Queen Caging and Oxalic Acid Treatment: Combined Effect on Vitellogenin Content and Enzyme Activities in the First Post-Treatment Workers and Drones, Apis mellifera L.
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
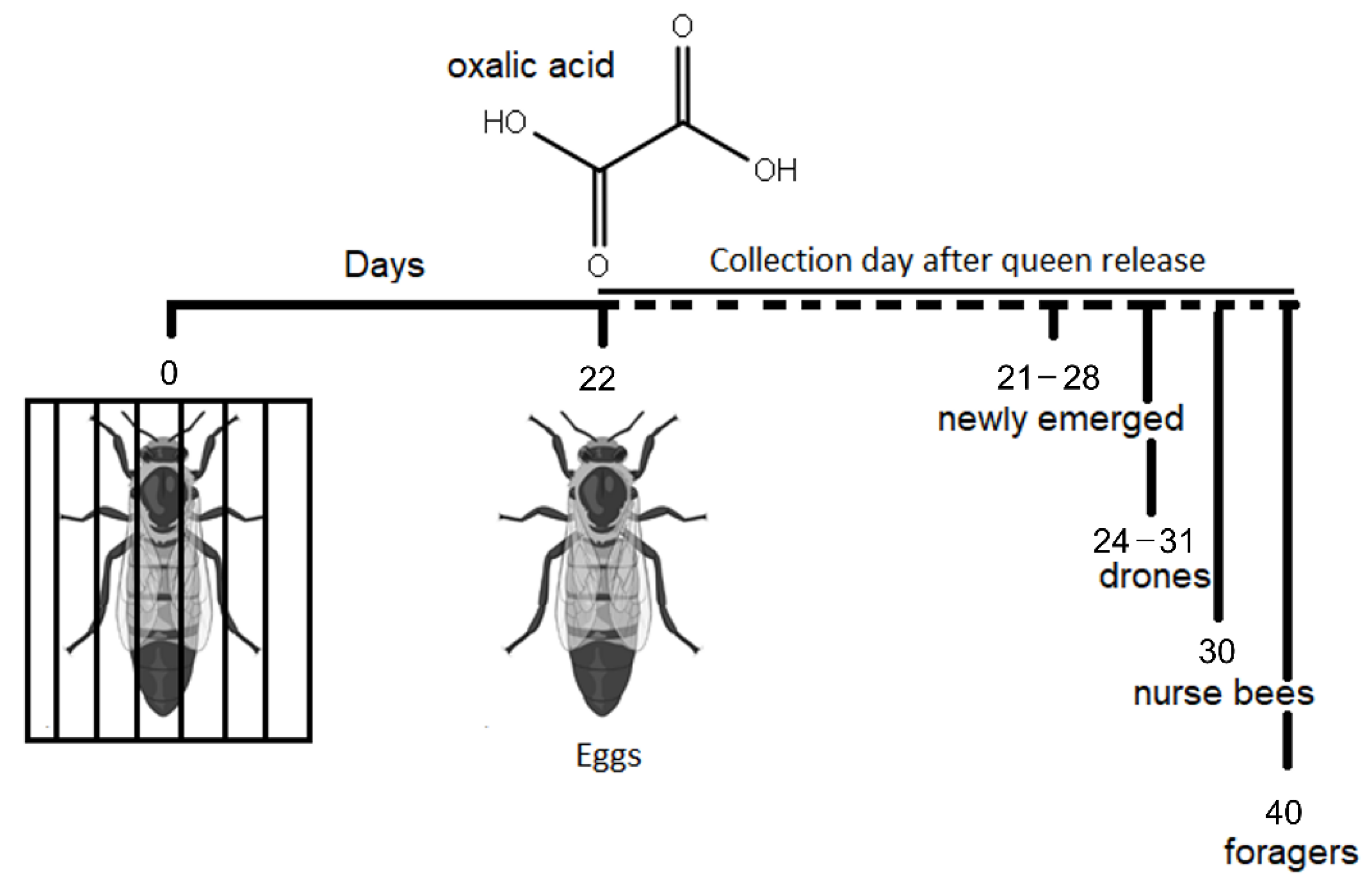
2.1. Bee Collection and Haemolymph Sampling
2.2. Immune System Enzymes
2.3. Vitellogenin Assay Kit
2.4. Antioxidant Enzymes
2.5. Statistical Analysis
3. Results
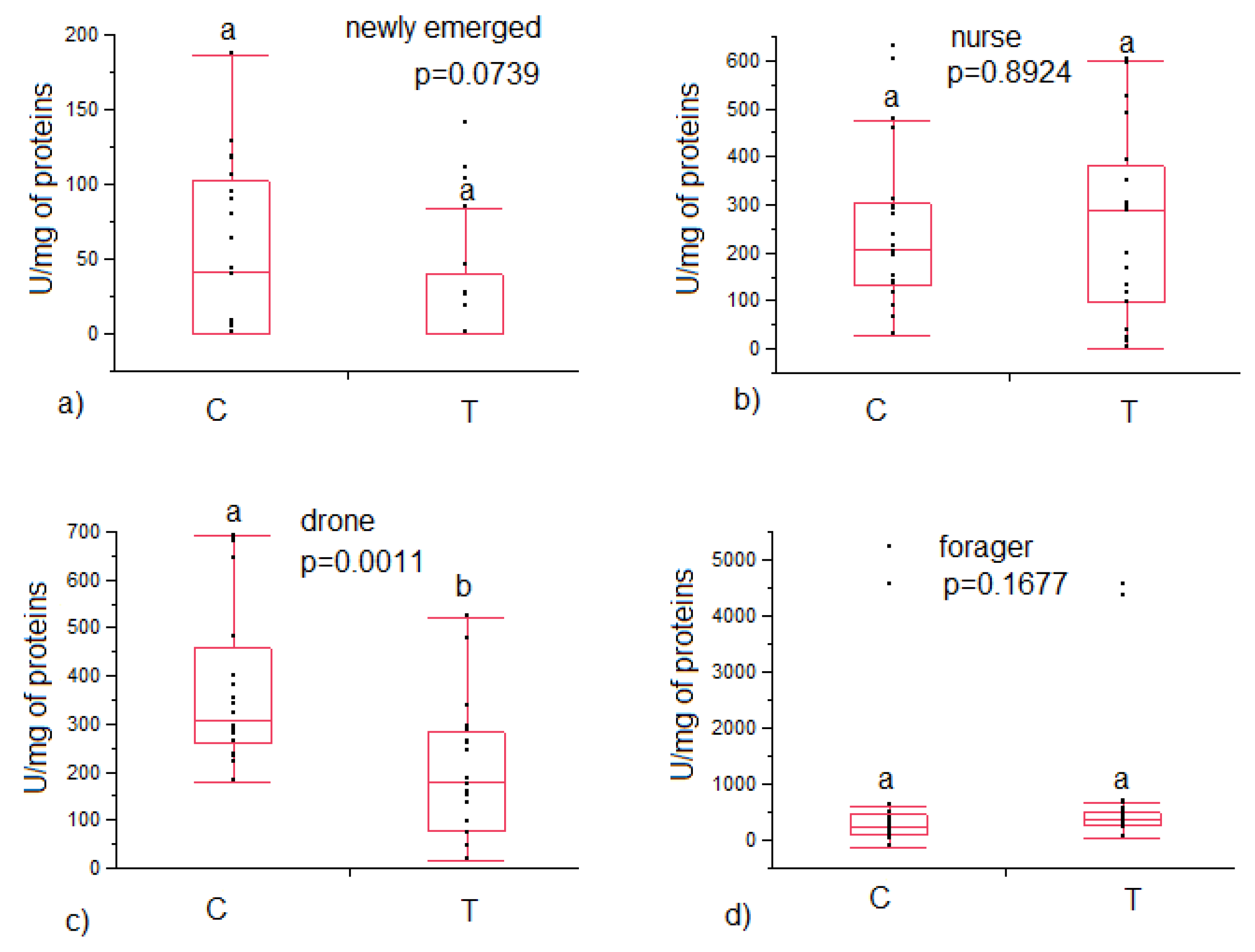
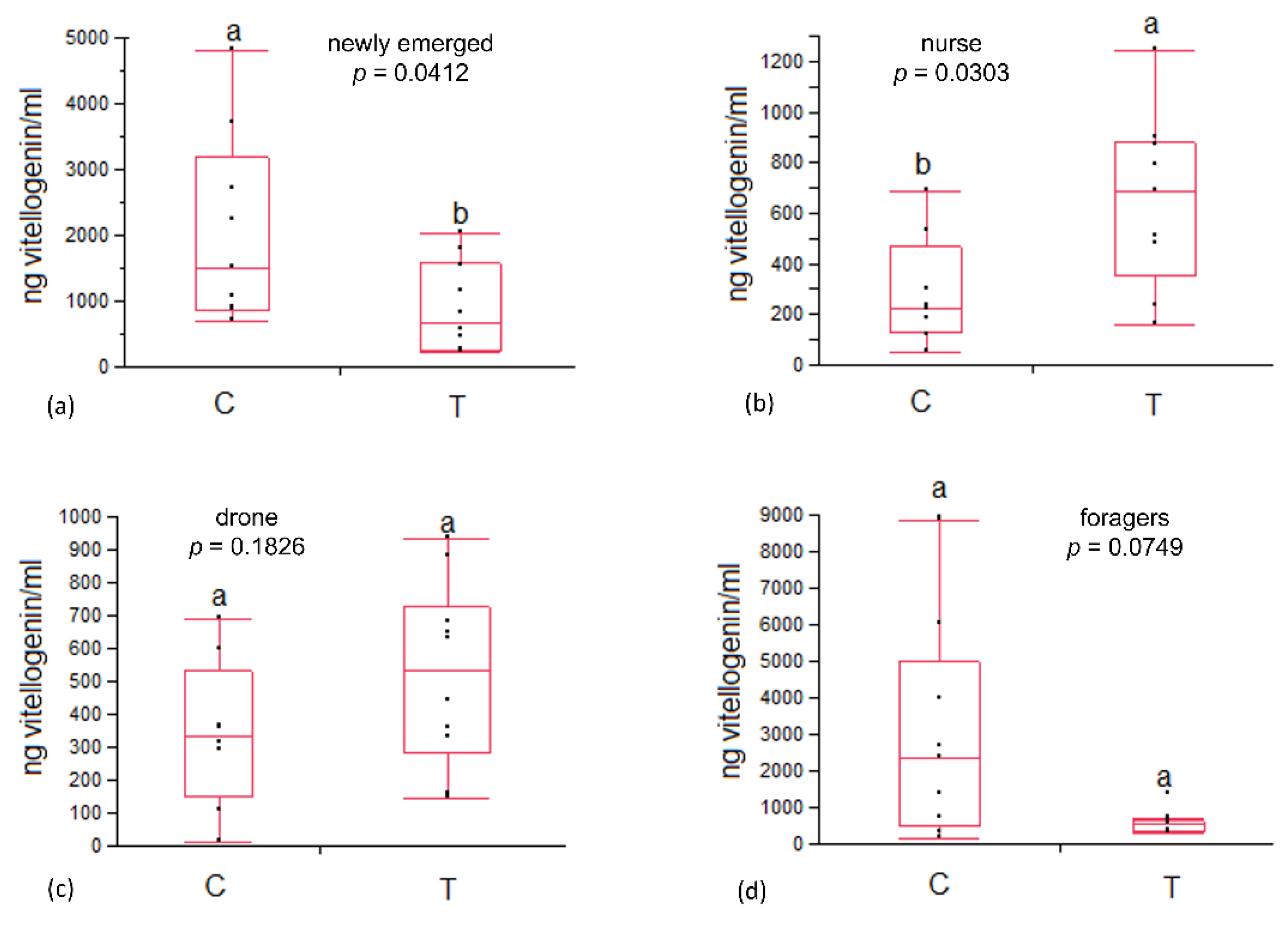
3.1. Immune System Enzymes Activity and Vitellogenin Content
3.2. Antioxidant Enzymes Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boecking, O.; Genersch, E. Varroosis—The ongoing crisis in bee keeping. J. Verbrauch. Lebensm. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A.; Alburaki, M.; Werle, C.; Knight, P.R.; Adamczyk, J. Brood removal or queen caging combined with oxalic acid treatment to control varroa mites (Varroa destructor) in honey bee colonies (Apis mellifera). Apidologie 2017, 48, 821–832. [Google Scholar] [CrossRef]
- Imdorf, A.; Charriere, J.D.; Maqueln, C.; Kilchenmann, V.; Bachofen, B. Alternative varroa control. Am. Bee J. 1996, 136, 189–194. [Google Scholar]
- Bacandritsos, N.; Papanastasiou, I.; Saitanis, C.; Nanetti, A.; Roinioti, E. Efficacy of repeated trickle applications of oxalic acid in syrup for varroosis control in Apis mellifera: Influence of meteorological conditions and presence of brood. Vet. Parasitol. 2007, 148, 174–178. [Google Scholar] [CrossRef]
- Al Toufailia, H.; Scandian, L.; Ratnieks, F.L. Towards integrated control of varroa: 2) comparing application methods and doses of oxalic acid on the mortality of phoretic Varroa destructor mites and their honey bee hosts. J. Apic. Res. 2015, 54, 108–120. [Google Scholar] [CrossRef]
- Gregorc, A.; Planinc, I. Acaricidal effect of oxalic acid in honeybee (Apis mellifera) colonies. Apidologie 2001, 32, 333–340. [Google Scholar] [CrossRef][Green Version]
