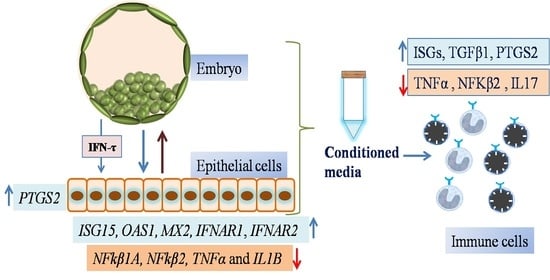
Embryo–Uterine Cross-Talk: Exploration of the Immunomodulatory Mechanism in Buffalo
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Uterine Epithelial Cells
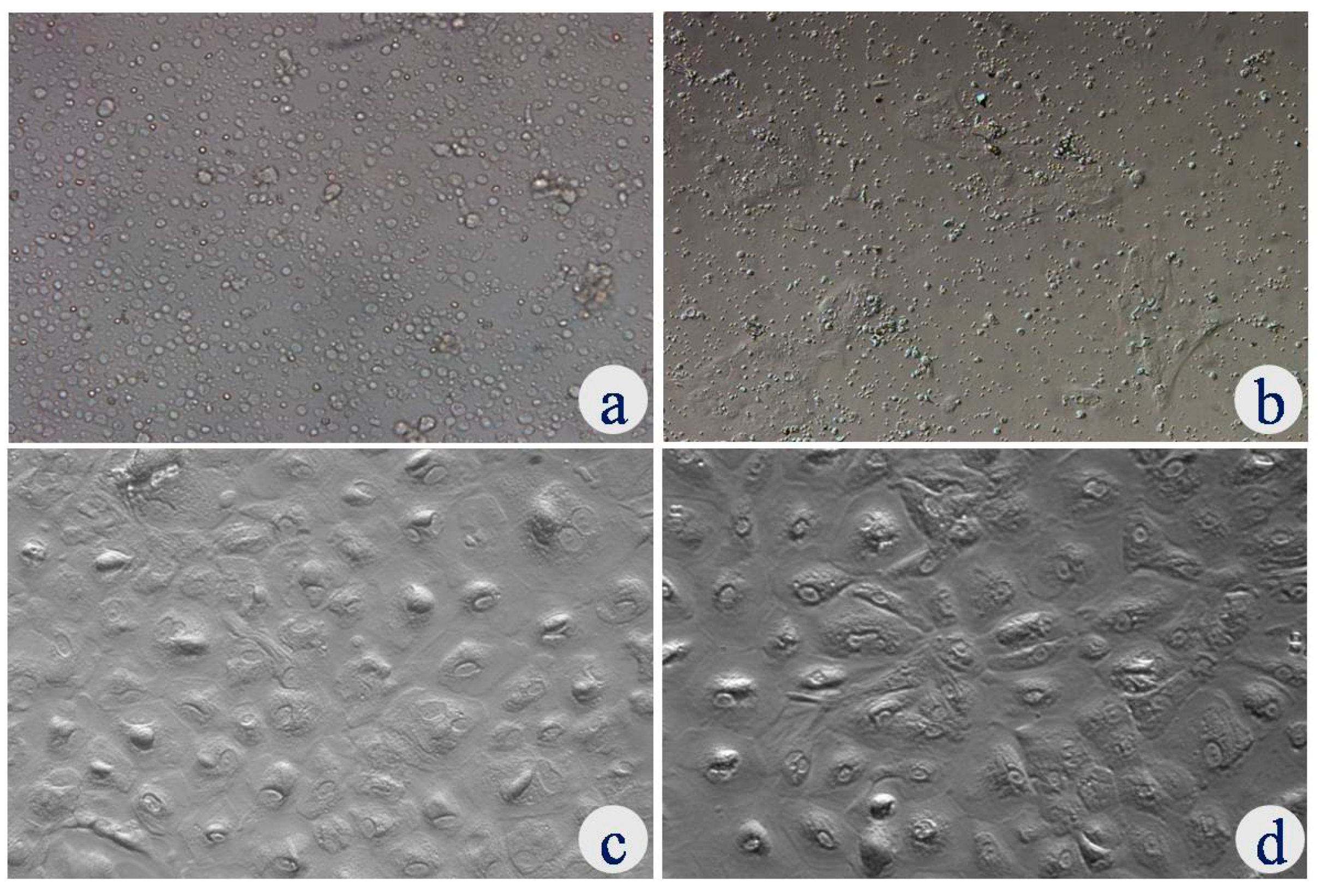
2.1.1. Isolation and Culture of Uterine Luminal Epithelial Cells (UECs)
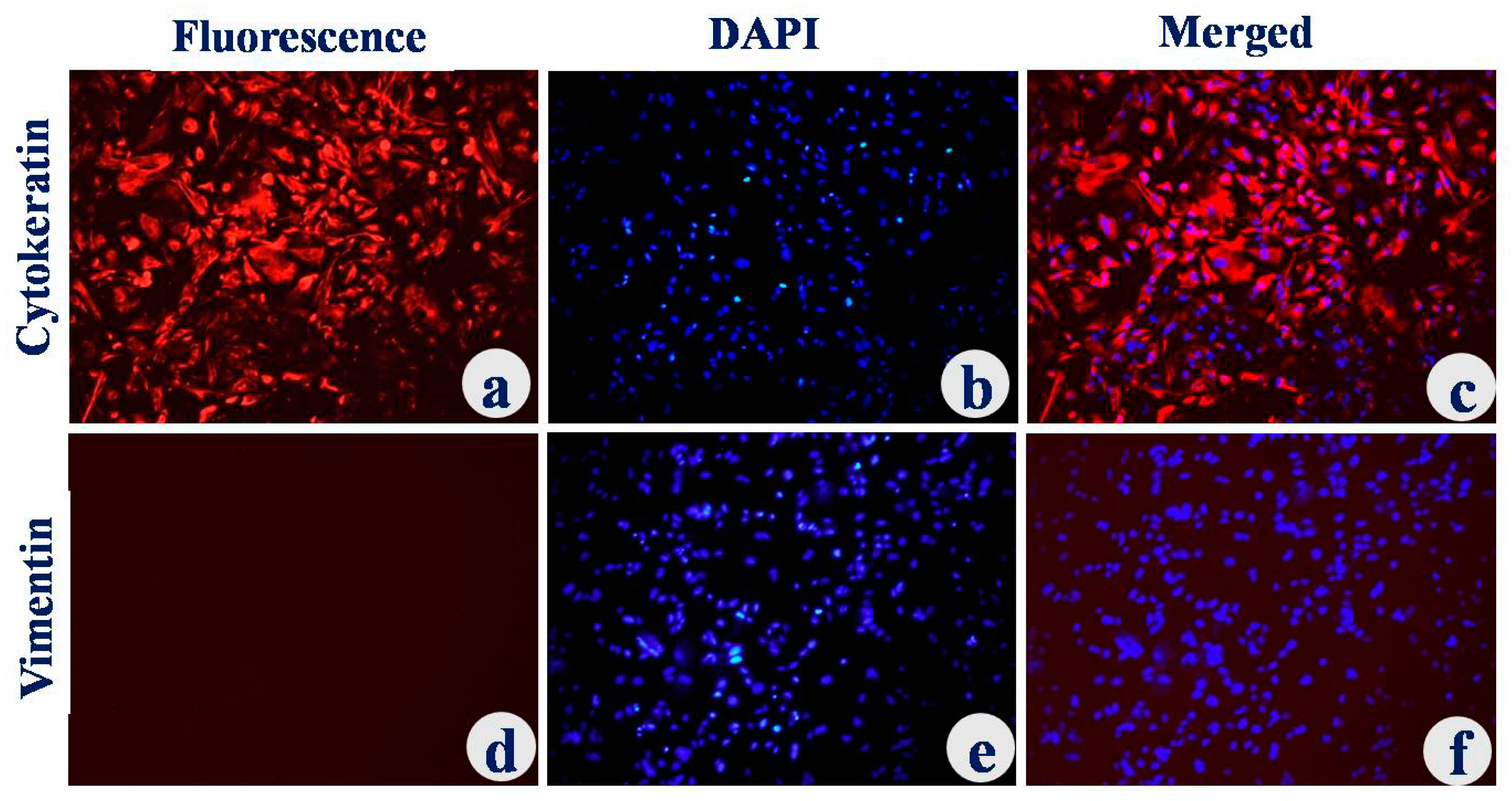
2.1.2. Characterization of Uterine Luminal Epithelial Cells (UECs)
2.1.3. Immunocytochemistry (ICC)
2.1.4. Treatment of Epithelial Cells with Steroid Hormones
2.1.5. In Vitro Embryo Production
2.2. Experiment 1: Effect of Pre-Implantation Embryo on the Expression Profile of Immune-Related Genes in Uterine Epithelial Cells
2.2.1. Co-Culture of Embryos with Steroid-Treated UECs
2.2.2. Real-Time Polymerase Chain Reaction (RT-PCR)
2.2.3. Gene Expression Analysis
2.3. Experiment 2: The Effect of Conditioned Media (CM) from Embryos and UEC–Embryo Co-Culture in PBMCs
2.3.1. Collection of CM from Embryos without UECs
2.3.2. Collection of CM from Steroid-Treated UEC Co-Cultured Embryos
2.3.3. Isolation of PBMCs
2.3.4. Treatment of PBMCs with CM
2.3.5. Total RNA Isolation and RT-PCR Assay
2.3.6. Gene Expression Analysis
2.4. Statistical Analysis
3. Results
3.1. Isolation and Characterization of Uterine Epithelial Cells
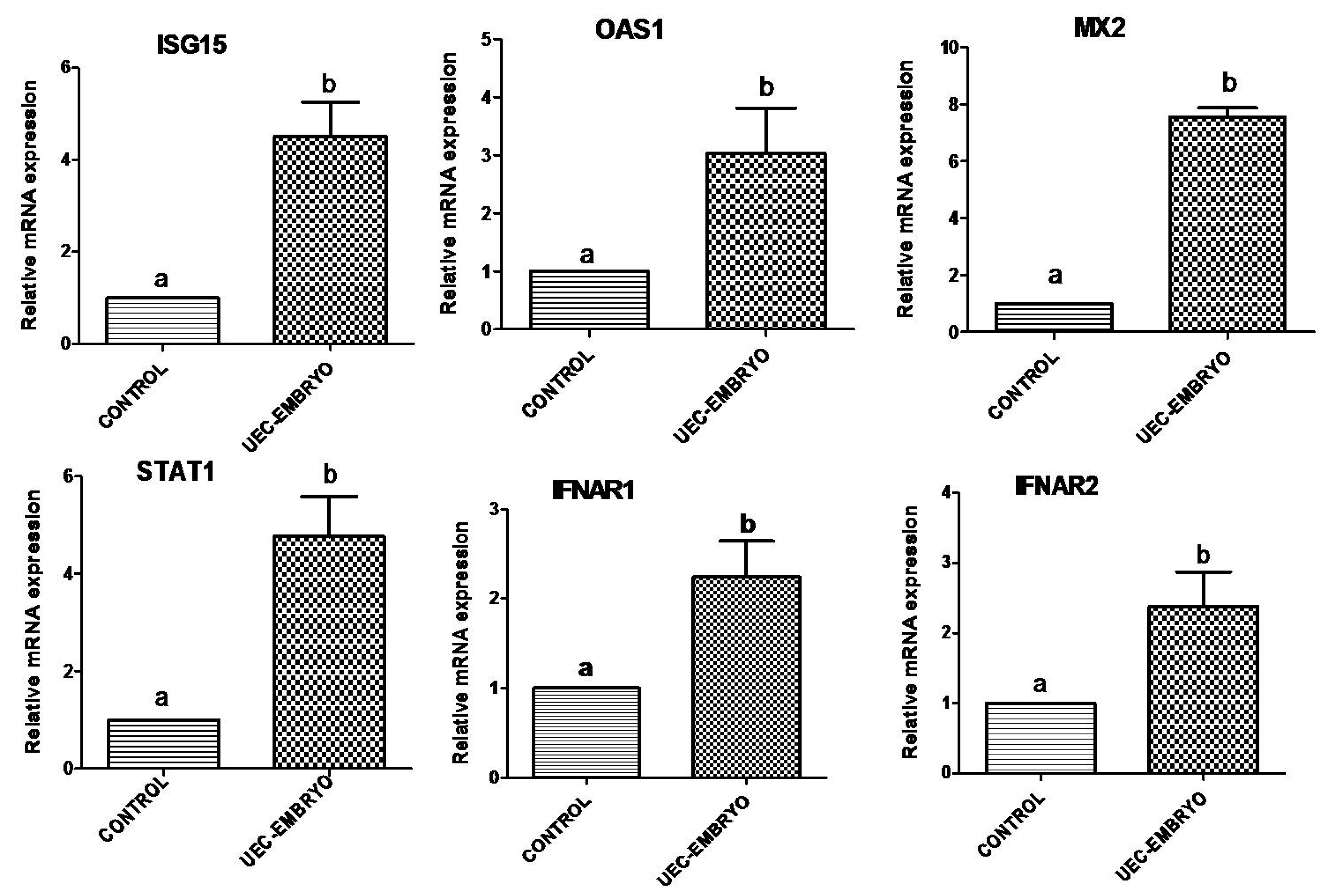
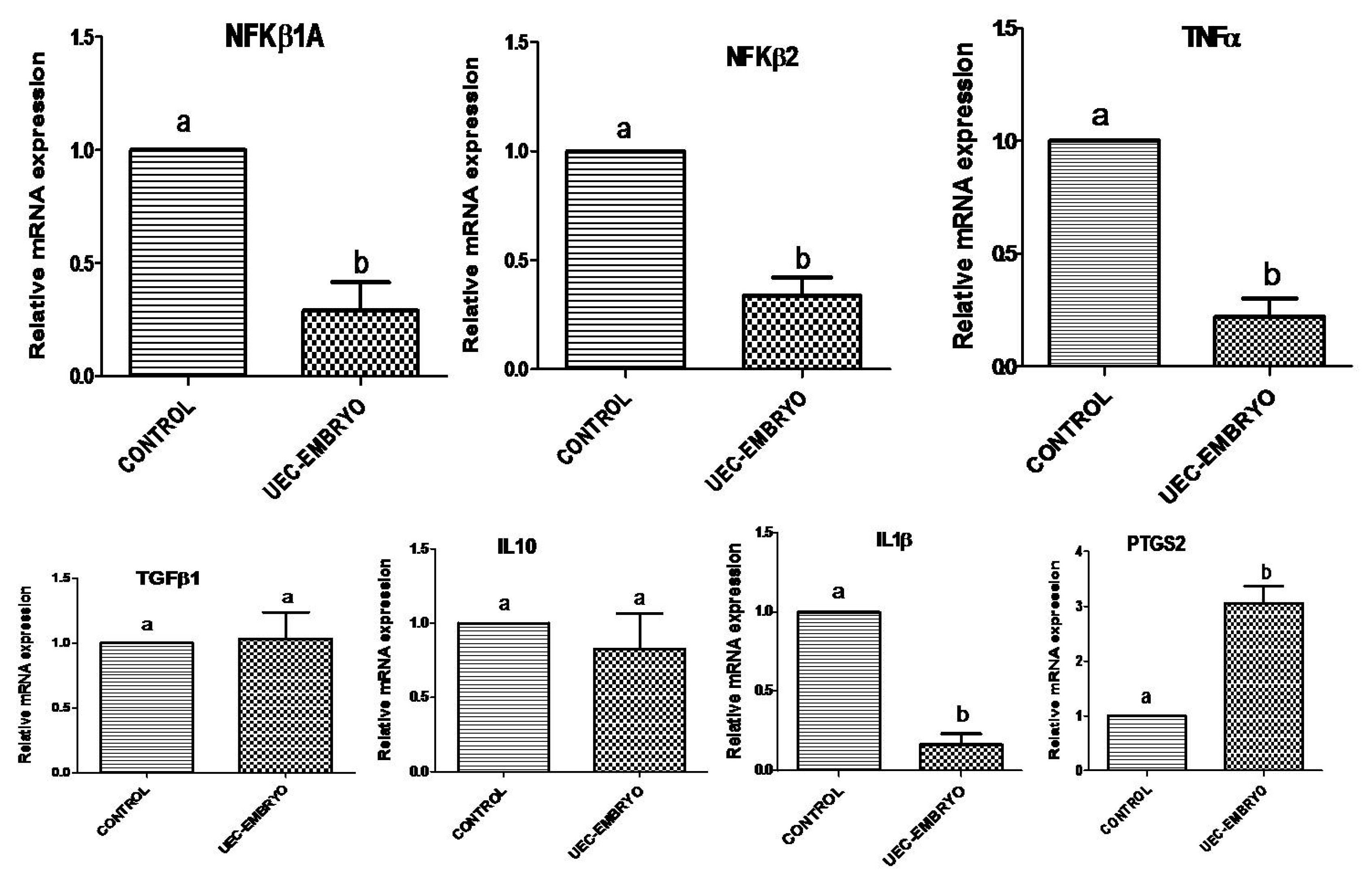
3.2. Effect of Embryos on Expression of Immune-Related Genes in UECs When Co-Cultured with Steroid-Treated UECs
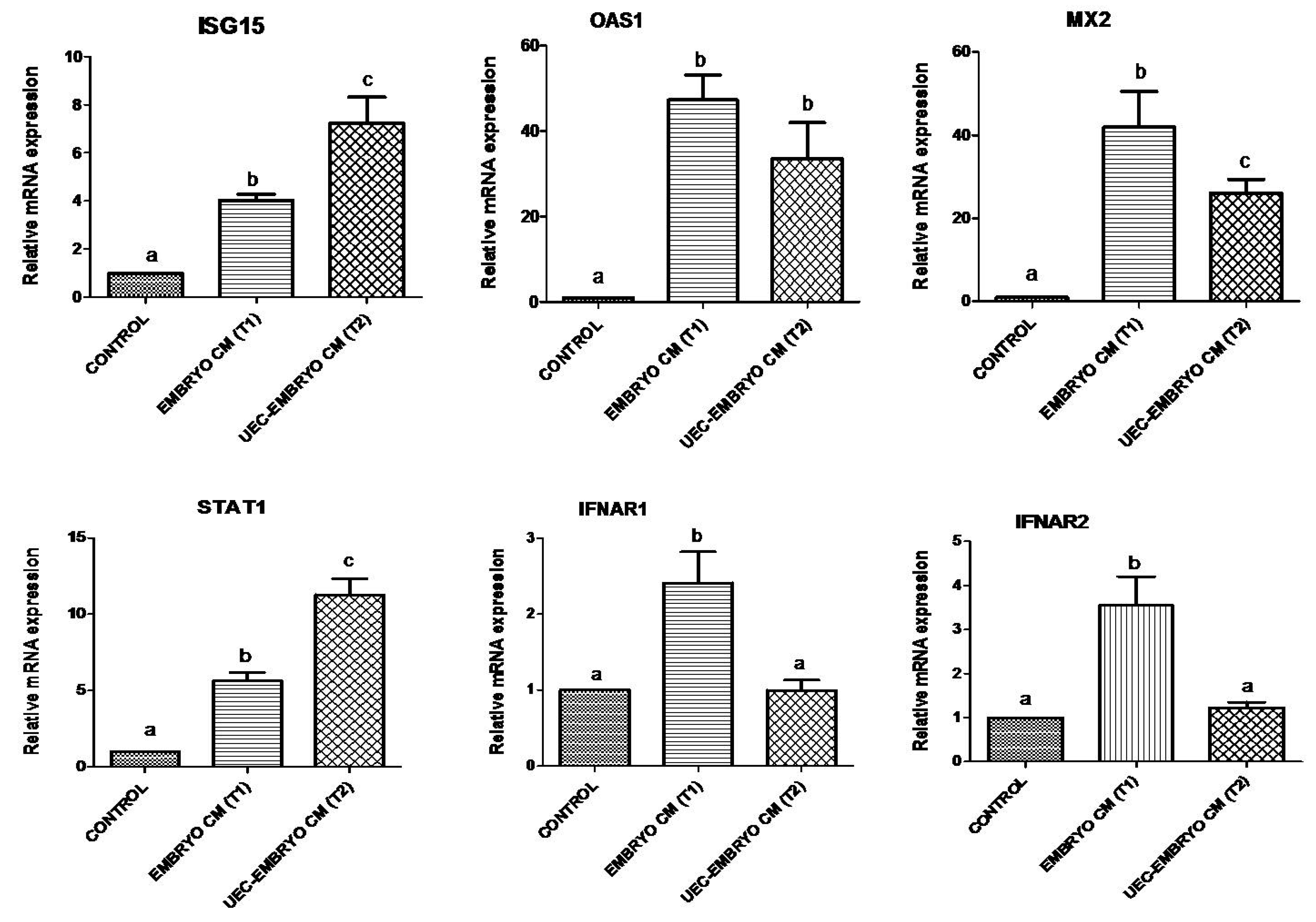
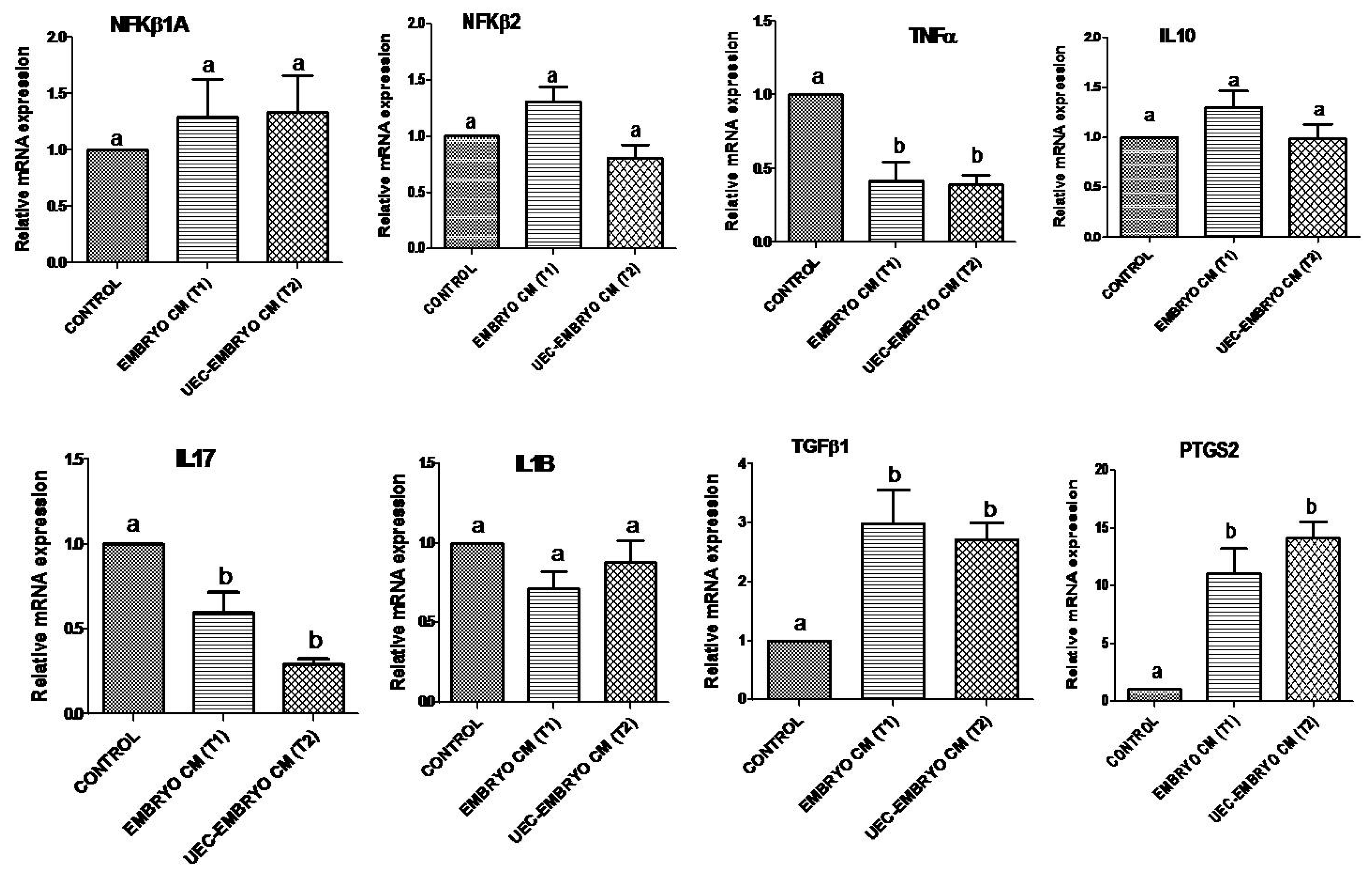
3.3. Effect of Conditioned Medium (CM) from Embryos and UEC Co-Cultured Embryos on Expression of Immune-Related Genes in PBMCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Campanile, G.; Neglia, G. Embryonic mortality in buffalo cows. Ital. J. Anim. Sci. 2007, 6, 119–129. [Google Scholar] [CrossRef]
