A New Species of the Genus Microhyla (Amphibia: Anura: Microhylidae) from the Dabie Mountains, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
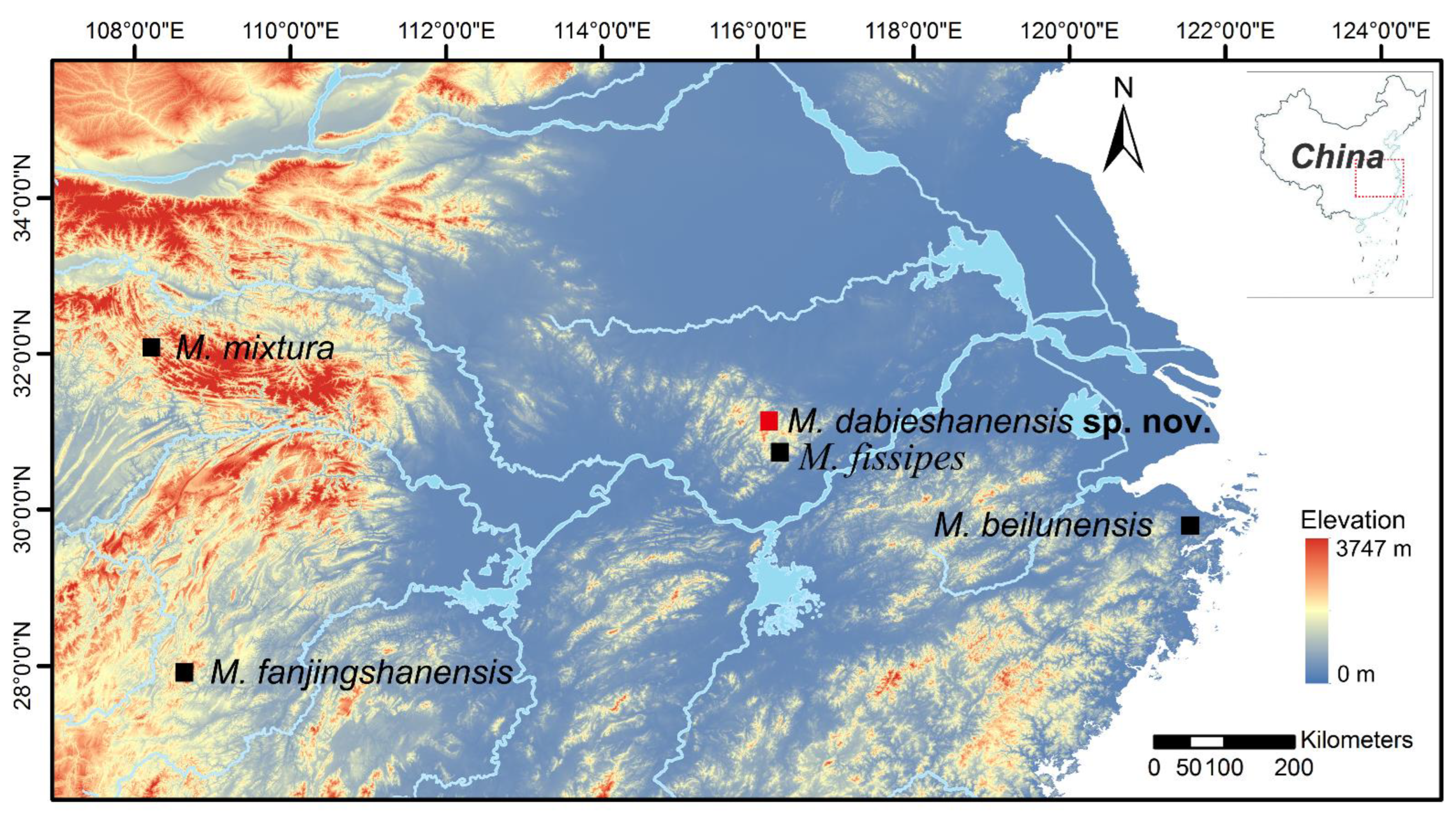
2.1. Sampling
2.2. Molecular Phylogenetic Analyses
2.3. Phylogenetic Analyses
2.4. Species Delimitation Analysis
2.5. Morphological Analyses
| SVL | snout–vent length; |
| HDL | head length; |
| HDW | maximum head width; |
| SNL | snout length; |
| ED | eye diameter; |
| UEW | upper eyelid width |
| IOD | interorbital distance; |
| IND | internasal distance; |
| LAL | length of lower arm and hand; |
| LW | lower arm width; |
| HAL | hand length; |
| HLL | hindlimb length; |
| TW | tibia width; |