- Büchler, R.; Uzunov, A.; Kovačić, M.; Prešern, J.; Pietropaoli, M.; Hatjina, F.; Pavlov, B.; Charistos, L.; Formato, G.; Galarza, E.; et al. Summer brood interruption as integrated management strategy for effective Varroa control in Europe. J. Apic. Res. 2020, 59, 764–773. [Google Scholar] [CrossRef]
- Berry, J.A.; Bartlett, L.J.; Bruckner, S.; Baker, C.; Braman, S.K.; Delaplane, K.S.; Williams, G.R. Assessing Repeated Oxalic Acid Vaporization in Honey Bee (Hymenoptera: Apidae) Colonies for Control of the Ectoparasitic Mite Varroa destructor. J. Insect Sci. 2022, 22, 15. [Google Scholar] [CrossRef]
- Nanetti, A.; Higes, M.; Baracani, G.; Besana, A. Control de la varroa con ácido oxálico en combinación con la interrupción artificial de la puesta de cría. In Proceedings of the II Congreso Ibérico de Apicultura, Guadalajara, Spain, 18–20 October 2012. [Google Scholar]
- Pietropaoli, M.; Giacomelli, A.; Milito, M.; Gobbi, C.; Scholl, F.; Formato, G. Integrated pest management strategies against Varroa destructor, the use of oxalic acid combined with innovative cages to obtain the absence of brood. Eur. J. lntegr. Med. 2012, 15, 93. [Google Scholar]
- Giacomelli, A.; Pietropaoli, M.; Carvelli, A.; Iacoponi, F.; Formato, G. Combination of thymol treatment (Apiguard®) and caging the queen technique to fight Varroa destructor. Apidologie 2016, 47, 606–616. [Google Scholar] [CrossRef]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase-its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef]
- Takenaka, T.; Ito, H.; Yatsunami, K.; Echigo, T. Changes of glucose oxidase activity and amount of gluconic acid formation in the hypopharyngeal glands during the lifespan of honey bee workers (Apis mellifera L.). Agric. Biol. Chem. 1990, 54, 2133–2134. [Google Scholar]
- Ohashi, K.; Natori, S.; Kubo, T. Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honeybee (Apis mellifera L.). Eur. J. Biochem. 1999, 265, 127–133. [Google Scholar] [CrossRef]
