The Effects of Sedation with Dexmedetomidine–Butorphanol and Anesthesia with Propofol–Isoflurane on Feline Grimace Scale© Scores
Abstract
Simple Summary
Abstract
1. Introduction
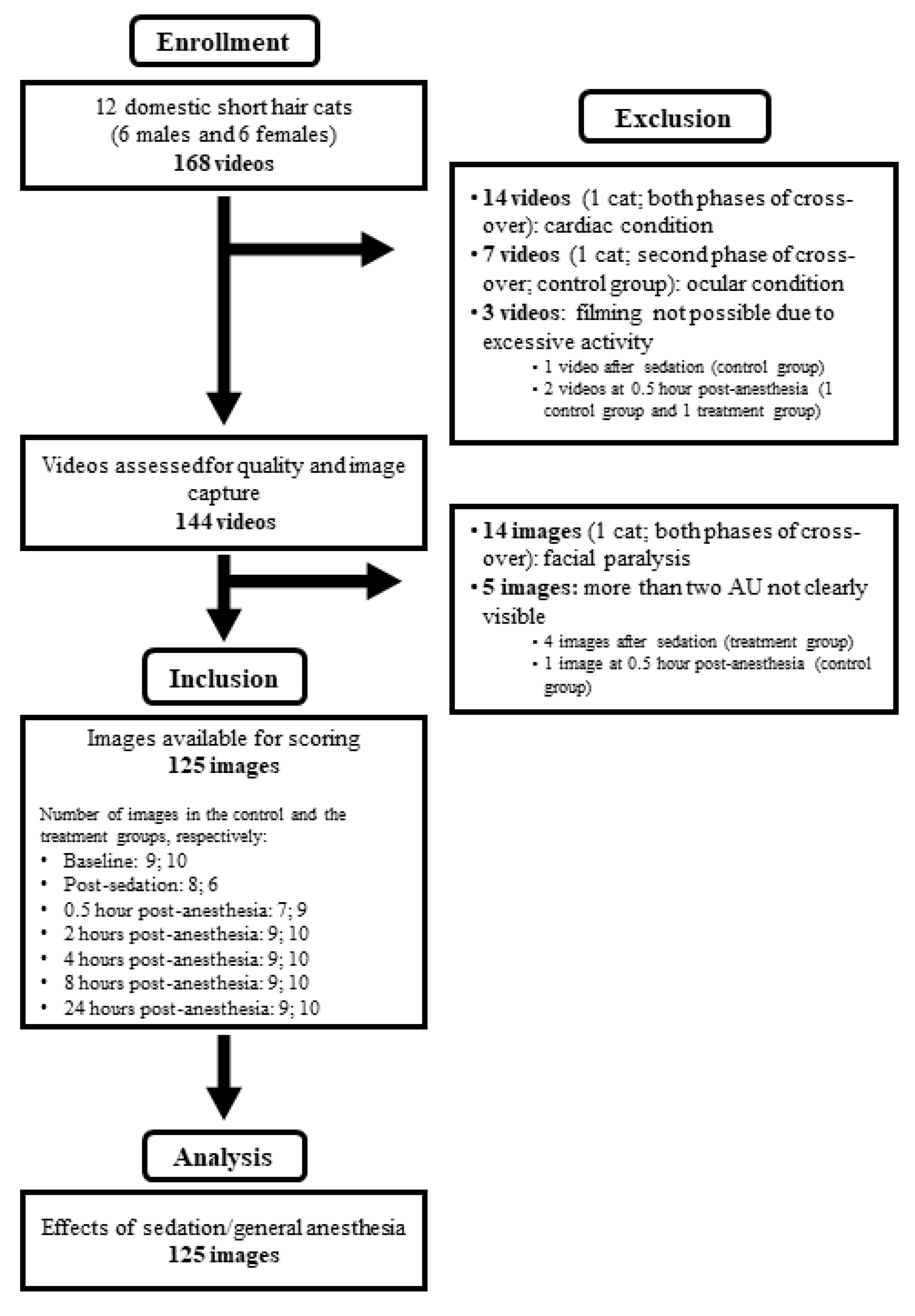
2. Materials and Methods
2.1. Animals
2.2. Sedation and Anesthesia
2.3. Assessment of Sedation
2.4. Video Recording and Image Capture
2.5. Image Scoring
3. Statistical Analyses
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steagall, P.V.; Monteiro, B.P. Acute pain in cats: Recent advances in clinical assessment. J. Feline Med. Surg. 2019, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Steagall, P.V. Analgesia: What Makes Cats Different/Challenging and What Is Critical for Cats? Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 749–767. [Google Scholar] [CrossRef]
- Reid, J.; Scott, E.M.; Calvo, G.; Nolan, A.M. Definitive Glasgow acute pain scale for cats: Validation and intervention level. Vet. Rec. 2017, 180, 449. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, M.C.; Watanabe, R.; Leung, V.S.Y.; Monteiro, B.P.; O’Toole, E.; Pang, D.S.J.; Steagull, P.V. Facial expressions of pain in cats: The development and validation of a Feline Grimace Scale. Sci. Rep. 2019, 9, 19128. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; de Oliveira, A.R.; de Lima, M.T.; Trindade, P.H.E.; Steagall, P.V.; Luna, S.P.L. Clinical validation of the short and long UNESP-Botucatu scales for feline pain assessment. PeerJ 2021, 9, e11225. [Google Scholar] [CrossRef]
- Watanabe, R.; Doodnaught, G.M.; Evangelista, M.C.; Monteiro, B.P.; Ruel, H.L.M.; Steagall, P.V. Inter-Rater Reliability of the Feline Grimace Scale in Cats Undergoing Dental Extractions. Front. Vet. Sci. 2020, 7, 302. [Google Scholar] [CrossRef]
- Evangelista, M.C.; Benito, J.; Monteiro, B.P.; Watanabe, R.; Doodnaught, G.M.; Pang, D.S.J. Clinical applicability of the Feline Grimace Scale: Real-time versus image scoring and the influence of sedation and surgery. PeerJ 2020, 8, e8967. [Google Scholar] [CrossRef]
- Evangelista, M.C.; Steagall, P.V. Agreement and reliability of the Feline Grimace Scale among cat owners, veterinarians, veterinary students and nurses. Sci. Rep. 2021, 11, 5262. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Lee, N.H.Y.; Steagall, P.V. Can cat carers reliably assess acute pain in cats using the Feline Grimace Scale? A large bilingual global survey. In Proceedings of the Association of Veterinary Anaesthetists Spring Meeting 2022, Nafplio, Greece, 18–20 May 2022. [Google Scholar]