| TFL | length of foot and tarsus and; |
| FL | foot length. |
2.6. Bioacoustics Analyses
3. Results
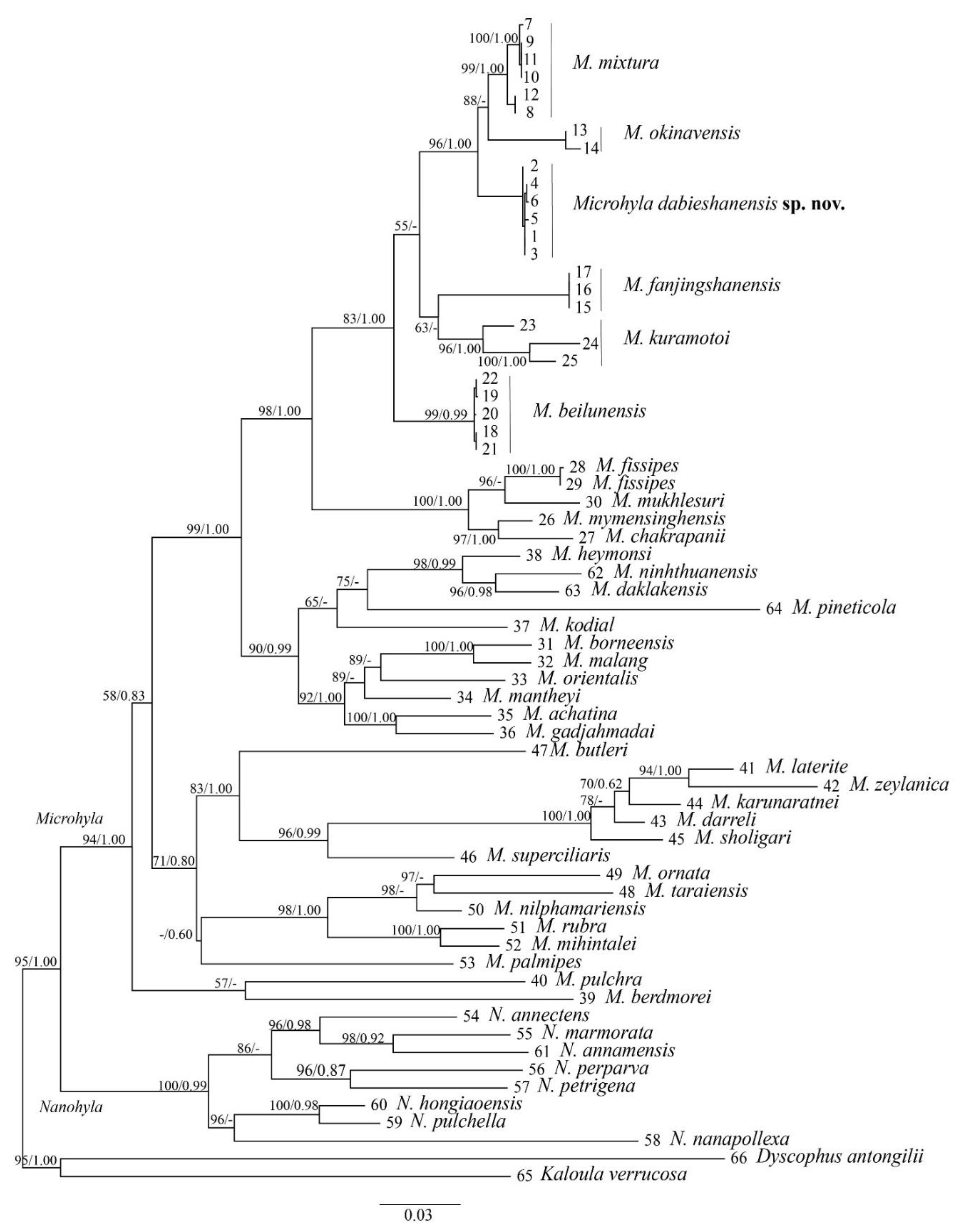
3.1. Phylogeny Analysis and Species Delimitation
3.2. Morphology
3.3. Bioacoustics
3.4. Taxonomic Account
3.4.1. Diagnosis
3.4.2. Holotype Description
3.4.3. Holotype Coloration
3.4.4. Intraspecific Morphological Variations
3.4.5. Sexual Dimorphism
3.4.6. Distribution and Habit
3.4.7. Morphological Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.1. 2022. Electronic Database Accessible. American Museum of Natural History, New York. Available online: https://amphibiansoftheworld.amnh.org/ (accessed on 22 February 2022).
