nutr. 2000, 55, 147–157. [Google Scholar] [CrossRef]
- Biswas, A.; Sahoo, J.; Chatli, M. A simple UV-Vis spectrophotometric method for determination of β-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT Food Sci. Technol. 2011, 44, 1809–1813. [Google Scholar] [CrossRef]
- Grebenstein, N.; Frank, J. Rapid baseline-separation of all eight tocopherols and tocotrienols by reversed-phase liquid-chromatography with a solid-core pentafluorophenyl column and their sensitive quantification in plasma and liver. J. Chromatogr. A 2012, 1243, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Agboola, A.; Omidiwura, B.; Oyeyemi, A.; Iyayi, E.; Adelani, A. Effects of four dietary oils on cholesterol and fatty acid composition of egg yolk in layers. Int. J. Agric. Biol. Eng. 2016, 10, 43–50. [Google Scholar]
- Yifei, L.; Shunan, D.; Haiteng, Z.; Ligang, Y.; Zhaodan, W.; Da, P.; Xian, Y.; Hui, X.; Guiju, S.; Shaokang, W. Red palm oil in laying ducks diets: Effects on productive performance, egg quality, concentrations of yolk carotenoids. J. Oil Palm Res. 2021, 33, 703–712. [Google Scholar]
- Karadas, F.; Erdoğan, S.; Kor, D.; Oto, G.; Uluman, M. The effects of different types of antioxidants (Se, vitamin E and carotenoids) in broiler diets on the growth performance, skin pigmentation and liver and plasma antioxidant concentrations. Braz. J. Poult. Sci. 2016, 18, 101–116. [Google Scholar] [CrossRef]
- Kang, K.R.; Cherian, G.; Sim, J.S. Tocopherols, retinol and carotenes in chicken egg and tissues as influenced by dietary palm oil. J. Food Sci. 1998, 63, 592–596. [Google Scholar] [CrossRef]
- Clunies, M.; Parks, D.; Leeson, S. Calcium and phosphorus metabolism and eggshell formation of hens fed different amounts of calcium. Poult. Sci. 1992, 71, 482–489. [Google Scholar] [CrossRef]
- Yeasmin, A.; Azhar, K.; Hishamuddin, O.; Awis Qurni, S. Effect of dietary crude palm oil on quality and oxidative stability of chicken eggs. J. Food Agric. Environ. 2014, 12, 179–181. [Google Scholar]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.-A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar]
- Garlich, J. Chicken serum alkaline phosphatase: Application of a kinetic assay and investigation of phenylalanine and homoarginine as selective inhibitors. Poult. Sci. 1974, 53, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Saminathan, M.; Mohamed, W.N.W.; NOH, A.M.; Ibrahim, N.A.; Fuat, M.A.; Dian, N.L.H.M.; Ramiah, S.K. Potential of feeding crude palm oil and co-products of palm oil milling on laying hens’ performance and egg quality: A review. J. Oil Palm Res. 2020, 32, 547–558. [Google Scholar] [CrossRef]