- Benito, J.; Steagall, P.V. Postoperative analgesia between non-pregnant healthy cats versus pregnant or cats with upper respiratory tract disease. Vet. Anaesth. Analg. 2017, 44, 195.e4. [Google Scholar] [CrossRef]
- Buisman, M.; Hasiuk, M.M.M.; Gunn, M.; Pang, D.S.J. The influence of demeanor on scores from two validated feline pain assessment scales during the perioperative period. Vet. Anaesth. Analg. 2017, 44, 646–655. [Google Scholar] [CrossRef]
- Ansah, O.B.; Raekallio, M.; Vainio, O. Comparison of three doses of dexmedetomidine with medetomidine in cats following intramuscular administration. J. Vet. Pharmacol. Ther. 1998, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Nagore, L.; Soler, C.; Gil, L.; Serra, I.; Soler, G.; Redondo, J.I. Sedative effects of dexmedetomidine, dexmedetomidine-pethidine and dexmedetomidine-butorphanol in cats. J. Vet. Pharmacol. Ther. 2013, 36, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Steagall, P.V.; Taylor, P.M.; Rodrigues, L.C.; Ferreira, T.H.; Minto, B.W.; Aguiar, A.J. Analgesia for cats after ovariohysterectomy with either buprenorphine or carprofen alone or in combination. Vet. Rec. 2009, 164, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, R.J.; Trimble, T.A.; Leece, E.A.; Vettorato, E. Comparison of intramuscular butorphanol and buprenorphine combined with dexmedetomidine for sedation in cats. J. Feline Med. Surg. 2018, 20, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for recognition, assessment and treatment of pain: WSAVA Global Pain Council members and co-authors of this document. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef]
- Grubb, T.; Sager, J.; Gaynor, J.S.; Montgomery, E.; Parker, J.A.; Shafford, H.; Tearney, C. 2020 AAHA Anesthesia and Monitoring Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2020, 56, 59–82. [Google Scholar] [CrossRef]
- Escobar, A.; Pypendop, B.H.; Siao, K.T.; Stanley, S.D.; Ilkiw, J.E. Effect of dexmedetomidine on the minimum alveolar concentration of isoflurane in cats. J. Vet. Pharmacol. Ther. 2012, 35, 163–168. [Google Scholar] [CrossRef]
- Holaday, D.A.; Fiserova-Bergerova, V.; Latto, I.P.; Zumbiel, M.A. Resistance of isoflurane to biotransformation in man. Anesthesiology 1975, 43, 325–332. [Google Scholar] [CrossRef]
- Miller, A.; Kitson, G.; Skalkoyannis, B.; Leach, M. The effect of isoflurane anaesthesia and buprenorphine on the mouse grimace scale and behaviour in CBA and DBA/2 mice. Appl. Anim. Behav. Sci. 2015, 172, 58–62. [Google Scholar] [CrossRef]
- Miller, A.L.; Golledge, H.D.; Leach, M.C. The Influence of Isoflurane Anaesthesia on the Rat Grimace Scale. PLoS ONE 2016, 11, e0166652. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, H.; Safar, P.; Miller, A. Delayed recovery of behaviour after anesthesia in rats. Resuscitation 1984, 12, 213–221. [Google Scholar] [CrossRef]