- Gorin, V.A.; Solovyeva, E.N.; Hasan, M.; Okamiya, H.; Karunarathna, D.M.S.S.; Pawangkhanant, P.; Silva, A.D.; Juthong, W.; Milto, K.D.; Nguyue, L.T.; et al. A little frog leaps a long way: Compounded colonizations of the Indian Subcontinent discovered in the tiny Oriental frog genus Microhyla (Amphibia: Microhylidae). PeerJ 2020, 8, e9411. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.C.; Luong, M.A.; Nguyen, Q.T.; Orlov, N.; Chen, Y.; Wang, B.; Jiang, J.P. A new species of Microhyla (Amphibia: Anura: Microhylidae) from Langbian Plateau, Central Vietnam. Asian Herpetol. Res. 2020, 11, 161–182. [Google Scholar]
- Zhang, M.H.; Fei, L.; Ye, C.Y.; Wang, Y.F.; Wang, B.; Jiang, J.P. A New Species of Genus Microhyla (Amphibia: Anura: Microhylidae) from Zhejiang Province, China. Asian Herpetol. Res. 2018, 9, 135–148. [Google Scholar]
- Li, S.Z.; Zhang, M.H.; Xu, N.; Lv, J.; Jiang, J.P.; Liu, J.; Wei, G.; Wang, B. A new species of the genus Microhyla (Amphibia: Anura: Microhylidae) from Guizhou Province, China. Zootaxa 2019, 4624, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Poyarkov, N.A.; Nguyen, T.T.; Nguyen, T.A.; Tran, V.H.; Gorin, V.A.; Murphy, R.W.; Nguyen, S.N. A new species of the genus Microhyla Tschudi, 1838 (Amphibia: Anura: Microhylidae) from Tay Nguyen Plateau, Central Vietnam. Zootaxa 2019, 4543, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Hamidy, A.; Belabut, D.M.; Ahmad, N.; Panha, S.; Sudin, A.; Khonsue, W.; Oh, H.S.; Yong, H.S.; Jiang, J.P.; et al. Systematic relationships of Oriental tiny frogs of the family Microhylidae (Amphibia, Anura) as revealed by mtDNA genealogy. Mol. Phylogenetics Evol. 2011, 61, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Tominaga, A. Distinct Species Status of a Microhyla from the Yaeyama Group of the Southern Ryukyus, Japan (Amphibia, Anura, Microhylidae). Curr. Herpetol. 2020, 39, 120–136. [Google Scholar] [CrossRef]
- Gorin, V.A.; Scherz, M.D.; Korost, D.V.; Poyarkov, N.A. Consequences of parallel miniaturisation in Microhylinae (Anura, Microhylidae), with the description of a new genus of diminutive South East Asian frogs. Zoosystematics Evol. 2021, 97, 21–54. [Google Scholar] [CrossRef]
- Hoang, C.V.; Nguyen, T.T.; Ninh, H.T.; Luong, A.M.; Pham, C.T.; Nguyen, T.Q.; Orlov, N.L.; Chen, Y.H.; Wang, B.; Ziegler, T.; et al. Two new cryptic species of Microhyla Tschudi, 1838 (Amphibia, Anura, Microhylidae) related to the M. heymonsi group from central Vietnam. ZooKeys 2021, 1036, 47–74. [Google Scholar] [CrossRef]
- AmphibiaChina. The Database of Chinese Amphibians. Kunming Institute of Zoology (CAS), Kunming, Yunnan, China. Electronic Database. 2022. Available online: http://www.amphibiachina.org/ (accessed on 22 February 2022).
