Stress Assessment of Wild Boar (Sus scrofa) in Corral-Style Traps Using Serum Cortisol Levels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Trapping
2.3. Sampling
2.4. Laboratory Analysis—SPE
2.5. Laboratory Analysis—RIA
2.6. Statistical Analysis
3. Results
3.1. Pre-Study
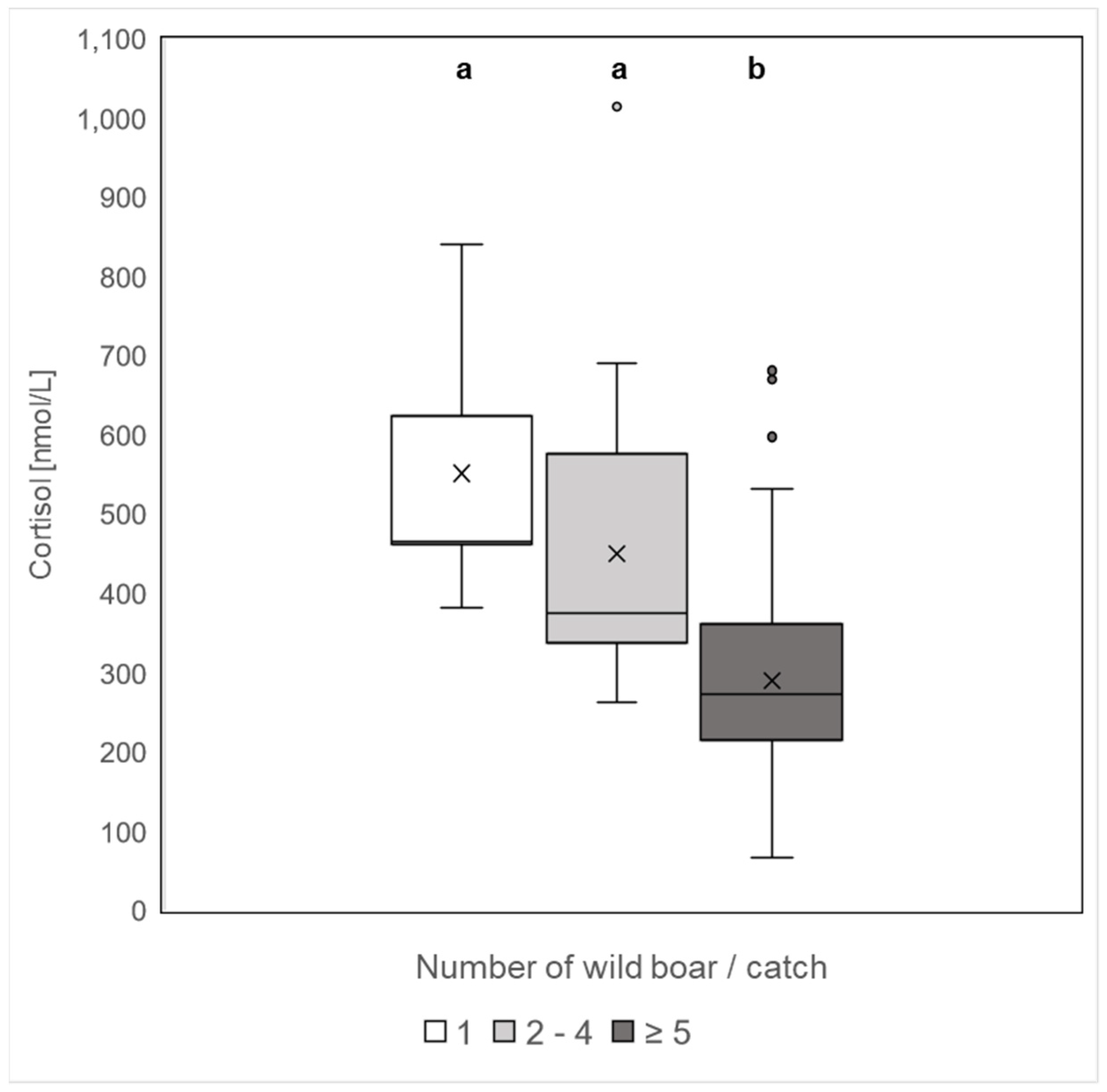
3.2. Differences between Hunting Methods and Group Size
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Da Rosa, C.A.; Wallau, M.O.; Pedrosa, F. Hunting as the main technique used to control wild pigs in Brazil. Wildl. Soc. Bull. 2018, 42, 111–118. [Google Scholar] [CrossRef]
- Keuling, O.; Strauß, E.; Siebert, U. How Do Hunters Hunt Wild Boar? Survey on Wild Boar Hunting Methods in the Federal State of Lower Saxony. Animals 2021, 11, 2658. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, T.; Kamerov, P.; Stefanov, D.; Depner, K. Trapping as an alternative method of eradicating classical swine fever in a wild boar population in Bulgaria. Rev. Sci. Tech. Off. Int. Epiz. 2011, 30, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Zani, L.; Dietze, K.; Dimova, Z.; Forth, J.H.; Denev, D.; Depner, K.; Alexandrov, T. African Swine Fever in a Bulgarian Backyard Farm—A Case Report. Vet. Sci. 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Śmietanka, K.; Woźniakowski, G.; Kozak, E.; Niemczuk, K.; Frączyk, M.; Bocian, Ł.; Kowalczyk, A.; Pejsak, Z. African Swine Fever Epidemic, Poland, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1201–1207. [Google Scholar] [CrossRef]
- More, S.; Miranda, M.A.; Bicout, D.; Bøtner, A.; Butterworth, A.; Calistri, P.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Michel, V.; et al. African swine fever in wild boar. EFS2 2018, 16, e05344. [Google Scholar] [CrossRef]
- Iossa, G.; Soulsbury, C.D.; Harris, S. Mammal trapping: A review of animal welfare standards of killing and restraining traps. Anim. Welf. 2007, 16, 335–352. [Google Scholar]
- Littin, K.E.; Mellor, D. Strategic animal welfare issues: Ethical and animal welfare issues arising from the killing of wildlife for disease control and environmental reasons. Rev. Sci. Tech. Off. Int. Epiz. 2005, 24, 767–782. [Google Scholar] [CrossRef]
- Anonymous. Agreement on International Humane Trapping Standards between the European Community, Canada and the Russian Federation. Off. J. Eur. Communities 1998, 42, 43–57. [Google Scholar]