- Wiederstein, I.; Auer, U.; Moens, Y. Laryngeal mask airway insertion requires less propofol than endotracheal intubation in dogs. Vet. Anaesth. Analg. 2006, 33, 201–206. [Google Scholar] [CrossRef] [PubMed]



| Time Point | Treatment Group | DIVAS | p Value between Groups | p Value Compared with Baseline |
|---|---|---|---|---|
| Baseline | Control | 0.0 (0.0–0.0) | 0.54 | - |
| Dexmedetomidine–butorphanol | 0.0 (0.0–0.0) | - | ||
| Post-sedation | Control | 0.0 (0.0–0.0) | <0.0001 | 0.31 |
| Dexmedetomidine–butorphanol | 91.2 (18.0–99.6) | <0.0001 | ||
| 0.5 h post-anesthesia | Control | 17.0 (9.5–91.2) | 0.42 | <0.0001 |
| Dexmedetomidine–butorphanol | 9.5 (0.0–36.0) | <0.0001 | ||
| 2 h post-anesthesia | Control | 0.0 (0.0–5.3) | 0.72 | 0.47 |
| Dexmedetomidine–butorphanol | 0.0 (0.0–9.5) | 0.56 | ||
| 4 h post-anesthesia | Control | 0.0 (0.0–4.2) | 0.99 | 0.61 |
| Dexmedetomidine–butorphanol | 0.0 (0.0–0.0) | 0.75 | ||
| 8 h post-anesthesia | Control | 0.0 (0.0–0.0) | 1.00 | 0.60 |
| Dexmedetomidine–butorphanol | 0.0 (0.0–0.0) | 0.79 | ||
| 24 h post-anesthesia | Control | 0.0 (0.0–0.0) | 1.00 | 0.61 |
| Dexmedetomidine–butorphanol | 0.0 (0.0–0.0) | 0.79 |
| Time Point | Ear Position | Orbital Tightening | Muzzle Tension | Whisker Changes | Head Position | Total FGS Scores (Ratio) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Dex-But | p Value between Groups | Control | Dex-But | p Value between Groups | Control | Dex-But | p Value between Groups | Control | Dex-But | p Value between Groups | Control | Dex-But | p Value between Groups | Control | Dex-But | p Value between Groups | |||||||||||||
| Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | Score | p Value Baseline | |||||||
| Baseline | 0.11 (0.09) | 0.08 (0.08) | 0.77 | 0.03 (0.07) | 0.00 (0.07) | 0.79 | 0.61 (0.17) | 0.45 (0.16) | 0.30 | 0.47 (0.12) | 0.51 (0.13) | 0.74 | 0.14 (0.16) | 0.13 (0.13) | 0.92 | 0.14 (0.04) | 0.11 (0.04) | |||||||||||||
| Post-sedation | 0.28 (0.09) | 0.21 | 1.0 (0.11) | < 0.0001 | < 0.0001 | 0.00 (0.08) | 0.80 | 0.92 (0.09) | < 0.0001 | < 0.0001 | 0.40 (0.17) | 0.21 | 0.91 (0.19) | 0.02 | 0.01 | 0.31 (0.14) | 0.20 | 1.13 (0.14) | < 0.0001 | < 0.0001 | 0.38 (0.17) | 0.22 | 1.48 (0.19) | < 0.0001 | < 0.0001 | 0.14 (0.04) | 0.50 | 0.51 (0.05) | < 0.0001 | < 0.0001 |
| 0.5 hour post-anesthesia | 0.43 (0.12) | 0.04 * | 0.29 (0.12) | 0.09 | 0.31 | 0.60 (0.11) | < 0.0001 | 0.45 (0.11) | 0.002 | 0.32 | 0.68 (0.21) | 0.78 | 0.52 (0.21) | 0.72 | 0.44 | 0.87 (0.12) | 0.001 | 0.76 (0.13) | 0.04 * | 0.43 | 0.77 (0.13) | < 0.0001 | 0.36 (0.14) | 0.14 | 0.03 * | 0.34 (0.05) | < 0.0001 | 0.23 (0.05) | 0.01 * | 0.03 * |