- Yeum, K.-J.; Russell, R.M. Carotenoid bioavailability and bioconversion. Annu. Rev. Nutr. 2002, 22, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Brubacher, G.; Weiser, H. The vitamin A activity of beta-carotene. Int. J. Vitam. Nutr. Res. 1985, 55, 5–15. [Google Scholar] [PubMed]
- Green, A.S.; Fascetti, A.J. Meeting the vitamin A requirement: The efficacy and importance of β-carotene in animal species. Sci. World J. 2016, 2016, 7393620. [Google Scholar] [CrossRef] [PubMed]
- Woutersen, R.; Wolterbeek, A.; Appel, M.; Berg, H.v.d.; Goldbohm, R.; Feron, V. Safety evaluation of synthetic β-carotene. Crit. Rev. Toxicol. 1999, 29, 515–542. [Google Scholar] [CrossRef]
- Lim, Y.; Traber, M.G. Alpha-tocopherol transfer protein (α-TTP): Insights from alpha-tocopherol transfer protein knockout mice. Nutr. Res. Pract. 2007, 1, 247–253. [Google Scholar] [CrossRef]
- Zanotti, G.; Calderone, V.; Beda, M.; Malpeli, G.; Folli, C.; Berni, R. Structure of chicken plasma retinol-binding protein. Biochim. Biophys. Acta 2001, 1550, 64–69. [Google Scholar] [CrossRef]
- Swindell, E.C.; Thaller, C.; Sockanathan, S.; Petkovich, M.; Jessell, T.M.; Eichele, G. Complementary domains of retinoic acid production and degradation in the early chick embryo. Dev. Biol. 1999, 216, 282–296. [Google Scholar] [CrossRef]
- Lampen, A.; Meyer, S.; Nau, H. Effects of receptor-selective retinoids on CYP26 gene expression and metabolism of all-trans-retinoic acid in intestinal cells. Drug Metab. Dispos. 2001, 29, 742–747. [Google Scholar]
- Zhou, X.; Dong, X.; Tong, J.; Xu, P.; Wang, Z. High levels of vitamin E affect retinol binding protein but not CYP26A1 in liver and hepatocytes from laying hens. Poult. Sci. 2012, 91, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
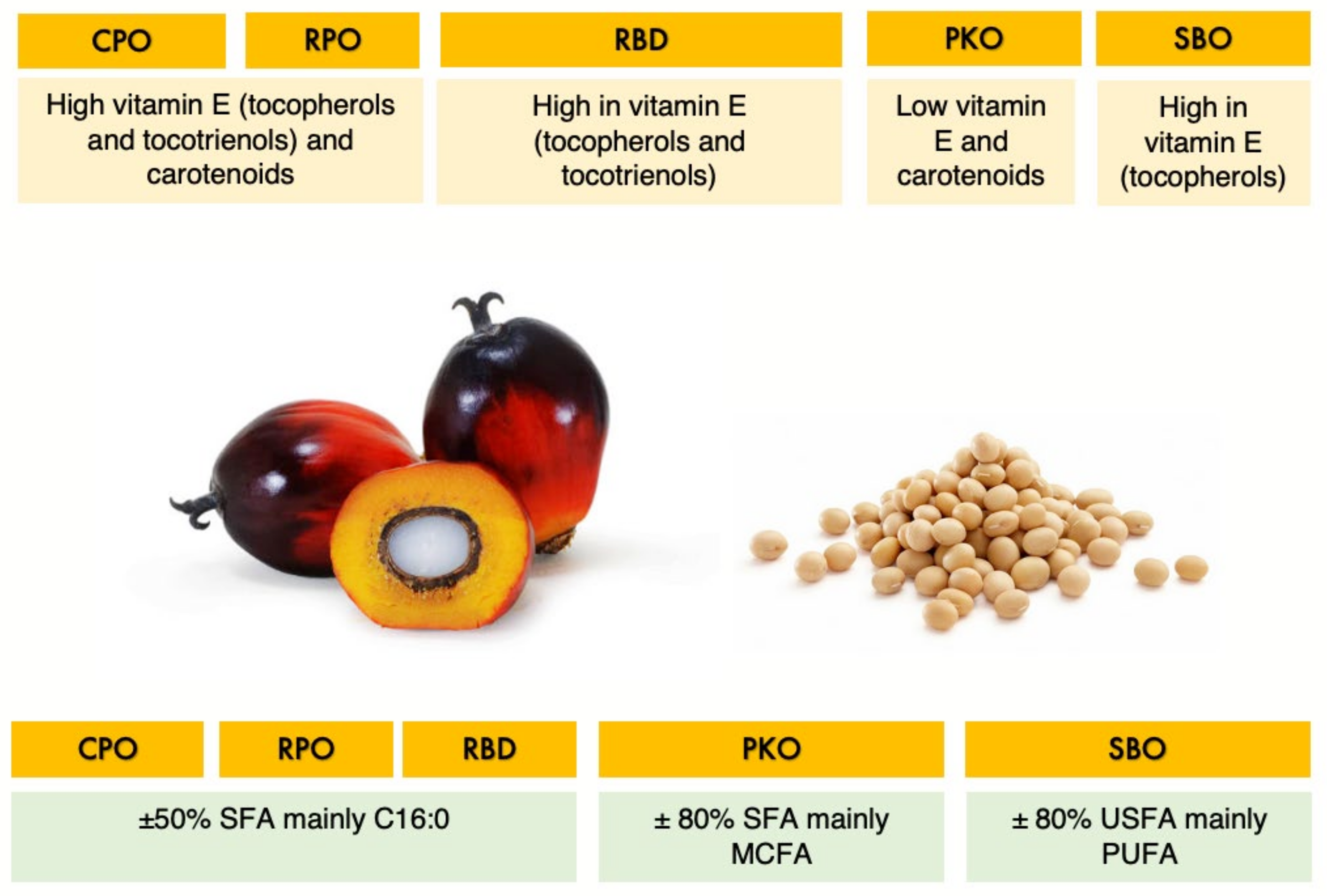
| Treatment | CPO | RPO | RBD | PKO | SBO |
|---|---|---|---|---|---|
| Ingredients (%) | |||||
| Corn | 48.90 | 48.90 | 48.90 | 48.90 | 48.90 |
| Soybean meal | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 |
| Wheat pollard | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 |
| CPO | 3.000 | - | - | - | - |
| RPO | - | 3.000 | - | - | - |
| RBD | - | - | 3.000 | - | - |
| PKO | - | - | - | 3.000 | - |
| SBO | - | - | - | - | 3.000 |
| DL-Methionine | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 |
| MDCP | 2.300 | 2.300 | 2.300 | 2.300 | 2.300 |
| Calcium carbonate | 8.350 | 8.350 | 8.350 | 8.350 | 8.350 |
| Choline chloride | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 |
| Salt | 0.350 | 0.350 | 0.350 | 0.350 | 0.350 |
| Mineral mix | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 |
| Vitamin mix | 0.200 | 0.200 | 0.200 | 0.200 | 0.200 |
