Microplastics as Contaminants in Water Bodies and Their Threat to the Aquatic Animals: A Mini-Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Status of Global Water Pollution Caused by MPs
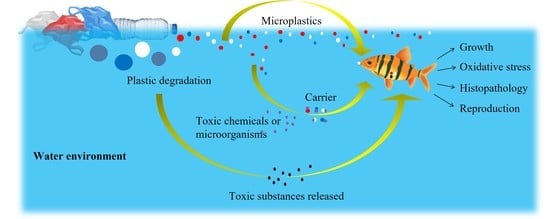
3. Stress Responsiveness of MPs in Water Environment on Aquatic Animals
3.1. Stress of MPs through Food Chain
3.2. Stress of Toxic Substances Released during Plastic Degradation Process
3.3. MPs as Carriers of Multi-Stressors
4. Summary and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- PlasticsEurope. Plastics—The Facts 2021. 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021 (accessed on 11 February 2022).
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2018, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Sinclair, C.J.; Bradley, E.L.; Boxall, A.B. Effects of environmental conditions on latex degradation in aquatic systems. Sci. Total. Environ. 2013, 447, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Chen, M.Y.; Fang, C.; Zheng, R.H.; Wang, S.M.; Hong, F.K.; Zhang, Y.S. Progress in the study of the toxicological effects of microplastics on marine organisms. J. Appl. Oceanogr. 2018, 37, 594–600. [Google Scholar]
- Xue, Y.P. Research progress of microplastics in freshwater river. Resour. Econ. Environ. Prot. 2020, 1, 12. [Google Scholar]
- Tu, R.H. Overview of the Firth United Nations Environment Assembly. World Environ. 2022, 2, 18–23. [Google Scholar]
- Zhou, D.Q.; Lv, S.W.; Liu, N.; Li, N. Review on pollution hazards and detection and analysis methods of microplastics in ocean. Chin. Fish. Qual. Stand. 2020, 10, 60–68. [Google Scholar]
- Jiang, X.P. Source and harm of marine microplastics. South Agric. Mach. 2019, 50, 276–277. [Google Scholar]
- Chen, L.; Deng, P.H.; Huang, F.Y.; Bai, S.Y. Pollution status and control of micro-plastics. Environ. Develop. 2020, 32, 34–35. [Google Scholar]
- Jia, Q.L.; Chen, H.; Zhao, X.; Li, L.; Nie, Y.H.; Ye, J.F. Removal of microplastics by treatment process of large scale municipal wastewater treatment plant. Environ. Sci. 2019, 40, 4105–4112. [Google Scholar]
- Xuan, L.Q.; Liu, S.; Luo, Y.; Li, Y.D.; Xia, Q. Removal of microplastics in different wastewater treatment processes in Harbin municipal wastewater treatment plants. Acta Sci. Circumstantiae 2020, 40, 3964–3970. [Google Scholar]
- Wang, W.F.; Yuan, W.K.; Chen, Y.L.; Wang, J. Microplastics in surface waters of Dongting Lake and Hong Lake, China. Sci. Total Environ. 2018, 633, 539–545. [Google Scholar] [CrossRef]
- Zhang, K.; Gong, W.; Lv, J.Z.; Xiong, X.; Wu, C.X. Accumulation of floating microplastics behind the Three Gorges Dam. Environ. Pollut. 2015, 204, 117–123. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Zhu, L.X.; Wang, T.; Li, D.J. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Li, Z.; Gao, C.M.; Yang, J.L.; Wu, L.Z.; Zhang, S.; Liu, Y.H.; Jin, D.D. Distribution characteristics of microplastics in surface water and sediments of Haizhou Bay, Lianyungang. Environ. Sci. 2020, 41, 3212–3221. [Google Scholar]
- Wang, W.F.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Yan, M.T.; Nie, H.Y.; Xu, K.H.; He, Y.H.; Hu, Y.T.; Huang, Y.M.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef]
- Zhang, D.D.; Fraser, M.A.; Huang, W.; Ge, C.J.; Wang, Y.; Zhang, C.F.; Guo, P. Microplastic pollution in water, sediment, and specific tissues of crayfish (Procambarus clarkii) within two different breeding modes in Jianli, Hubei province, China. Environ. Pollut. 2021, 272, 115939. [Google Scholar] [CrossRef]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Arendt, N.; Foeldi, C.; Dierkes, G.; Wagner, M.; et al. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total Environ. 2020, 738, 139866. [Google Scholar] [CrossRef]