- International Organization for Standardization. Animal (Mammal) Traps—Part 5: Methods for Testing Restraining Traps (ISO 10990-5); International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- Proulx, G.; Cattet, M.; Serfass, T.L.; Baker, S.E. Updating the AIHTS Trapping Standards to Improve Animal Welfare and Capture Efficiency and Selectivity. Animals 2020, 10, 1262. [Google Scholar] [CrossRef]
- Fahlman, Å.; Lindsjö, J.; Norling, T.A.; Kjellander, P.; Ågren, E.O.; Bergvall, U.A. Wild boar behaviour during live-trap capture in a corral-style trap: Implications for animal welfare. Acta Vet. Scand. 2020, 62, 59. [Google Scholar] [CrossRef]
- Conejero, C.; López-Olvera, J.R.; González-Crespo, C.; Ráez-Bravo, A.; Castillo-Contreras, R.; Tampach, S.; Velarde, R.; Mentaberre, G. Assessing mammal trapping standards in wild boar drop-net capture. Sci. Rep. 2022, 12, 15090. [Google Scholar] [CrossRef]
- Fraser, D.; Fraser, A.F.; Ritchie, J. The Term “Stress” in a Veterinary Context. Br. Vet. J. 1975, 131, 653–662. [Google Scholar] [CrossRef]
- Fried, T.H. Stress: What Is It and How Can It Be Quantified? Int. J. Study Anim. Probl. 1980, 1, 366–374. [Google Scholar]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Hattingh, J.; Pitts, N.I.; Ganhao, M.F. Immediate response to repeated capture and handling of wild impala. J. Exp. Zool. 1988, 248, 109–112. [Google Scholar] [CrossRef]
- Casas-Díaz, E.; Closa-Sebastià, F.; Marco, I.; Lavín, S.; Bach-Raich, E.; Cuenca, R. Hematologic and biochemical reference intervals for Wild Boar (Sus scrofa) captured by cage trap. Vet. Clin. Pathol. 2015, 44, 215–222. [Google Scholar] [CrossRef]
- Gentsch, R.P.; Kjellander, P.; Röken, B.O. Cortisol response of wild ungulates to trauma situations: Hunting is not necessarily the worst stressor. Eur. J. Wildl. Res. 2018, 64, 11. [Google Scholar] [CrossRef]
- Güldenpfennig, J.; Schmicke, M.; Hoedemaker, M.; Siebert, U.; Keuling, O. An approach to assess stress in response to drive hunts using cortisol levels of wild boar (Sus scrofa). Sci. Rep. 2021, 11, 16381. [Google Scholar] [CrossRef]
- Hamilton, G.D.; Weeks, H.P. Cortisol and aldosterone comparisons of cottontail rabbits collected by shooting, trapping, and falconry. J. Wildl. Dis. 1985, 21, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.J.D.; Anderson, E.; Foggin, C.; Kock, M.; Tiran, E. Plasma cortisol as an indicator of stress due to capture and translocation in wildlife species. Vet. Rec. 1995, 136, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.E.; Ågren, E.; Amundin, M.; Röken, B.; Palme, R.; Mörner, T. Behavioral and Physiological Responses of Trap-Induced Stress in European Badgers. J. Wildl. Manag. 2006, 70, 884–891. [Google Scholar] [CrossRef]