- Forde, N.; Lonergan, P. Interferon-tau and fertility in ruminants. Reproduction 2017, 154, F33–F43. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Devi, H.L.; Bhat, I.A.; Indu, B.; Bharti, M.K.; Shabir, U.; Sharma, G.T. Expression profile of adhesion molecules in blastocyst vis-a-vis uterine epithelial cells. Theriogenology 2021, 170, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.M.; Simintiras, C.A.; Lonergan, P. Aspects of embryo-maternal communication in establishment of pregnancy in cattle. Anim. Reprod. 2019, 16, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.C.; Baez, G.M.; Garcia-Guerra, A.; Toledo, M.Z.; Monteiro, P.L.; Melo, L.F.; Ochoa, J.C.; Santos, J.E.; Sartori, R. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 2016, 86, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Vallet, J.L.; Roberts, R.M.; Sharp, D.C.; Thatcher, W.W. Role of conceptus secretory products in establishment of pregnancy. Reproduction 1986, 76, 841–850. [Google Scholar] [CrossRef]
- Funeshima, N.; Miura, R.; Katoh, T.; Yaginuma, H.; Kitou, T.; Yoshimura, I.; Konda, K.; Hamano, S.; Shirasuna, K. Metabolomic profiles of plasma and uterine luminal fluids from healthy and repeat breeder Holstein cows. BMC Vet. Res. 2021, 17, 54. [Google Scholar] [CrossRef]
- Wonfor, R.E.; Creevey, C.J.; Natoli, M.; Hegarty, M.; Nash, D.M.; Rose, M.T. Interaction of preimplantation factor with the global bovine endometrial transcriptome. PLoS ONE 2020, 15, e0242874. [Google Scholar] [CrossRef]
- Spencer, T.E.; Sandra, O.; Wolf, E. Genes involved in conceptus-endometrial interactions in ruminants: Insights from reductionism and thoughts on holistic approaches. Reproduction 2008, 135, 165–179. [Google Scholar] [CrossRef]