- Anderson, P.J.; Sarah, W.; Victoria, L.; Jonathan, K.C.; Mark, L.H.; Michael, D.R. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef]
- Marrone, A.; La Russa, M.F.; Randazzo, L.; La Russa, D.; Cellini, E.; Pellegrino, D. Microplastics in the Center of Mediterranean: Comparison of the Two Calabrian Coasts and Distribution from Coastal Areas to the Open Sea. Int. J. Environ. Res. Public Health 2021, 18, 10712. [Google Scholar] [CrossRef]
- Suaria, G.; Perold, V.; Lee, J.R.; Lebouard, F.; Aliani, S.; Ryan, P.G. Floating macro- and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020, 136, 105494. [Google Scholar] [CrossRef]
- Pan, Z.; Guo, H.G.; Chen, H.Z.; Wang, S.M.; Sun, X.W.; Zou, Q.P.; Zhang, Y.B.; Lin, H.; Cai, S.Z.; Huang, J. Microplastics in the Northwestern Pacific: Abundance, distribution, and characteristics. Sci. Total Environ. 2019, 650, 1913–1922. [Google Scholar] [CrossRef]
- Wang, S.M.; Chen, H.Z.; Zhou, X.W.; Tian, Y.Q.; Lin, C.; Wang, W.L.; Zhou, K.W.; Zhang, Y.B.; Lin, H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef]
- Kor, K.; Mehdinia, A. Neustonic microplastic pollution in the Persian Gulf. Mar. Pollut. Bull. 2020, 150, 11066. [Google Scholar] [CrossRef]
- Ta, A.T.; Babel, S. Microplastic contamination on the lower Chao Phraya: Abundance, characteristic and interaction with heavy metals. Chemosphere 2020, 257, 127234. [Google Scholar] [CrossRef]
- Eibes, P.M.; Gabel, F. Floating microplastic debris in a rural river in Germany: Distribution, types and potential sources and sinks. Sci. Total Environ. 2022, 816, 151641. [Google Scholar] [CrossRef]
- Park, T.J.; Lee, S.H.; Lee, M.S.; Lee, J.K.; Lee, S.H.; Zoh, K.D. Occurrence of microplastics in the Han River and riverine fish in South Korea. Sci. Total Environ. 2020, 708, 134535. [Google Scholar] [CrossRef]
- Buckingham, J.W.; Manno, C.; Waluda, C.M.; Waller, C.L. A record of microplastic in the marine nearshore waters of South Georgia. Environ. Pollut. 2022, 306, 119379. [Google Scholar] [CrossRef]
- Sainio, E.; Lehtiniemi, M.; Setälä, O. Microplastic ingestion by small coastal fish in the northern Baltic Sea, Finland. Mar. Pollut. Bull. 2021, 172, 112814. [Google Scholar] [CrossRef]
- Manbohi, A.; Mehdinia, A.; Rahnama, R.; Dehbandi, R. Microplastic pollution in inshore and offshore surface waters of the southern Caspian Sea. Chemosphere 2021, 281, 130896. [Google Scholar] [CrossRef]
- Karaoğlu, K.; Gül, S. Characterization of microplastic pollution in tadpoles living in small water-bodies from Rize, the northeast of Turkey. Chemosphere 2020, 255, 126915. [Google Scholar] [CrossRef]
- Irfan, T.; Khalid, S.; Taneez, M.; Hashmi, M.Z. Plastic driven pollution in Pakistan: The first evidence of environmental exposure to microplastic in sediments and water of Rawal Lake. Environ. Sci. Pollut. Res. Int. 2020, 27, 15083–15092. [Google Scholar] [CrossRef] [PubMed]
- Taha, Z.D.; Md Amin, R.; Anuar, S.T.; Nasser, A.A.A.; Sohaimi, E.S. Microplastics in seawater and zooplankton: A case study from Terengganu estuary and offshore waters, Malaysia. Sci. Total Environ. 2021, 786, 147466. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Nationwide monitoring of microplastics in bivalves from the coastal environment of Korea. Environ. Pollut. 2021, 270, 116175. [Google Scholar] [CrossRef] [PubMed]