- Torres-Blas, I.; Mentaberre, G.; Castillo-Contreras, R.; Fernández-Aguilar, X.; Conejero, C.; Valldeperes, M.; González-Crespo, C.; Colom-Cadena, A.; Lavín, S.; López-Olvera, J.R. Assessing methods to live-capture wild boars (Sus scrofa) in urban and peri-urban environments. Vet. Rec. 2020, 187, e85. [Google Scholar] [CrossRef]
- Romero, M.L. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002, 128, 1–24. [Google Scholar] [CrossRef]
- Spencer, G.S. Circadian variation of somatomedin and cortisol in pigs. Horm. Metab. Res. 1979, 11, 586–587. [Google Scholar] [CrossRef]
- Hay, M.; Meunier-Salaün, M.C.; Brulaud, F.; Monnier, M.; Mormède, P. Assessment of hypothalamic-pituitary-adrenal axis and sympathetic nervous system activity in pregnant sows through the measurement of glucocorticoids and catecholamines in urine. J. Anim. Sci. 2000, 78, 420–428. [Google Scholar] [CrossRef]
- Marple, D.N.; Aberle, E.D.; Forrest, J.C.; Blake, W.H.; Judge, M.D. Effects of humidity and temperature on porcine plasma adrenal corticoids, ACTH and growth hormone levels. J. Anim. Sci. 1972, 34, 809–812. [Google Scholar] [CrossRef][Green Version]
- Ruis, M. The Circadian Rhythm of Salivary Cortisol in Growing Pigs: Effects of Age, Gender, and Stress. Physiol. Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
- Hennessy, D.P.; Stelmasiak, T.; Johnston, N.E.; Jackson, P.N.; Outch, K.H. Consistent capacity for adrenocortical response to ACTH administration in pigs. Am. J. Vet. Res. 1988, 49, 1276–1283. [Google Scholar]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Sibug, R.M.; Helmerhorst, F.M.; Schmidt, M.V.; Schmidt, M. Stress, genes and the mechanism of programming the brain for later life. Neurosci. Biobehav. Rev. 2005, 29, 271–281. [Google Scholar] [CrossRef]
- Romano, M.C.; Rodas, A.Z.; Valdez, R.A.; Hernández, S.E.; Galindo, F.; Canales, D.; Brousset, D.M. Stress in wildlife species: Noninvasive monitoring of glucocorticoids. Neuroimmunomodulation 2010, 17, 209–212. [Google Scholar] [CrossRef]
- Bateson, P.; Bradshaw, E.L. The effects of wound site and blood collection method on biochemical measures obtained from wild, free-ranging red deer (Cervus elaphus) shot by rifle. J. Zool. 2000, 252, 285–292. [Google Scholar] [CrossRef]