| Antioxidants | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 |
| Toxin binder | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 |
| TOTAL | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Calculated nutrients (in % unless stated) | |||||
| ME (kcal/kg) | 2790 | 2790 | 2790 | 2790 | 2790 |
| CP | 17.17 | 17.17 | 17.17 | 17.17 | 17.17 |
| EE | 4.980 | 4.98 | 4.98 | 4.98 | 4.98 |
| CF | 3.800 | 3.80 | 3.80 | 3.80 | 3.80 |
| Ca | 4.000 | 4.00 | 4.00 | 4.00 | 4.00 |
| Total phosphorus | 0.840 | 0.84 | 0.84 | 0.84 | 0.84 |
| Avail. phosphorus | 0.460 | 0.46 | 0.46 | 0.46 | 0.46 |
| Methionine | 0.581 | 0.581 | 0.581 | 0.581 | 0.581 |
| Lysine | 0.933 | 0.933 | 0.933 | 0.933 | 0.933 |
| Target Gene | Primer Sequence | Product Size (bp) | Accession No. |
|---|---|---|---|
| GAPDH F | CTGGCAAAGTCCAAGTGGTG | 275 | NM_204305.1 |
| GAPDH R | AGCACCACCCTTCAGATGAG | ||
| TTPA F | TCCAGCAGTGGCCAAGAAAA | 108 | XM_040663107.1 |
| TTPA R | GCGAAGACTGGGTGGAAGAA | ||
| BCO1 F | ACAAAGAAGAGCATCCAGAGC | 142 | NM_001364902.1 |
| BCO1 R | GCCAAGCCATCAAACCAGTG | ||
| CYP26A1 F | ATGGAGCACACACAGGGTAAT | 423 | NM_001001129.1 |
| CYP26A1 R | GTTGAACTCGTCCTTGTCGGT | ||
| RBP4A F | CTTCAATAACTGGGATGTCTG | 287 | NM_205238.1 |
| RBP4A R | GGTCTATCTGCCTTTGTCTAAC |
| Treatment | CPO | RPO | RBD | PKO | SBO | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| HDEP (%) | |||||||
| Week 22–25 | 88.69 | 87.03 | 91.67 | 90.12 | 87.26 | 1.49 | 0.866 |
| Week 26–29 | 92.74 | 90.60 | 93.21 | 94.52 | 88.22 | 1.57 | 0.764 |
| Week 30–33 | 93.69 | 90.60 | 93.57 | 93.93 | 86.43 | 1.70 | 0.609 |
| Week 34–37 | 91.31 | 88.10 | 91.07 | 92.02 | 85.24 | 1.51 | 0.616 |
| Overall | 91.61 | 89.08 | 92.38 | 92.65 | 86.79 | 1.49 | 0.713 |
| Egg weight (g/hen/day) | |||||||
| Week 22–25 | 54.01 | 53.47 | 55.16 | 54.23 | 54.75 | 0.258 | 0.271 |
| Week 26–29 | 57.44 | 55.67 | 57.25 | 56.65 | 57.36 | 0.275 | 0.210 |
| Week 30–33 | 57.99 a,b | 56.44 b | 57.46 a,b | 57.41 a,b | 59.12 a | 0.279 | 0.045 |
| Week 34–37 | 57.51 | 56.30 | 57.38 | 57.04 | 57.96 | 0.261 | 0.348 |
| Overall | 56.74 | 55.46 | 56.82 | 56.33 | 57.24 | 0.250 | 0.213 |
| Egg mass (g/hen/day) | |||||||
| Week 22–25 | 48.12 b | 46.93 b | 51.43 a | 49.21 a,b | 48.04 b | 0.439 | 0.015 |
| Week 26–29 | 53.50 a,b | 50.74 b | 53.77 a | 53.87 a | 50.83 b | 0.450 | 0.034 |
| Week 30–33 | 54.60 | 51.40 | 54.17 | 54.24 | 51.86 | 0.496 | 0.119 |
| Week 34–37 | 52.76 | 49.80 | 52.63 | 52.77 | 49.82 | 0.454 | 0.042 |
| Overall | 52.24 a,b | 49.72 b | 53.02 a | 52.52 a,b | 50.12 b | 0.433 | 0.045 |
| Feed intake (g/hen/day) | |||||||
| Week 22–25 | 102.67 | 100.67 | 105.48 | 103.45 | 103.26 | 0.667 | 0.258 |
| Week 26–29 | 108.42 | 106.37 | 108.36 | 107.82 | 106.88 | 0.375 | 0.329 |