- Fei, L.; Hu, S.Q.; Ye, C.Y.; Huang, Y.Z. Fauna Sinica. In Amphibia, Anura; Science Press: Beijing, China, 2009; Volume 957, pp. 899–904. [Google Scholar]
- Fei, L.; Ye, C.Y.; Jiang, J.P. Colored Atlas of Chinese Amphibians and Their Distributions; Sichuan Publishing House of Science and Technology: Chengdu, China, 2012; p. 510. [Google Scholar]
- Chen, X.H.; Tao, L.K.; Shi, L.; Meng, X.P. Discovery of Microhyla mixtura in Henan Province. Chin. J. Zool. 2003, 38, 89–90. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; p. 5. [Google Scholar]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Paabo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in mammals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Che, J.; Chen, H.M.; Yang, J.X.; Jin, J.Q.; Jiang, K.E.; Yuan, Z.Y.; Murphy, R.W.; Zhang, Y.P. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Resour. 2012, 12, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Tu, N.; Yang, M.H.; Liang, D.; Zhang, P. A large-scale phylogeny of Microhylidae inferred from a combined dataset of 121 genes and 427 taxa. Mol. Phylogenetics Evol. 2018, 126, 85–91. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Mark, P.V.D.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*Phylogenetic Analysis Using Parsimony (*and Other Methods); Sinauer Associates: Sunderland, MA, USA, 2003. [Google Scholar]
- Nylander, J. MrModeltest 1.0 b. a Simplified Version of David Posada’s ôô Modeltest 3.06; Uppsala University Sweden, Department of Systematic Zoology: Uppsala, Sweden, 2003. [Google Scholar]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Hillis, D.M. Success of phylogenetic methods in the four-taxon case. Syst. Biol. 1993, 42, 247–264. [Google Scholar] [CrossRef]
- Yang, Z.H. The BPP program for species tree estimation and species delimitation. Curr. Zool. 2015, 61, 854–865. [Google Scholar] [CrossRef]
- Hu, S.Q.; Zhao, E.M.; Liu, C.C. A herpetological survey of the Tsinling and Ta-Pa Shan region. Acat Zool. Sin. 1966, 18, 57–89. [Google Scholar]
- Wu, L.; Dong, Q.; Xu, R.H. Amphibians of Guizhou Province; Guizhou People Press: Guiyang, China, 1986; p. 192. [Google Scholar]
- Peloso, P.L.; Frost, D.R.; Richards, S.J.; Rodrigues, M.T.; Donnellan, S.; Matsui, M.; Cristopher, J.; Raxworthy, S.D.; Biju; Lemmon, E.M.; et al. The impact of anchored phylogenomics and taxon sampling on phylogenetic inference in narrow-mouthed frogs (Anura, Microhylidae). Cladistics 2016, 32, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.G. A small collection of frogs from Annam collected in the years 1938–1939 by Bertil Björkegren. Ark. Zool. 1942, 34, 1–11. [Google Scholar]
- Atmaja, V.Y.; Hamidy, A.; Arisuryanti, T.; Matsui, M.; Smith, E.N. A new species of Microhyla (Anura: Microhylidae) from Sumatra, Indonesia. Treubia 2018, 45, 25–46. [Google Scholar] [CrossRef]
- Bain, R.H.; Nguyen, T.Q. Three new species of narrow-mouthed frogs (genus Microhyla) from Indochina, with comments on Microhyla annamensis and Microhyla palmipes. Copeia 2004, 507–524. [Google Scholar] [CrossRef]
- Blyth, E. Report for October meeting, 1855. J. Asiat. Soc. Bengal 1856, 24, 711–723. [Google Scholar]