- Sevwandi Dharmadasa, W.L.S.; Andrady, A.L.; Kumara, P.B.T.P.; Maes, T.; Gangabadage, C.S. Microplastic pollution in Marine Protected Areas of Southern Sri Lanka. Mar. Pollut. Bull. 2021, 168, 112462. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Luo, H.T.; Zou, J.T.; Chen, J.M.; Pan, X.L.; Rousseau, D.P.L.; Li, J. Characteristics and removal of microplastics in rural domestic wastewater treatment facilities of China. Sci. Total Environ. 2020, 739, 139935. [Google Scholar] [CrossRef] [PubMed]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in four estuarine rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- Shruti, V.C.; Jonathan, M.P.; Rodriguez-Espinosa, P.F.; Rodríguez-González, F. Microplastics in freshwater sediments of Atoyac River basin, Puebla City, Mexico. Sci. Total Environ. 2019, 654, 154–163. [Google Scholar] [CrossRef]
- Rey, S.F.; Franklin, J.; Rey, S.J. Microplastic pollution on island beaches, Oahu, Hawai’i. PLoS ONE 2021, 16, e0247224. [Google Scholar] [CrossRef]
- Nabizadeh, R.; Sajadi, M.; Rastkari, N.; Yaghmaeian, K. Microplastic pollution on the Persian Gulf shoreline: A case study of Bandar Abbas city, Hormozgan Province, Iran. Mar. Pollut. Bull. 2019, 145, 536–546. [Google Scholar] [CrossRef]
- Li, B.W.; Su, L.; Zhang, H.B.; Deng, H.; Chen, Q.Q.; Shi, H.H. Microplastics in fishes and their living environments surrounding a plastic production area. Sci. Total Environ. 2020, 727, 138662. [Google Scholar] [CrossRef]
- Deng, H.; Wei, R.; Luo, W.Y.; Hu, L.L.; Li, B.W.; Di, Y.N.; Shi, H.H. Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut. 2020, 258, 113658. [Google Scholar] [CrossRef]
- Ding, J.F.; Li, J.X.; Sun, C.J.; Jiang, F.H.; He, C.F.; Zhang, M.; Ju, P.; Ding, N.X.Y. An examination of the occurrence and potential risks of microplastics across various shellfish. Sci. Total Environ. 2020, 739, 139887. [Google Scholar] [CrossRef]
- Liu, Q.B. Distribution of Microplastics and Their Uptake and Elimination in Zooplankton in the Bohai Sea and the Yellow Sea. Master Thesis, Dalian Maritime University, Dalian, China, 2020. [Google Scholar]
- Collard, F.; Gasperi, J.; Gilbert, B.; Eppe, G.; Azimi, S.; Rocher, V.; Tassin, B. Anthropogenic particles in the stomach contents and liver of the freshwater fish Squalius Cephalus. Sci. Total Environ. 2018, 643, 1257–1264. [Google Scholar] [CrossRef]
- Jabeen, K.; Su, L.; Li, J.N.; Yang, D.Q.; Tong, C.F.; Mu, J.L.; Shi, H.H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- Huang, J.S.; Koongolla, J.B.; Li, H.X.; Lin, L.; Pan, Y.P.; Liu, S.; He, W.H.; Maharana, D.; Xu, X.R. Microplastic accumulation in fish from Zhanjiang mangrove wetland, South China. Sci. Total Environ. 2020, 708, 134839. [Google Scholar] [CrossRef]
- Koraltan, İ.; Mavruk, S.; Güven, O. Effect of biological and environmental factors on microplastic ingestion of commercial fish species. Chemosphere 2022, 303, 135101. [Google Scholar] [CrossRef]
- Hanachi, P.; Karbalaei, S.; Walker, T.R.; Cole, M.; Hosseini, S.V. Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ. Sci. Pollut. Res. Int. 2019, 26, 23777–23787. [Google Scholar] [CrossRef]
- Castelvetro, V.; Corti, A.; Bianchi, S.; Giacomelli, G.; Manariti, A.; Vinciguerra, V. Microplastics in fish meal: Contamination level analyzed by polymer type, including polyester (PET), polyolefins, and polystyrene. Environ. Pollut. 2020, 273, 115792. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.J.; Zhu, X.P.; Sun, C.F.; Teng, J.; Chen, L.M.; Shan, E.C.; Zhao, J.M. Microplastics in fish meals: An exposure route for aquaculture animals. Sci Total Environ. 2022, 807, 151049. [Google Scholar] [CrossRef]