- Biase, F.H.; Hue, I.; Dickinson, S.E.; Jaffrezic, F.; Laloe, D.; Lewin, H.A.; Sandra, O. Fine-tuned adaptation of embryo-endometrium pairs at implantation revealed by transcriptome analyses in Bos taurus. PLoS Biol. 2019, 17, e3000046. [Google Scholar] [CrossRef]
- Amjadi, F.; Salehi, E.; Mehdizadeh, M.; Aflatoonian, R. Role of the innate immunity in female reproductive tract. Adv. Biomed. Res. 2014, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, D.R. Evolution of mammalian pregnancy in the presence of the maternal immune system. Rev. Reprod. 2000, 5, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Corrales, F.J.; Caamaño, J.N.; Díez, C.; Trigal, B.; Mora, M.I.; Martín, D.; Carrocera, S.; Gómez, E. Proteome of the early embryo-maternal dialogue in the cattle uterus. J. Prot. Res. 2012, 11, 751–766. [Google Scholar] [CrossRef]
- Spencer, T.E.; Bazer, F.W. Conceptus signals for establishment and maintenance of pregnancy. Reprod. Biol. Endo. 2004, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Talukder, A.K.; Yousef, M.S.; Rashid, M.B.; Awai, K.; Acosta, T.J.; Shimizu, T.; Okuda, K.; Shimada, M.; Imakawa, K.; Miyamoto, A. Bovine embryo induces an anti-inflammatory response in uterine epithelial cells and immune cells in vitro: Possible involvement of interferon tau as an intermediator. J. Reprod. Dev. 2017, 63, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Pugliesi, G.; Miagawa, B.T.; Paiva, Y.N.; França, M.R.; Silva, L.A.; Binelli, M. Conceptus-induced changes in the gene expression of blood immune cells and the ultrasound-accessed luteal function in beef cattle: How early can we detect pregnancy? Biol. Reprod. 2014, 91, 95. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Mau, V.J.; Hudson, S.N.; Tremellen, K.P. Cytokine-leukocyte networks and the establishment of pregnancy. Am. J. Reprod. Immunol. 1997, 37, 438–442. [Google Scholar] [CrossRef]