- Boulenger, G.A. Descriptions of new species of reptiles and batrachians in the British Museum—Part II. Ann. Mag. Nat. Hist. Ser. 5 1884, 13, 396–398. [Google Scholar] [CrossRef]
- Boulenger, G.A. Descriptions of new Malay frogs. Ann. Mag. Nat. Hist. Ser. 6 1897, 19, 106–108. [Google Scholar] [CrossRef]
- Boulenger, G.A. Descriptions of New Batrachians and Reptiles from Larut Hills, Perak. Ann. Mag. Nat. Hist. Ser. 7 1900, 6, 186–193. [Google Scholar] [CrossRef]
- Boulenger, G.A. Descriptions of three new frogs in the collection of the British Museum. Ann. Mag. Nat. Hist. Ser. 9 1920, 6, 106–108. [Google Scholar] [CrossRef][Green Version]
- Brown, A.E. A collection of reptiles and batrachians from Borneo and the Loo Choo Islands. Proc. Acad. Nat. Sci. Phila. 1902, 54, 175–186. [Google Scholar]
- Das, I.; Yaakob, N.; Sukumaran, J. A new species of Microhyla (Anura: Microhylidae) from the Malay Peninsula. Hamadryad 2007, 31, 304–314. [Google Scholar]
- Duméril, A.M.C.; Bibron, G. Erpétologie Genérale ou Histoire Naturelle Complète des Reptiles, Tome 8; Librarie Enclyclopedique de Roret: Paris, France, 1841; p. 792. [Google Scholar]
- Dutta, S.; Ray, P. Microhyla sholigari, a new species of microhylid frog (Anura: Microhylidae) from Karnataka, India. Hamadryad 2000, 25, 38–44. [Google Scholar]
- Fernando, P.; Siriwardhane, M. Microhyla karunaratnei (Anura: Microhylidae), a new species of frog endemic to Sri Lanka. J. South Asian Nat. Hist. 1996, 2, 135–142. [Google Scholar]
- Garg, S.; Suyesh, R.; Das, A.; Jiang, J.P.; Wijayathilaka, N.; Amarasinghe, A.A.T.; Farits, A.; Vineeth, K.K.; Aravind, N.A.; Senevirathne, G.; et al. Systematic revision of Microhyla (Microhylidae) frogs of South Asia: A molecular, morphological, and acoustic assessment. Vertebr. Zool. 2019, 69, 1–71. [Google Scholar]
- Hallowell, E. Report upon the Reptilia of the North Pacific Exploring Expedition, under command of Capt. John Rogers, U.S.N 1860. Proc. Acad. Nat. Sci. Phila. 1861, 12, 480–510. [Google Scholar]
- Hasan, M.; Islam, M.M.; Kuramoto, M.; Kurabayashi, A.; Sumida, M. Description of two new species of Microhyla (Anura: Microhylidae) from Bangladesh. Zootaxa 2014, 3755, 401–418. [Google Scholar] [CrossRef]
- Howlader, M.S.A.; Nair, A.; Gopalan, S.V.; Merilä, J. A new species of Microhyla (Anura: Microhylidae) from Nilphamari, Bangladesh. PLoS ONE 2015, 10, e0119825. [Google Scholar] [CrossRef]
- Inger, R.F.; Frogner, K.J. New species of narrow-mouth frogs (genus Microhyla) from Borneo. Sarawak Mus. J. 1979, 27, 311–322. [Google Scholar]
- Inger, R.F. Four new species of frogs from Borneo. Malay. Nat. J. 1989, 42, 229–243. [Google Scholar]
- Jerdon, T.C. Catalogue of reptiles inhabiting the peninsula of India. J. Asiat. Soc. Bengal 1853, 22, 462–479. [Google Scholar]
- Khatiwada, J.R.; Shu, G.C.; Wang, S.H.; Thapa, A.; Wang, B.; Jiang, J.P. A new species of the genus Microhyla (Anura: Microhylidae) from Eastern Nepal. Zootaxa 2017, 4254, 221–239. [Google Scholar] [CrossRef]
- Matsui, M.; Hamidy, A.; Eto, K. Description of a new species of Microhyla from Bali, Indonesia (Amphibia, Anura). Zootaxa 2013, 3670, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M. Taxonomic revision of one of the Old World’s smallest frogs, with description of a new Bornean Microhyla (Amphibia, Microhylidae). Zootaxa 2011, 2814, 33–49. [Google Scholar] [CrossRef]