- Karbalaei, S.; Golieskardi, A.; Watt, D.U.; Boiret, M.; Hanachi, P.; Walker, T.R.; Karami, A. Analysis and inorganic composition of microplastics in commercial Malaysian fish meals. Mar. Pollut. Bull. 2020, 150, 110687. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Eroldoğan, O.T.; Evliyaoğlu, E.; Turchini, G.M.; Wu, X.G. Fish out, plastic in: Global pattern of plastics in commercial fishmeal. Aquaculture 2021, 534, 736316. [Google Scholar] [CrossRef]
- De Carvalho, A.R.; Imbert, A.; Parker, B.; Euphrasie, A.; Boulêtreau, S.; Britton, J.R.; Cucherousset, J. Microplastic in angling baits as a cryptic source of contamination in European freshwaters. Sci. Rep. 2021, 11, 11255. [Google Scholar] [CrossRef]
- Garcia, A.G.; Suárez, D.C.; Li, J.; Rotchell, J.M. A comparison of microplastic contamination in freshwater fish from natural and farmed sources. Environ. Sci. Pollut. Res. Int. 2021, 28, 14488–14497. [Google Scholar] [CrossRef]
- Savoca, S.; Matanović, K.; D’Angelo, G.; Vetri, V.; Anselmo, S.; Bottari, T.; Mancuso, M.; Kužir, S.; Spanò, N.; Capillo, G.; et al. Ingestion of plastic and non-plastic microfibers by farmed gilthead sea bream (Sparus aurata) and common carp (Cyprinus carpio) at different life stages. Sci. Total Environ. 2021, 782, 146851. [Google Scholar] [CrossRef]
- Lei, L.L. Toxic Effects and Mechanisms of Microplastic Particles on Caenorhabditis elegans and Danio rerio. Master’s Thesis, East China Normal University, Shanghai, China, 2019. [Google Scholar]
- Li, Q.J.; Zheng, S.; Zhu, M.L.; Sun, X.X. Study on the feeding of microplastics by turbot juveniles (Scophthalmus Maximus). Environ. Prot. 2020, 48, 40–46. [Google Scholar]
- Yang, H.; Xiong, H.R.; Mi, K.H.; Xue, W.; Wei, W.Z.; Zhang, Y.Y. Toxicity comparison of nano-sized and micron-sized microplastics to Goldfish Carassius auratus Larvae. J. Hazard. Mater. 2020, 388, 122058. [Google Scholar] [CrossRef]
- Yin, L.Y.; Liu, H.Y.; Cui, H.W.; Chen, B.J.; Li, L.L.; Wu, F. Impacts of polystyrene microplastics on the behavior and metabolism in a marine demersal teleost, black rockfish (Sebastes schlegelii). J. Hazard. Mater. 2019, 380, 120861. [Google Scholar] [CrossRef]
- Jin, Y.X.; Xia, J.Z.; Pan, Z.H.; Yang, J.J.; Wang, W.C.; Fu, Z.W. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Yu, P.; Cai, M.Q.; Wu, D.L.; Zhang, M.; Chen, M.H.; Zhao, Y.L. Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir sinensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.Q. Effects of Polystyrene Microplastics on the Microbiota and Metabolism in Zebrafish. Master Thesis, Zhejiang University of Technology, Hangzhou, China, 2019. [Google Scholar]
- Qiao, R.X.; Sheng, C.; Lu, Y.F.; Zhang, Y.; Ren, H.Q.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.X.; Wang, S.X.; Wang, X.H.; Yu, X.; Hu, M.H.; Huang, W.; Wang, Y.J. Nanoplastics impair the intestinal health of the juvenile large yellow croaker Larimichthys crocea. J. Hazard. Mater. 2020, 397, 122773. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.N.; Wen, B.; Zhu, J.G.; Zhang, Y.S.; Gao, J.Z.; Chen, Z.Z. Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci. Total Environ. 2020, 733, 138929. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Rao, B.Q.; Guo, X.M.; Gao, J.Y. Effects of microplastics on embryo hatching and intestinal accumulation in larval zebrafish Danio rerio. Huan Jing Ke Xue 2021, 42, 485–491. [Google Scholar] [PubMed]