- Pandey, S.; Bhat, I.A.; Bharti, M.K.; Shabir, U.; Peer, B.A.; Indu, B.; Sonwane, A.; Chandra, V.; SaiKumar, G.; Sharma, G.T. Progesterone modulates adhesion molecules in uterine epithelial cells and in vitro embryo production in buffalo. Reprod. Dom. Anim. 2020, 55, 833–843. [Google Scholar] [CrossRef]
- Lakshmi Devi, H.; Shital, N.D.; Pandey, S.; Yasotha, T.; Chandra, V.; Sharma, G.T. Impact of uterine epithelial cells and its conditioned medium on the in vitro embryo production in buffalo (Bubalus bubalis). Theriogenology 2022, 183, 61–68. [Google Scholar]
- Bhardwaj, R.; Ansari, M.M.; Parmar, M.S.; Chandra, V.; Sharma, G.T. Stem Cell Conditioned Media Contains Important Growth Factors and Improves In Vitro Buffalo Embryo Production. Anim. Biotechnol. 2016, 27, 118–125. [Google Scholar] [CrossRef]
- Pandey, S.; Somal, A.; Parmar, M.S.; Gupta, S.; Chandra, V.; SaiKumar, G.; Sharma, G.T. Comparative analysis of developmental and molecular correlates of developmental competence of buffalo oocytes derived from small and large follicles. Ind. J. Anim. Sci. 2017, 87, 1194–1199. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Passaro, C.; Tutt, D.; Mathew, D.J.; Sanchez, J.M.; Browne, J.A.; Boe-Hansen, G.B.; Fair, T.; Lonergan, P. Blastocyst-induced changes in the bovine endometrial transcriptome. Reproduction 2018, 156, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Munoz, M. Multiple-embryo transfer for studying very early maternal–embryo interactions in cattle. Reproduction 2015, 150, R35–R43. [Google Scholar] [CrossRef]