- Al-Sherif, A.A.; Mazeed, A.M.; Ewis, M.A.; Nafea, E.A.; Hagag, E.S.E.; Kamel, A.A. Activity of salivary glands in secreting honey-elaborating enzymes in two subspecies of honeybee (Apis mellifera L). Physiol. Entomol. 2017, 42, 397–403. [Google Scholar] [CrossRef]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Amdam, G.V.; Simões, Z.L.; Hagen, A.; Norberg, K.; Schrøder, K.; Mikkelsen, Ø.; Kirkwood, T.B.L.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef]
- Salmela, H.; Sundström, L. Vitellogenin in inflammation and immunity in social insects. Inflamm. Cell Signal. 2018, 4, e1506. [Google Scholar] [CrossRef][Green Version]
- Weirich, G.F.; Collins, A.M.; Williams, V.P. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie 2002, 33, 3–14. [Google Scholar] [CrossRef]
- Sagona, S.; Betti, L.; Casini, L.; Palego, L.; Giannaccini, G.; Felicioli, A. Antioxidant enzymes activity during age polyethism in Apis mellifera L., 1758. J. Apic. Res. 2021, 60, 879–889. [Google Scholar] [CrossRef]
- Jia, H.; Sun, R.; Shi, W.; Yan, Y.; Li, H.; Guo, X.; Xu, B. Characterization of a mitochondrial manganese superoxide dismutase gene from Apis cerana cerana and its role in oxidative stress. J. Insect Physiol. 2014, 60, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Corona, M.; Robinson, G.E. Genes of the antioxidant system of the honey bee: Annotation and phylogeny. Insect Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Felicioli, A.; Sagona, S.; Galloni, M.; Bortolotti, L.; Bogo, G.; Guarnieri, M.; Nepi, M. Effects of nonprotein amino acids on survival and locomotion of Osmia bicornis. Insect Mol. Biol. 2018, 27, 556–563. [Google Scholar] [CrossRef]
- Sagona, S.; Minieri, S.; Coppola, F.; Gatta, D.; Casini, L.; Palego, L.; Betti, L.; Giannaccini, G.; Felicioli, A. Effects of chestnut hydrolysable tannin enrichment in the artificial diet of forager bees, Apis mellifera. J. Apic. Res. 2021, 1–7. [Google Scholar] [CrossRef]
- Mazzei, M.; Fronte, B.; Sagona, S.; Carrozza, M.L.; Forzan, M.; Pizzurro, F.; Bibbiani, C.; Miragliotta, V.; Abramo, F.; Millanta, F.; et al. Effect of 1, 3-1, 6 β-glucan on natural and experimental deformed wing virus infection in newly emerged honeybees (Apis mellifera ligustica). PLoS ONE 2016, 11, e0166297. [Google Scholar] [CrossRef]