- Parker, H.; Osman, H.W. Frogs of the genus Microhyla from Ceylon. J. Nat. Hist. 1948, 1, 759–764. [Google Scholar] [CrossRef]
- Parker, H. The brevicipitid frogs of the genus: Microhyla. Ann. Mag. Nat. Hist. 1928, 10, 473–499. [Google Scholar] [CrossRef]
- Pillai, R. On two frogs of the family Microhylidae from Andamans including a new species. Proc. Indian Acad. Sci. Sect. B 1977, 86, 135–138. [Google Scholar] [CrossRef]
- Poyarkov, N.A.; Gorin, A.V.; Zaw, T.; Kretova, D.V.; Gogoleva, S.S.; Pawangkhanant, P.; Che, J. On the road to Mandalay: Contribution to the Microhyla Tschudi, 1838 (Amphibia: Anura: Microhylidae) fauna of Myanmar with description of two new species. Zool. Res. 2019, 40, 1–33. [Google Scholar]
- Poyarkov, N.A.; Nguyen, T.V.; Trofimets, A.V.; Gorin, V.A. A new cryptic species of the genus Microhyla (Amphibia: Microhylidae) from Langbian Plateau, Vietnam. Taprobanica J. Asian Biodivers. 2020, 9, 136–163. [Google Scholar] [CrossRef]
- Poyarkov, N.A.; Vassilieva, A.B.; Orlov, N.L.; Galoyan, E.A.; Dao, T.T.A.; Le, D.T.T.; Kretova, V.D. Taxonomy and distribution of narrow-mouth frogs of the genus Microhyla Tschudi, 1838 (Anura: Microhylidae) from Vietnam with descriptions of five new species. Russ. J. Herpetol. 2014, 21, 89–148. [Google Scholar]
- Schenkel, E. Achter Nachtrag zum Katalog der herpetologischen Sammlung des Basler Museums. Verh. Der Nat. Ges. Basel 1901, 13, 142–199. [Google Scholar]
- Seshadri, K.S.; Singal, R.; Priti, H.; Ravikanth, G.; Vidisha, M.K.; Saurabh, S.; Pratik, M.; Gururaja, K. Microhyla laterite sp. nov., a new species of Microhyla Tschudi, 1838 (Amphibia: Anura: Microhylidae) from a laterite rock formation in South West India. PLoS ONE 2016, 11, e0149727. [Google Scholar] [CrossRef] [PubMed]
- Stejneger, L. Diagnoses of eight new batrachians and reptiles from the Riu Kiu Archipelago, Japan. Proc. Biol. Soc. Wash. 1901, 14, 189–191. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Fiipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis using evolutionary distance. Mol. Biol. Evol. 2011, 28, 2725–2729. [Google Scholar] [CrossRef]
- Vineeth, K.K.; Radhakrishna, U.K.; Godwin, R.D.; Anwesha, S.; Rajashekhar, K.P.; Aravind, N.A. A new species of Microhyla Tschudi, 1838 (Anura: Microhylidae) from West Coast of India: An integrative taxonomic approach. Zootaxa 2018, 4420, 151–179. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Beitrag zur Amphibien-fauna der Insel Formosa. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin 1911, 179–184. Available online: https://www.zobodat.at/pdf/Sitzber-Ges-Naturforsch-Freunde-Berlin_1911_0179-0184.pdf (accessed on 1 October 2022).
- Wijayathilaka, N.; Meegaskumbura, M. An acoustic analysis of the genus Microhyla (Anura: Microhylidae) of Sri Lanka. PLoS ONE 2016, 11, e0159003. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.Z.; Jiang, F.; Yang, P.; Cui, J.G.; Brauth, S.E.; Tang, Y.Z. Male vocal competition is dynamic and strongly affected by social contexts in music frogs. Anim. Cogn. 2014, 17, 483–494. [Google Scholar] [CrossRef]




| A10 (Species Delimitation Using a User-Specified Guide Tree) | ||||||
| nDNA-algorithm0 | ε = 1 | ε = 2 | ε = 5 | ε = 1 | ε = 2 | ε = 5 |