- Muñoz, P.M.; Boadella, M.; Arnal, M.; de Miguel, M.J.; Revilla, M.; Martínez, D.; Vicente, J.; Acevedo, P.; Oleaga, A.; Ruiz-Fons, F.; et al. Spatial distribution and risk factors of Brucellosis in Iberian wild ungulates. BMC Infec. Dis. 2010, 10, 46. [Google Scholar] [CrossRef]
- Boadella, M.; Gortázar, C. Effect of haemolysis and repeated freeze-thawing cycles on wild boar serum antibody testing by ELISA. BMC Res. Notes 2011, 4, 498. [Google Scholar] [CrossRef]
- Bateson, P.; Bradshaw, E.L. Physiological effects of hunting red deer (Cervus elaphus). Proc. Biol. Sci. 1997, 264, 1707–1714. [Google Scholar] [CrossRef]
- Wudy, S.A.; Schuler, G.; Sánchez-Guijo, A.; Hartmann, M.F. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018, 179, 88–103. [Google Scholar] [CrossRef]
- Newman, A.E.M.; Chin, E.H.; Schmidt, K.L.; Bond, L.; Wynne-Edwards, K.E.; Soma, K.K. Analysis of steroids in songbird plasma and brain by coupling solid phase extraction to radioimmunoassay. Gen. Comp. Endocrinol. 2008, 155, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Koren, L.; Ng, E.S.M.; Soma, K.K.; Wynne-Edwards, K.E. Sample preparation and liquid chromatography-tandem mass spectrometry for multiple steroids in mammalian and avian circulation. PLoS ONE 2012, 7, e32496. [Google Scholar] [CrossRef] [PubMed]
- Stroud, L.R.; Solomon, C.; Shenassa, E.; Papandonatos, G.; Niaura, R.; Lipsitt, L.P.; Lewinn, K.; Buka, S.L. Long-term stability of maternal prenatal steroid hormones from the National Collaborative Perinatal Project: Still valid after all these years. Psychoneuroendocrinology 2007, 32, 140–150. [Google Scholar] [CrossRef][Green Version]
- Reimers, T.J.; McCann, J.P.; Cowan, R.G. Effects of storage times and temperatures on T3, T4, LH, prolactin, insulin, cortisol and progesterone concentrations in blood samples from cows. J. Anim. Sci. 1983, 57, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Greiser, G.; Krüger, S.; Martin, I.; Thelke, F. Status und Entwicklung Ausgewählter Wildtierarten in Deutschland: Jahresbericht 2018; Wildtier-Informationssystem der Länder Deutschlands (WILD); Deutscher Jagdverband: Berlin, Germany, 2020. [Google Scholar]
- World Health Organisation. Use of Anticoagulants in Diagnostic Laboratory Investigations: Stability of Blood, Plasma and Serum Samples; WHO: Geneva, Switzerland, 2002.