- Dade, H.A. Anatomy and Dissection of the Honeybee; Bee Research Association: London, UK, 1962. [Google Scholar]
- Hrassnigg, N.; Crailsheim, K. Differences in drone and worker physiology in honeybees (Apis mellifera). Apidologie 2005, 36, 255–277. [Google Scholar] [CrossRef]
- Kairo, G.; Biron, D.G.; Ben Abdelkader, F.; Bonnet, M.; Tchamitchian, S.; Cousin, M.; Dussaubat, C.; Benoit, B.; Kretzschmar, A.; Belzunces, L.P.; et al. Nosema ceranae, Fipronil and their combination compromise honey bee reproduction via changes in male physiology. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Fang, Y.; Feng, M.; Han, B.; Qi, Y.; Hu, H.; Fan, P.; Huo, X.; Meng, L.; Li, J. Proteome analysis unravels mechanism underling the embryogenesis of the honeybee drone and its divergence with the worker (Apis mellifera lingustica). J. Proteome Res. 2015, 14, 4059–4071. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.E. Anatomy and Physiology of the Honeybee; McGraw-Hill Nook Company, Inc.: New York, NY, USA; London, UK, 1925. [Google Scholar]
- Candy, D.J. Glucose oxidase and other enzymes of hydrogen peroxide metabolism from cuticle of Schistocerca americana gregaria. Insect Biochem. 1979, 9, 661–665. [Google Scholar] [CrossRef]
- Sagona, S.; Fronte, B.; Coppola, F.; Tafi, E.; Giusti, M.; Palego, L.; Betti, L.; Giannaccini, G.; Guglielminetti, L.; Felicioli, A. Effect of Honey and Syrup Diets Enriched with 1, 3-1, 6 β-Glucans on Honeybee Survival Rate and Phenoloxidase Activity (Apis mellifera L. 1758). Vet. Sci. 2021, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Cabbri, R.; Ferlizza, E.; Nanetti, A.; Monari, E.; Andreani, G.; Galuppi, R.; Isani, G. Biomarkers of nutritional status in honeybee haemolymph: Effects of different biotechnical approaches for Varroa destructor treatment and wintering phase. Apidologie 2018, 49, 606–618. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef]