| heredity = 0 | heredity = 0 | heredity = 0 | locusrate = 0 | locusrate = 0 | locusrate = 0 | |
| posterior probability [number of species] | P [5] = 0.99 | P [5] = 1 | P [5] = 0.99 | P [5] = 1 | P [5] = 0.99 | P [5] = 0.99 |
| nDNA-algorithm1 | (α m) = (1 0.5) | (α m) = (1.5 1) | (α m) = (2 2) | (α m) = (1 0.5) | (α m) = (1.5 1) | (α m) = (2 2) |
| heredity = 0 | heredity = 0 | heredity = 0 | locusrate = 0 | locusrate = 0 | locusrate = 0 | |
| posterior probability [number of species] | P [5] = 1 | P [5] = 0.99 | P [5] = 0.99 | P [5] = 1 | P [5] = 0.99 | P [5] = 1 |
| A11 (Joint Species Delimitation and Species Tree Inference) | ||||||
| nDNA-algorithm0 | ε = 1 | ε = 2 | ε = 5 | ε = 1 | ε = 2 | ε = 5 |
| heredity = 0 | heredity = 0 | heredity = 0 | locusrate = 0 | locusrate = 0 | locusrate = 0 | |
| posterior probability [number of species] | P [5] = 0.99 | P [5] = 0.99 | P [5] = 0.99 | P [5] = 1 | P [5] = 0.98 | P [5] = 0.99 |
| nDNA-algorithm1 | (α m) = (1 0.5) | (α m) = (1.5 1) | (α m) = (2 2) | (α m) = (1 0.5) | (α m) = (1.5 1) | (α m) = (2 2) |
| heredity = 0 | heredity = 0 | heredity = 0 | locusrate = 0 | locusrate = 0 | locusrate = 0 | |
| posterior probability [number of species] | P [5] = 0.99 | P [5] = 0.99 | P [5] = 0.99 | P [5] = 1 | P [5] = 0.99 | P [5] = 0.99 |
| Morphometric Characteristics | Male | Female | ||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | |
| SVL | 0.205 | −0.035 | 0.093 | 0.641 | −0.269 | −0.098 |
| HDL | −0.103 | −0.462 | 0.153 | 0.057 | 0.203 | −0.392 |
| HDW | −0.155 | −0.405 | 0.222 | −0.017 | 0.292 | −0.162 |
| SL | −0.325 | 0.148 | 0.117 | −0.046 | 0.264 | 0.255 |
| IND | −0.191 | −0.107 | 0.39 | −0.296 | 0.274 | −0.23 |
| IOD | 0.178 | −0.278 | −0.415 | −0.281 | 0.006 | 0.447 |
| UEW | −0.199 | 0.416 | 0.145 | −0.068 | 0.243 | −0.194 |
| ED | 0.229 | −0.277 | −0.176 | −0.297 | 0.094 | −0.491 |
| LAL | −0.288 | 0.036 | −0.356 | −0.152 | 0.263 | 0.227 |
| LW | −0.247 | −0.058 | 0.355 | −0.232 | 0.273 | 0.222 |
| HAL | −0.283 | −0.038 | −0.291 | −0.224 | 0.276 | 0.061 |
| HLL | −0.325 | 0.216 | −0.088 | 0.049 | 0.253 | 0.295 |
| TL | −0.261 | −0.252 | −0.22 | 0.287 | 0.286 | −0.084 |
| TW | −0.172 | −0.365 | 0.2 | 0.116 | 0.29 | −0.095 |
| TFL | −0.332 | −0.036 | −0.28 | 0.258 | 0.247 | 0.068 |
| FL | −0.346 | −0.101 | −0.129 | 0.18 | 0.279 | 0.033 |
| Eigenvalues | 6.605 | 3.753 | 1.559 | 1.158 | 10.751 | 2.022 |
| Percentage of total variance | 41.28 | 23.458 | 9.745 | 7.235 | 67.196 | 1.889 |
| Cumulative percentage | 41.28 | 64.738 | 74.483 | 81.718 | 61.196 | 86.083 |
| Species | M.dabieshanensis sp. nov | M. mixtura | M. beilunensis | M. fanjingshanensis | |
|---|---|---|---|---|---|
| Call Parameters | |||||
| Notes per call | 7.5 ± 0.8 | 8.5 ± 0.8 | 6.0 ± 1.8 | 10.4 ± 0.8 | |
| 6–9 | 7–10 | 4–10 | 9–11 | ||
| Call duration (s) | 0.744 ± 0.083 | 1.048 ± 0.192 | 0.261 ± 0.069 | 0.574 ± 0.022 | |
| 0.598–0.863 | 0.872–1.422 | 0.195–0.444 | 0.872–1.422 | ||
| Call interval (s) | 1.656 ± 0.347 | 1.402 ± 0.390 | 0.274 ± 0.028 | 3.087 ± 0.065 | |
| 1.231–2.253 | 0.788–1.869 | 0.238–0.320 | 3.005–3.198 | ||
| Call rate (call/min) | 25.0 | 24.5 | 112.1 | 16.4 | |
| Dominant frequency (Hz) | 2764.0 ± 102.9 | 2137.7 ± 70.6 | 2049.7 ± 291.3 | 2391.9 ± 69.3 | |