- Lu, Y.F.; Zhang, Y.; Feng, D.Y.; Jiang, W.; Zhao, Y.P.; Geng, J.J.; Ding, L.L.; Ren, H.Q. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Wang, L.L. Study on Toxicological Effects of PVC Microplastic on Juvenile Yellow River Carp. Master’s Thesis, Henan Nornal University, Xinxiang, China, 2019. [Google Scholar]
- Wan, Z.Q.; Wang, C.Y.; Zhou, J.J.; Shen, M.L.; Wang, X.Y.; Fu, Z.W.; Jin, Y.X. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Yang, B.Z.; Huang, H. Effect of microplastics on antioxidant enzyme system in juvenile red crucian carp. Environ. Sci. Technol. 2019, 42, 23–27. [Google Scholar]
- Wang, J.; Li, Y.J.; Lu, L.; Zheng, M.Y.; Zhang, X.N.; Tian, H.; Wang, W.; Ru, S.G. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Qiao, R.X.; Deng, Y.F.; Zhang, S.H.; Wolosker, M.B.; Zhu, Q.D.; Ren, H.Q.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut. 2018, 236, 425–431. [Google Scholar] [CrossRef]
- Albano, M.; Panarello, G.; Di Paola, D.; Capparucci, F.; Crupi, R.; Gugliandolo, E.; Spanò, N.; Capillo, G.; Savoca, S. The influence of polystyrene microspheres abundance on development and feeding behavior of Artemia salina (Linnaeus, 1758). Appl. Sci. 2021, 11, 3352. [Google Scholar] [CrossRef]
- Jeyavani. J.; Sibiya, A.; Bhavaniramya, S.; Mahboob, S.; Al-Ghanim, K.A.; Nisa, Z.U.; Riaz, M.N.; Nicoletti, M.; Govindarajan, M.; Vaseeharan, B. Toxicity evaluation of polypropylene microplastic on marine microcrustacean Artemia salina: An analysis of implications and vulnerability. Chemosphere 2022, 296, 133990. [Google Scholar]
- Suman, T.Y.; Jia, P.P.; Li, W.G.; Junaid, M.; Xin, G.Y.; Wang, Y.; Pei, D.S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater. 2020, 400, 123220. [Google Scholar] [CrossRef]
- Zhang, X.L.; Xia, M.L.; Zhao, J.Y.; Cao, Z.G.; Zou, W.; Zhou, Q.X. Photoaging enhanced the adverse effects of polyamide microplastics on the growth, intestinal health, and lipid absorption in developing zebrafish. Environ. Int. 2022, 158, 106922. [Google Scholar] [CrossRef]
- Lu, X.; Deng, D.F.; Huang, F.; Casu, F.; Kraco, E.; Newton, R.J.; Zohn, M.; Teh, S.J.; Watson, A.M.; Shepherd, B.; et al. Chronic exposure to high-density polyethylene microplastic through feeding alters the nutrient metabolism of juvenile yellow perch (Perca flavescens). Anim. Nutr. 2022, 9, 143–158. [Google Scholar] [CrossRef]
- Mbugani, J.J.; Machiwa, J.F.; Shilla, D.A.; Joseph, D.; Kimaro, W.H.; Khan, F.R. Impaired growth performance of Wami Tilapia juveniles (Oreochromis urolepis) (Norman, 1922) due to microplastic induced degeneration of the small intestine. Microplastics. 2022, 1, 334–345. [Google Scholar] [CrossRef]
- DiBona, E.; Pinnell, L.J.; Heising-Huang, A.; Geist, S.; Turner, J.W.; Seemann, F. A holistic assessment of polyethylene fiber ingestion in larval and juvenile Japanese medaka fish. Front Physiol. 2021, 12, 668645. [Google Scholar] [CrossRef]
- Han, X. Bioaccumulation of Microplastics in Different Tissues of Zebrafish. Master Thesis, Dalian Maritime University, Dalian, China, 2020. [Google Scholar]
- Hou, M.M.; Wang, C.L.; Xu, C.S.; Qiu, N.; Xia, Z.J.; Su, L.X.; Wang, J.W. Effects of polystyrene microplastics exposure on the growth of Gobiocypris rarus larvae. Sichuan J. Zool. 2020, 39, 140–147. [Google Scholar]
- Liu, Q.B.; Zhang, M.X.; Ding, G.H.; Li, X.S.; Zhang, D.; Zhang, W.W.; Wang, Y.; Wang, J.Y. Uptake and elimination of microplastics by Tigriopus japonicus and its impact on feeding behavior. Asian J. Ecotoxicol. 2020, 15, 184–191. [Google Scholar]