- Trenczek, T.; Engels, W. Occurrence of vitellogenin in drone honeybees (Apis mellifica). Int. J. Invertebr. Reprod. 1986, 10, 307–311. [Google Scholar] [CrossRef]
- Bitondi, M.M.G.; Simoes, Z.P. The relationship between level of pollen in the diet, vitellogenin and juvenile hormone titres in Africanized Apis mellifera workers. J. Apic. Res. 1996, 35, 27–36. [Google Scholar] [CrossRef]
- Szolderits, M.J.; Crailsheim, K. A comparison of pollen consumption and digestion in honeybee (Apis mellifera carnica) drones and workers. J. Insect Physiol. 1993, 39, 877–881. [Google Scholar] [CrossRef]
- Brouwers, E.V.M.; Ebert, R.; Beetsma, J. Behavioural and physiological aspects of nurse bees in relation to the composition of larval food during caste differentiation in the honeybee. J. Apic. Res. 1987, 26, 11–23. [Google Scholar] [CrossRef]
| Control Colonies | Experimental Colonies | p Value | Test Value | |
|---|---|---|---|---|
| Phenoloxidase activity U/mg of proteins | ||||
| Newly emerged bees | 124 ± 43(129) | 236 ± 66(138) | 0.3607 | χ2 = 0.8354, df = 1 |
| Nurse bees | 350 ± 111(264) | 205 ± 51(234) | 0.5939 | χ2 = 0.2843, df = 1 |
| Foragers | 248 ± 82(205) | 144 ± 43(116) | 0.3250 | χ2 = 0.9686, df = 1 |
| Drones | 310 ± 108(260) | 172 ± 48(136) | 0.3073 | χ2 = 1.0422, df = 1 |
| Control Colonies | Experimental Colonies | p Value | Test Value | |
|---|---|---|---|---|
| SOD activity U/mg of proteins | ||||
| Newly emerged bees | 3.96 ± 0.36 (3.78) | 4.23 ± 0.42 (4.52) | 0.7624 | χ2 = 0.0914, df = 1 |
| Nurse bees | 4.03 ± 0.31 (4.13) | 4.49 ± 0.21 (4.46) | 0.4905 | χ2 = 0.4754, df = 1 |
| Foragers | 5.08 ± 0.33 (5.53) | 5.37 ± 0.30 (5.58) | 0.4057 | χ2 = 0.6914, df = 1 |
| Drones | 4.29 ± 0.43 (4.39) | 3.52 ± 0.35 (3.48) | 0.5453 | χ2 = 0.3657, df = 1 |
| Catalase activity nmol/min/mg of proteins | ||||
| Newly emerged bees | 16.66 ± 3.85 (13.79) | 12.08 ± 3.88 (9.76) | 0.2263 | χ2 = 1.4640, df = 1 |
| Nurse bees | 56.89 ± 4.32 (55.40) | 52.07 ± 7.31 (47.37) | 0.4497 | χ2 = 0.5714, df = 1 |