| 2670–2971 | 2024–2239 | 1730–2399 | 2306–2501 | ||
| Species | SVL | Species | SVL | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| 1 | M. dabieshanensis sp. nov | 19.1–22.5 | 24.9–26.7 | 25 | M. maculifera | 12.0–13.3 | 11.8 |
| 2 | M. achatina | 16 | 23 | 26 | M. malang | 18.7–22.2 | 19.0–23.4 |
| 3 | M. aurantiventris | 25.2–27.0 | 30 | 27 | M. mantheyi | 15.0–29.2 | 14.8–24.1 |
| 4 | M. beilunensis | 19.08–23.73 | 26.39–28.25 | 28 | M. mihintalei | 21.7–27.3 | 24.4 |
| 5 | M. berdmorei | 23.8–28.9 | 26.2–45.6 | 29 | M. minuta | 14.7–15.9 | 15.7–17.2 |
| 6 | M. borneensis | 10.6–12.8 | 17.9–18.8 | 30 | M. mixtura | 20.5–23.7 | 23.8–26.6 |
| 7 | M. butleri | 20.0–25.0 | 21.0–26.0 | 31 | M. mukhlesuri | 16.5–21.0 | 17.3–18.4 |
| 8 | M. chakrapanii | 22 | ? | 32 | M. mymensinghensis | 14.2–17.6 | 15.2–21.3 |
| 9 | M. daklakensi | 17.7–20.1 | 22.9–26.8 | 33 | M. nilphamariensis | 14.8–20.0 | 18.7–21.0 |
| 10 | M. darevskii | 27.0–32.6 | ? | 34 | M. ninhthuanensis | 17.3–18.8 | 21.6–23.6 |
| 11 | M. darreli | 15.0–15.7 | ? | 35 | M. okinavensis | 22.5–28.2 | 26.5–30.8 |
| 12 | M. fanjingshanensis | 19.0–22.7 | 22.5–23.0 | 36 | M. orientalis | 15.8–17.4 | 15.8–17.4 |
| 13 | M. fissipes | 18.0–27.5 | 20.0–28.0 | 37 | M. ornata | 13.4–24.9 | 24.9–26.2 |
| 14 | M. fodiens | 12.6–20.8 | ? | 38 | M. palmipes | 16 | 21.8 |
| 15 | M. fowleri | 29.5–32.5 | 32.2–41.5 | 39 | M. picta | 25.2–30.1 | 27.2–33.4 |
| 16 | M. fusca | 23.0 ** | 40 | M. pineticola | 16.0–18.0 | 22.9–26.8 | |
| 17 | M. gadjahmadai | 18.20–21.32 | 20.37–25.51 | 41 | M. pulchra | 23.0–32.0 | 28.0–36.5 |
| 18 | M. heymonsi | 16.5–22.0 | 18.0–26.5 | 42 | M. pulverata | 17.5–19.5 | 18.8–20.2 |
| 19 | M. irrawaddy | 12.3–17.1 | 16.7–20.9 | 43 | M. rubra | 20.0–27.5 | 20.5–29.5 |
| 20 | M. karunaratnei | 15.8–19.1 | 19.6–21.0 | 44 | M. sholigari | ? | 11.0–15.0 |
| 21 | M. kodial | 16.9–17.4 | 18.0–20.4 | 45 | M. superciliaris | ? | 12 |
| 22 | M. kuramotoi | 23.8–27.8 | 29.6 | 46 | M. taraiensis | 19.9–20.9 | 22.1–24.9 |
| 23 | M. neglecta | 18.7–20.2 | 23.4–26.2 | 47 | M. zeylanica | 14.4–18.3 | 15.8–20.0 |
| 24 | M. laterite | 15.3–16.6 | 18.4 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Chen, C.; Zhang, M.; Wang, Z.; Ma, H.; Sun, R.; Jiang, J.; Zhang, B. A New Species of the Genus Microhyla (Amphibia: Anura: Microhylidae) from the Dabie Mountains, China. Animals 2022, 12, 2894. https://doi.org/10.3390/ani12212894
Zhang C, Chen C, Zhang M, Wang Z, Ma H, Sun R, Jiang J, Zhang B. A New Species of the Genus Microhyla (Amphibia: Anura: Microhylidae) from the Dabie Mountains, China. Animals. 2022; 12(21):2894. https://doi.org/10.3390/ani12212894
Chicago/Turabian StyleZhang, Caiwen, Cheng Chen, Meihua Zhang, Zhiyue Wang, Haohao Ma, Ruolei Sun, Jianping Jiang, and Baowei Zhang. 2022. "A New Species of the Genus Microhyla (Amphibia: Anura: Microhylidae) from the Dabie Mountains, China" Animals 12, no. 21: 2894. https://doi.org/10.3390/ani12212894
APA StyleZhang, C., Chen, C., Zhang, M., Wang, Z., Ma, H., Sun, R., Jiang, J., & Zhang, B. (2022). A New Species of the Genus Microhyla (Amphibia: Anura: Microhylidae) from the Dabie Mountains, China. Animals, 12(21), 2894. https://doi.org/10.3390/ani12212894