- Maillo, V.; Gaora, P.Ó.; Forde, N.; Besenfelder, U.; Havlicek, V.; Burns, G.W.; Spencer, T.E.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Oviduct-embryo interactions in cattle: Two-way traffic or a one-way street? Biol. Reprod. 2015, 92, 144. [Google Scholar] [CrossRef]
- Forde, N.; Carter, F.; Spencer, T.E.; Bazer, F.W.; Sandra, O.; Mansouri-Attia, N.; Okumu, L.A.; McGettigan, P.A.; Mehta, J.P.; McBride, R.; et al. Conceptus-induced changes in the endometrial transcriptome: How soon does the cow know she is pregnant? Biol. Reprod. 2011, 85, 144–156. [Google Scholar] [CrossRef]
- Bazer, F.W.; Thatcher, W.W. Chronicling the discovery of interferon tau. Reproduction 2017, 154, F11–F20. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.R.; Sinedino, L.D.P.; Spencer, T.E. Paracrine and endocrine actions of interferon tau (IFNτ). Reproduction 2017, 154, F45–F59. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, X.; Zhao, G.; Wu, H.; Mi, J.; Qiu, C.; Peng, X.; Deng, G. IFN-ô plays an anti-inflammatory role in Staphylococcus aureus-induced endometritis in mice through the suppression of NF-κB pathway and MMP9 expression. J. Inter. Cyto. Res. 2017, 37, 81–89. [Google Scholar] [CrossRef]
- McCracken, S.A.; Hadfield, K.; Rahimi, Z.; Gallery, E.D.; Morri, J.M. NF-kappaB-regulated suppression of T-bet in T cells represses Th1 immune responses in pregnancy. Eur. J. Immunol. 2007, 37, 1386–1396. [Google Scholar] [CrossRef]
- Beltman, M.E.; Forde, N.; Furney, P.; Carter, F.; Roche, J.F.; Lonergan, P.; Crowe, M.A. Characterisation of endometrial gene expression and metabolic parameters in beef heifers yielding viable or non-viable embryos on Day 7 after insemination. Reprod. Fertil. Dev. 2010, 22, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Binelli, M.; Scolari, S.C.; Pugliesi, G.; Van Hoeck, V.; Gonella-Diaza, A.M.; Andrade, S.C.S.; Gasparin, G.R.; Coutinho, L.L. The transcriptome signature of the receptive bovine uterus determined at early gestation. PLoS ONE 2015, 10, e0122874. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Sirois, J.; Houde, A.; Murphy, B.D. Cloning, developmental expression, and immunohistochemistry of cyclooxygenase2 in the endometrium during embryo implantation and gestation in the mink (Mustela vison). Endocrinology 1998, 139, 3629–3636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Ann. Rev. Pharm. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Li, X.; Ballantyne, L.L.; Crawford, M.C.; FitzGerald, G.A.; Funk, C.D. Isoform-specific compensation of cyclooxygenase (PTGS) genes during implantation and late-stage pregnancy. Sci. Rep. 2018, 8, 12097. [Google Scholar] [CrossRef]
- Low, B.G.; Hansen, P.J. Actions of steroids and prostaglandins secreted by the placenta and uterus of the cow and ewe on lymphocyte proliferation in vitro. Am. J. Reprod. Immunol. Microbiol. 1988, 18, 71–75. [Google Scholar] [CrossRef]
- Xiao, C.W.; Liu, J.M.; Sirois, J.; Goff, A.K. Regulation of cyclooxygenase-2 and prostaglandin F synthase gene expression by steroid hormones and interferon-tau in bovine endometrial cells. Endocrinology 1998, 139, 2293–2299. [Google Scholar] [CrossRef]
- Shirasuna, K.; Matsumoto, H.; Kobayashi, E.; Nitta, A.; Haneda, S.; Matsui, M.; Kawashima, C.; Kida, K.; Shimizu, T.; Miyamoto, A. Upregulation of interferon-stimulated genes and interleukin-10 in peripheral blood immune cells during early pregnancy in dairy cows. J. Reprod. Dev. 2011, 58, 84–90. [Google Scholar] [CrossRef]