| Week 30–33 | 106.64 | 106.52 | 108.43 | 107.26 | 107.20 | 0.308 | 0.316 |
| Week 34–37 | 103.29 | 103.04 | 106.86 | 104.53 | 104.40 | 0.616 | 0.316 |
| Overall | 105.25 | 104.15 | 107.28 | 105.76 | 105.44 | 0.468 | 0.336 |
| Feed conversion ratio | |||||||
| Week 22–25 | 2.03 | 2.06 | 2.03 | 2.06 | 2.05 | 0.018 | 0.971 |
| Week 26–29 | 1.99 | 2.01 | 1.98 | 1.96 | 1.97 | 0.016 | 0.908 |
| Week 30–33 | 1.98 | 2.03 | 2.00 | 1.99 | 1.97 | 0.017 | 0.867 |
| Week 34–37 | 2.05 | 2.11 | 2.04 | 2.05 | 2.01 | 0.018 | 0.479 |
| Overall | 2.01 | 2.06 | 2.01 | 2.01 | 2.00 | 0.014 | 0.664 |
| Treatment | CPO | RPO | RBD | PKO | SBO | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Albumen height (mm) | 5.240 a | 4.860 b | 4.910 b | 4.960 a,b | 4.820 b | 0.048 | 0.035 |
| Haugh unit | 70.20 a | 67.44 b | 67.27 b | 67.90 a,b | 66.38 b | 0.407 | 0.035 |
| Shell thickness (mm) | 0.385 | 0.380 | 0.382 | 0.382 | 0.392 | 0.002 | 0.418 |
| Shell weight (g) | 5.583 | 5.517 | 5.510 | 5.510 | 5.569 | 0.033 | 0.929 |
| Shell percentage (%) | 9.354 | 9.432 | 9.276 | 9.276 | 9.189 | 0.048 | 0.580 |
| Yolk color | |||||||
| RCF | 4.848 a | 4.915 a | 4.527 b | 4.624 b | 4.359 c | 0.036 | <0.001 |
| L* (lightness) | 61.65 | 60.55 | 61.87 | 61.36 | 61.01 | 0.258 | 0.534 |
| a* (redness) | 12.28 b | 14.72 a | 11.50 b | 12.21 b | 11.93 b | 0.256 | <0.001 |
| b* (yellowness) | 62.49 d | 67.96 a | 63.60 c,d | 65.56 b | 65.40 b,c | 0.437 | <0.001 |
| Treatment | CPO | RPO | RBD | PKO | SBO | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Liver and kidney functions | |||||||
| ALP (U/L) | 339.6 c | 504.1 b,c | 443.5 c | 736.3 a | 670.0 a,b | 44.83 | 0.004 |
| ALT (U/L) | 16.21 | 20.74 | 16.45 | 18.59 | 19.06 | 1.835 | 0.952 |
| AST (U/L) | 328.1 | 363.1 | 314.8 | 341.9 | 295.9 | 13.99 | 0.676 |
| GGT (U/L) | 25.55 | 18.98 | 27.11 | 21.01 | 27.24 | 1.313 | 0.140 |
| Serum proteins | |||||||
| TP (g/L) | 45.93 | 45.63 | 47.73 | 44.37 | 48.27 | 0.784 | 0.565 |
| Albumin (g/L) | 21.44 | 22.76 | 22.73 | 19.71 | 22.86 | 0.480 | 0.166 |
| Globulin (g/L) | 24.50 | 22.87 | 25.01 | 24.66 | 25.41 | 0.574 | 0.750 |
| A:G | 0.880 | 1.001 | 0.913 | 0.803 | 0.905 | 0.029 | 0.255 |
| Minerals | |||||||
| Ca (mmol/L) | 6.000 | 5.967 | 5.730 | 5.883 | 6.410 | 0.127 | 0.592 |
| P (mmol/L) | 1.380 | 1.867 | 1.177 | 1.337 | 1.430 | 0.097 | 0.232 |
| Ca: P | 4.396 | 3.580 | 4.909 | 4.444 | 4.491 | 0.222 | 0.483 |
| Treatment | CPO | RPO | RBD | PKO | SBO | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| β-carotene (μg/mL) | |||||||
| Feed | 28.48 a | 29.83 a | 3.659 b | 3.474 b | 4.272 b | 3.339 | <0.001 |
| Serum | 1.238 | 1.251 | 0.469 | 1.103 | 0.546 | 0.127 | 0.095 |