| 2 hours post-anesthesia | 0.22 (0.11) | 0.66 | 0.10 (0.11) | 0.84 | 0.34 | 0.15 (0.11) | 0.43 | 0.13 (0.10) | 0.35 | 0.88 | 0.79 (0.20) | 0.39 | 0.68 (0.20) | 0.22 | 0.53 | 0.69 (0.12) | 0.07 | 0.64 (0.12) | 0.25 | 0.67 | 0.17 (0.13) | 0.86 | 0.20 (0.13) | 0.61 | 0.86 | 0.20 (0.05) | 0.23 | 0.16 (0.04) | 0.17 | 0.41 |
| 4 hours post-anesthesia | 0.27 (0.11) | 0.38 | 0.10 (0.11) | 0.84 | 0.17 | 0.29 (0.11) | 0.08 | 0.00 (0.10) | 1.00 | 0.04 * | 0.85 (0.20) | 0.25 | 0.58 (0.20) | 0.50 | 0.15 | 0.67 (0.12) | 0.08 | 0.52 (0.12) | 0.82 | 0.20 | 0.20 (0.13) | 0.72 | 0.18 (0.13) | 0.73 | 0.89 | 0.23 (0.05) | 0.08 | 0.13 (0.04) | 0.57 | 0.03 * |
| 8 hours post-anesthesia | 0.13 (0.11) | 0.83 | 0.05 (0.11) | 0.84 | 0.49 | 0.23 (0.11) | 0.17 | 0.03 (0.10) | 0.85 | 0.14 | 0.90 (0.20) | 0.15 | 0.63 (0.20) | 0.34 | 0.14 | 0.69 (0.12) | 0.07 | 0.61 (0.12) | 0.36 | 0.51 | 0.28 (0.13) | 0.37 | 0.13 (0.13) | 1.0 | 0.36 | 0.22 (0.05) | 0.12 | 0.13 (0.04) | 0.51 | 0.07 |
| 24 hours post-anesthesia | 0.11 (0.11) | 0.66 | 0.17 (0.11) | 0.41 | 0.60 | 0.09 (0.11) | 0.69 | 0.15 (0.10) | 0.26 | 0.67 | 0.60 (0.20) | 0.89 | 0.60 (0.20) | 0.41 | 0.99 | 0.34 (0.12) | 0.29 | 0.62 (0.12) | 0.30 | 0.02 * | 0.12 (0.13) | 0.86 | 0.23 (0.13) | 0.50 | 0.52 | 0.13 (0.05) | 0.66 | 0.17 (0.04) | 0.12 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, R.; Monteiro, B.P.; Ruel, H.L.M.; Cheng, A.; Marangoni, S.; Steagall, P.V. The Effects of Sedation with Dexmedetomidine–Butorphanol and Anesthesia with Propofol–Isoflurane on Feline Grimace Scale© Scores. Animals 2022, 12, 2914. https://doi.org/10.3390/ani12212914
Watanabe R, Monteiro BP, Ruel HLM, Cheng A, Marangoni S, Steagall PV. The Effects of Sedation with Dexmedetomidine–Butorphanol and Anesthesia with Propofol–Isoflurane on Feline Grimace Scale© Scores. Animals. 2022; 12(21):2914. https://doi.org/10.3390/ani12212914
Chicago/Turabian StyleWatanabe, Ryota, Beatriz P. Monteiro, Hélène L. M. Ruel, Alice Cheng, Sabrine Marangoni, and Paulo V. Steagall. 2022. "The Effects of Sedation with Dexmedetomidine–Butorphanol and Anesthesia with Propofol–Isoflurane on Feline Grimace Scale© Scores" Animals 12, no. 21: 2914. https://doi.org/10.3390/ani12212914
APA StyleWatanabe, R., Monteiro, B. P., Ruel, H. L. M., Cheng, A., Marangoni, S., & Steagall, P. V. (2022). The Effects of Sedation with Dexmedetomidine–Butorphanol and Anesthesia with Propofol–Isoflurane on Feline Grimace Scale© Scores. Animals, 12(21), 2914. https://doi.org/10.3390/ani12212914