- Schuler, G.; Dezhkam, Y.; Bingsohn, L.; Hoffmann, B.; Failing, K.; Galuska, C.E.; Hartmann, M.F.; Sánchez-Guijo, A.; Wudy, S.A. Free and sulfated steroids secretion in postpubertal boars (Sus scrofa domestica). Reproduction 2014, 148, 303–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richert-Hanauer, U.; Failing, K.; Hoffmann, B.; Moellmann, U. Untersuchungen zum Verlauf der Cortisolwerte im Blut beim Schaf während des Zyklus und der Trachtigkeit. Dtsch. Tierärztliche Wochenschau 1988, 95, 374–376. [Google Scholar]
- Statistical Analysis System Institute Inc. SAS® Statistical Analysis System (Base SAS® 9.4); Statistical Analysis System Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Marks, C.A. Haematological and biochemical responses of red foxes (Vulpes vulpes) to different capture methods and shooting. Anim. Welf. 2010, 19, 223–234. [Google Scholar]
- Huber, N.; Vetter, S.G.; Evans, A.L.; Kjellander, P.; Küker, S.; Bergvall, U.A.; Arnemo, J.M. Quantifying capture stress in free ranging European roe deer (Capreolus capreolus). BMC Vet. Res. 2017, 13, 127. [Google Scholar] [CrossRef]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Pub: Wallingford, UK; New York, NY, USA, 2000. [Google Scholar]
- Rash, J.M.; Jerkunica, I.; Sgoutas, D.S. Lipid interference in steroid radioimmunoassay. Clin. Chem. 1980, 26, 84–88. [Google Scholar] [CrossRef]
- Sweitzer, R.A.; Gonzales, B.J.; Gardner, I.; Van Vuren, D.; Waithman, J.D.; Boyce, W.M. A modified panel trap and immobilization technique for capturing multiple wild pig. Wildl. Soc. Bull. 1997, 25, 699–705. [Google Scholar]
- Ruis, M.A.; te Brake, J.H.; Engel, B.; Buist, W.G.; Blokhuis, H.J.; Koolhaas, J.M. Adaptation to social isolation. Physiol. Behav. 2001, 73, 541–551. [Google Scholar] [CrossRef]


| Storage Temperature | Time Point of Centrifugation (Storage Time) | Mean | |||
|---|---|---|---|---|---|
| t0 < 15 min | t1 = 33 h | t2 = 57 h | t3 = 87 h | ||
| Cooled (4–7 °C) | 406.4 ± 141.3 | 387.5 ± 141.6 | 370.7 ± 176.9 | 384.6 ± 160.4 | 380.9 ± 154.8 |
| Room temperature (18–25 °C) | 346.2 ± 115.9 | 341.4 ± 161.7 | 340.1 ± 158.8 | 342.6 ± 141.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westhoff, K.M.; Fetzer, A.; Büttner, K.; Schuler, G.; Lang, J.; Lierz, M. Stress Assessment of Wild Boar (Sus scrofa) in Corral-Style Traps Using Serum Cortisol Levels. Animals 2022, 12, 3008. https://doi.org/10.3390/ani12213008
Westhoff KM, Fetzer A, Büttner K, Schuler G, Lang J, Lierz M. Stress Assessment of Wild Boar (Sus scrofa) in Corral-Style Traps Using Serum Cortisol Levels. Animals. 2022; 12(21):3008. https://doi.org/10.3390/ani12213008
Chicago/Turabian StyleWesthoff, Katharina M., André Fetzer, Kathrin Büttner, Gerhard Schuler, Johannes Lang, and Michael Lierz. 2022. "Stress Assessment of Wild Boar (Sus scrofa) in Corral-Style Traps Using Serum Cortisol Levels" Animals 12, no. 21: 3008. https://doi.org/10.3390/ani12213008
APA StyleWesthoff, K. M., Fetzer, A., Büttner, K., Schuler, G., Lang, J., & Lierz, M. (2022). Stress Assessment of Wild Boar (Sus scrofa) in Corral-Style Traps Using Serum Cortisol Levels. Animals, 12(21), 3008. https://doi.org/10.3390/ani12213008