| Foragers | 15.82 ± 3.39 (13.24) | 19.75 ± 3.03 (22.34) | 0.2568 | χ2 = 1.2857, df = 1 |
| Drones | 10.20 ± 3.97 (5.04) | 7.37 ± 1.27 (7.51) | 0.8206 | χ2 = 0.0514, df = 1 |
| Glutathione S transferase activity nmol/min/mg of proteins | ||||
| Newly emerged bees | 161 ± 26 (142) | 128 ± 24 (136) | 0.5576 | F = 0.3563, df = 1 |
| Nurse bees | 145 ± 17 (138) | 197 ± 21 (180) | 0.0569 | F = 4.0394, df = 1 |
| Foragers | 156 ± 19 (159) | 166 ± 16 (171) | 0.6793 | F = 0.1760, df = 1 |
| Drones | 106 ± 16 (116) | 148 ± 19 (131) | 0.1091 | F = 2.8123, df = 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagona, S.; Coppola, F.; Nanetti, A.; Cardaio, I.; Tafi, E.; Palego, L.; Betti, L.; Giannaccini, G.; Felicioli, A. Queen Caging and Oxalic Acid Treatment: Combined Effect on Vitellogenin Content and Enzyme Activities in the First Post-Treatment Workers and Drones, Apis mellifera L. Animals 2022, 12, 3121. https://doi.org/10.3390/ani12223121
Sagona S, Coppola F, Nanetti A, Cardaio I, Tafi E, Palego L, Betti L, Giannaccini G, Felicioli A. Queen Caging and Oxalic Acid Treatment: Combined Effect on Vitellogenin Content and Enzyme Activities in the First Post-Treatment Workers and Drones, Apis mellifera L. Animals. 2022; 12(22):3121. https://doi.org/10.3390/ani12223121
Chicago/Turabian StyleSagona, Simona, Francesca Coppola, Antonio Nanetti, Ilaria Cardaio, Elena Tafi, Lionella Palego, Laura Betti, Gino Giannaccini, and Antonio Felicioli. 2022. "Queen Caging and Oxalic Acid Treatment: Combined Effect on Vitellogenin Content and Enzyme Activities in the First Post-Treatment Workers and Drones, Apis mellifera L." Animals 12, no. 22: 3121. https://doi.org/10.3390/ani12223121
APA StyleSagona, S., Coppola, F., Nanetti, A., Cardaio, I., Tafi, E., Palego, L., Betti, L., Giannaccini, G., & Felicioli, A. (2022). Queen Caging and Oxalic Acid Treatment: Combined Effect on Vitellogenin Content and Enzyme Activities in the First Post-Treatment Workers and Drones, Apis mellifera L. Animals, 12(22), 3121. https://doi.org/10.3390/ani12223121