- Rashid, M.B.; Talukder, A.K.; Kusama, K.; Haneda, S.; Takedomi, T.; Yoshino, H.; Moriyasu, S.; Matsui, M.; Shimada, M.; Imakawa, K.; et al. Evidence that interferon-tau secreted from Day-7 embryo in vivo generates anti-inflammatory immune response in the bovine uterus. Biochem. Res. Commun. 2018, 500, 879–884. [Google Scholar] [CrossRef]
- Baratelli, F.; Lin, Y.; Zhu, L.; Yang, S.C.; Heuzé-Vourch, N.; Zeng, G.; Reckamp, K.; Dohadwala, M.; Sharma, S.; Dubinett, S.M. Prostaglandin E2 induces FOXP3 gene expressionand T regulatory cell function in human CD4+ T cells. J. Immunol. 2005, 175, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
| Group | Details |
|---|---|
| Control | Steroid-treated luminal epithelial cells without embryo |
| Treatment (T) | Day 4 cleavage embryos + Steroid-treated luminal epithelial cells monolayer |
| Gene | Primer Sequence (5′-3′) | Annealing Temperature (°C) | Amplicon Size (bp) | Accession No. |
|---|---|---|---|---|
| IFNτ | F-GATGGCCCTGGTGCTGGTCA | 58 | 184 | [3] |
| R-GTCGCCCTCCACCATCTCCTG | ||||
| ISG15 | F-TCTGAGGGACTCCATGACGG | 55 | 51 | NM_174366 |
| R-TTCTGGGCGATGAACTGCTT | ||||
| OAS1 | F-TAGGCCTGGAACATCAGGTC | 57 | 105 | NM_178108 |
| R-TTTGGTCTGGCTGGATTACC | ||||
| MX2 | F-CTTCAGAGACGCCTCAGTCG | 56 | 232 | NM_173941 |
| R-TGAAGCAGCCAGGAATAGT | ||||
| STAT1 | F-CTCATTAGTTCTGGCACCAGC | 60 | 108 | AW289395 |
| R-CACACGAAGGTGATGAACATG | ||||
| IFNAR1 | F-GCGAAGAGTTTCCGCAACAG | 57 | 275 | NM_174552.2 |
| R-TCCAAGGCAGGTCCAATGAC | ||||
| IFNAR2 | F-TCGTATGTTGCGCCTGTTCT | 56 | 231 | NM_174553.2 |
| R-GTCCGTCGTGTTTACCCACA | ||||
| NFKβ2 | F-CCTGCTGAATGCTCTGTCTG | 58 | 102 | NM_001102101.1 |
| R-TCCTCCTTCACCTCTGTGCT | ||||
| NFKβIA | F-AAGTGGTCCGCCAAGTGAAG | 56 | 105 | NM_001045868.1 |
| R-CGATTTCTGGCTGGTTAGTGATC | ||||
| TNFα | F-CCCCAGGGCTCCAGAAGTTGC | 60 | 103 | XM_005696606.3 |