| Liver | 12.97 a | 11.39 a | 4.338 b | 6.179 b | 5.387 b | 0.955 | <0.001 |
| Yolk | 57.78 a | 54.17 a | 43.34 b | 56.42 a | 47.26 b | 1.643 | 0.001 |
| Retinol (mM) | |||||||
| Serum | 13.66 | 14.24 | 14.94 | 15.60 | 15.58 | 0.354 | 0.356 |
| Liver | 9.637 | 9.178 | 7.856 | 8.352 | 7.874 | 0.509 | 0.799 |
| Yolk | 7.562 | 6.865 | 5.929 | 7.048 | 6.883 | 0.206 | 0.138 |
| Treatment | CPO | RPO | RBD | PKO | SBO | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| α-tocopherol (mM) | |||||||
| Feed | 0.829 a | 0.737 a,b | 0.673 a,b | 0.547 b | 0.554 b | 0.037 | 0.033 |
| Serum | 0.310 | 0.330 | 0.463 | 0.427 | 0.324 | 0.040 | 0.716 |
| Liver | 0.121 | 0.205 | 0.152 | 0.144 | 0.130 | 0.013 | 0.295 |
| Yolk | 1.788 | 1.732 | 1.712 | 1.770 | 1.702 | 0.074 | 0.997 |
| Treatment | CPO | RPO | RBD | PKO | SBO | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| TTPA | 0.984 | 1.519 | 1.922 | 1.508 | 1.000 | 0.172 | 0.412 |
| BCO1 | 0.232 | 0.286 | 0.633 | 0.652 | 1.000 | 0.101 | 0.074 |
| RBP4A | 1.908 a | 0.980 b | 0.968 b | 2.067 a | 1.000 b | 0.143 | 0.001 |
| CYP26A1 | 0.131 b | 0.448 b | 0.170 b | 0.374 b | 1.000 a | 0.095 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izuddin, W.I.; Loh, T.C.; Akit, H.; Nayan, N.; Noor, A.M.; Foo, H.L. Influence of Dietary Palm Oils, Palm Kernel Oil and Soybean Oil in Laying Hens on Production Performance, Egg Quality, Serum Biochemicals and Hepatic Expression of Beta-Carotene, Retinol and Alpha-Tocopherol Genes. Animals 2022, 12, 3156. https://doi.org/10.3390/ani12223156
Izuddin WI, Loh TC, Akit H, Nayan N, Noor AM, Foo HL. Influence of Dietary Palm Oils, Palm Kernel Oil and Soybean Oil in Laying Hens on Production Performance, Egg Quality, Serum Biochemicals and Hepatic Expression of Beta-Carotene, Retinol and Alpha-Tocopherol Genes. Animals. 2022; 12(22):3156. https://doi.org/10.3390/ani12223156
Chicago/Turabian StyleIzuddin, Wan Ibrahim, Teck Chwen Loh, Henny Akit, Nazri Nayan, Ahmadilfitri Md Noor, and Hooi Ling Foo. 2022. "Influence of Dietary Palm Oils, Palm Kernel Oil and Soybean Oil in Laying Hens on Production Performance, Egg Quality, Serum Biochemicals and Hepatic Expression of Beta-Carotene, Retinol and Alpha-Tocopherol Genes" Animals 12, no. 22: 3156. https://doi.org/10.3390/ani12223156
APA StyleIzuddin, W. I., Loh, T. C., Akit, H., Nayan, N., Noor, A. M., & Foo, H. L. (2022). Influence of Dietary Palm Oils, Palm Kernel Oil and Soybean Oil in Laying Hens on Production Performance, Egg Quality, Serum Biochemicals and Hepatic Expression of Beta-Carotene, Retinol and Alpha-Tocopherol Genes. Animals, 12(22), 3156. https://doi.org/10.3390/ani12223156