| R-GGCCGATTACCCCGAAGTGC | ||||
| TGFβ1 | F-CGCTCCTGTGGCTACTAATGCTGA | 58 | 149 | NM_001314142.1 |
| R-CGGGGGACTGGCGAGCC | ||||
| IL1B | F-AATCGAAGAAAGGCCCGTCT | 58 | 51 | DQ837159.1 |
| R-ATATCCTGGCCACCTCGAAA | ||||
| IL10 | F-AGCCAGCCTGCCCCACAT | 58 | 140 | DQ837159.1 |
| R-TCCCCCAGCGAGTTCACG | ||||
| IL17 | F-CACAGCATGTGAGGGTCAAAC | 56 | 83 | NM_001008412 |
| R-GGTGGAGCGCTTGTGATAAT | ||||
| PTGS2 | F-CATGGGTGTGAAAGGGACGAAAGA | 60 | 182 | XM_018060731.1 |
| R-CCTTAGTGAAAGCTGGTCCTCGTT | ||||
| Cytokeratin | F-CCCCCAGGTCCTTCAGCAGCC | 64 | 147 | NM_001290975.1 |
| R-GGGCCCCACCGTAGCTTCCAG | ||||
| Vimentin | F-CCGACGCCATCAACACCGAGT | 60 | 163 | NM_173969.3 |
| R-TTGCCCTGGCCCTTGAGCTG | ||||
| GAPDH | F-GCGATACTCACTCTTCTACTTTCGA | 58 | 82 | U85042.1 |
| R-TCGTACCAGGAAATGAGCTTGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huidrom, L.D.; Dhanaji, S.N.; Pandey, S.; Chandra, V.; Gutulla, T.S. Embryo–Uterine Cross-Talk: Exploration of the Immunomodulatory Mechanism in Buffalo. Animals 2022, 12, 3138. https://doi.org/10.3390/ani12223138
Huidrom LD, Dhanaji SN, Pandey S, Chandra V, Gutulla TS. Embryo–Uterine Cross-Talk: Exploration of the Immunomodulatory Mechanism in Buffalo. Animals. 2022; 12(22):3138. https://doi.org/10.3390/ani12223138
Chicago/Turabian StyleHuidrom, Lakshmi Devi, Shital Nagargoje Dhanaji, Sriti Pandey, Vikash Chandra, and Taru Sharma Gutulla. 2022. "Embryo–Uterine Cross-Talk: Exploration of the Immunomodulatory Mechanism in Buffalo" Animals 12, no. 22: 3138. https://doi.org/10.3390/ani12223138
APA StyleHuidrom, L. D., Dhanaji, S. N., Pandey, S., Chandra, V., & Gutulla, T. S. (2022). Embryo–Uterine Cross-Talk: Exploration of the Immunomodulatory Mechanism in Buffalo. Animals, 12(22), 3138. https://doi.org/10.3390/ani12223138