- Felice, B.D.; Bacchetta, R.; Santo, N.; Tremolada, P.; Parolini, M. Polystyrene microplastics did not affect body growth and swimming activity in Xenopus laevis tadpoles. Environ. Sci. Pollut. Res. 2018, 25, 34644–34651. [Google Scholar] [CrossRef]
- Ding, J.N.; Zhang, S.S.; Razanajatovo, R.M.; Zuo, H.; Zhu, W.B. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Xia, X.H.; Sun, M.H.; Zhou, M.; Chang, Z.J.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef]
- Jabeen, K.; Li, B.W.; Chen, Q.Q.; Su, L.; Wu, C.X.; Hollert, H.; Shi, H.H. Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 2018, 213, 323–332. [Google Scholar] [CrossRef]
- Yin, L.Y.; Chen, B.J.; Xia, B.; Shi, X.T.; Qu, K.M. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef]
- Dang, B.Y. Review on distribution and environmental effects of microplastics. Technol. Econ. Guide 2019, 27, 124. [Google Scholar]
- Guo, Z.; Zhang, L.J.; Liu, X.Y.; Yu, Y.J.; Liu, S.; Chen, M.B.; Huang, C.S.; Hu, G.C. The enrichment and purification of hexabromocyclododecanes and its effects on thyroid in zebrafish. Ecotoxicol. Environ. Saf. 2019, 185, 109690. [Google Scholar] [CrossRef]
- Yang, W.T.; Kuang, W.; Zheng, X.Q. Pollution status and ecological risk research progress of phthalate esters in environment. Environ. Prot. Circ. Econ. 2020, 40, 34–60. [Google Scholar]
- Lee, Y.M.; Lee, J.E.; Choe, W.; Kim, T.; Lee, J.Y.; Kho, Y.; Choi, K.; Zoh, K.D. Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ. Int. 2019, 126, 635–643. [Google Scholar] [CrossRef]
- Jiang, L.L. Study on the cumulative effect of phthalate esters in the food chain composed of Chlorella vulgaris to Pagrosomus Major. Fish. Mod. 2014, 41, 5–11. [Google Scholar]
- Yang, W.K.; Chiang, L.F.; Tan, S.W.; Chen, P.J. Environmentally relevant concentrations of di(2-ethylhexyl)phthalate exposure alter larval growth and locomotion in medaka fish via multiple pathways. Sci. Total Environ. 2018, 640–641, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Hu, Y.; Chen, H.G.; Jia, X.P.; Cai, W.G. Transcriptome analysis in the liver of Nile tilapia (Oreochromis niloticus) after treated with di(2-ethylhexyl) phthaIate. China Environ. Sci. 2019, 39, 386–396. [Google Scholar]
- Guo, W.; Han, J.; Wu, S.M.; Shi, X.J.; Wang, Q.W.; Zhou, B.S. Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate affects lipid metabolism in zebrafish larvae via DNA methylation modification. Environ. Sci. Technol. 2020, 54, 355–363. [Google Scholar] [CrossRef]
- Sun, L.M.; Ling, Y.H.; Jiang, J.H.; Wang, D.T.; Wang, J.X.; Li, J.Y.; Wang, X.D.; Wang, H.L. Differential mechanisms regarding triclosan vs. bisphenol A and fluorene-9-bisphenol induced zebrafish lipid-metabolism disorders by RNA-Seq. Chemosphere 2020, 251, 126318. [Google Scholar] [CrossRef]
- Frenzilli, G.; Martorell-Ribera, J.; Bernardeschi, M.; Scarcelli, V.; Jönsson, E.; Diano, N.; Moggio, M.; Guidi, P.; Sturve, J.; Asker, N. Bisphenol A and Bisphenol S Induce Endocrine and Chromosomal Alterations in Brown Trout. Front Endocrinol. 2021, 12, 645519. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.R.; Yang, M.; Yu, Y.P.; Yan, M.T.; Zhou, L.; Liu, X.C.; Xiao, S.Q.; Yang, Y.; Wang, Y.X.; et al. Toxic effects of bisphenol A on goldfish gonad development and the possible pathway of BPA disturbance in female and male fish reproduction. Chemosphere 2019, 221, 235–245. [Google Scholar] [CrossRef]
- Yuan, W.K.; Zhou, Y.F.; Chen, Y.L.; Liu, X.N.; Wang, J. Toxicological effects of microplastics and heavy metals on the Daphnia magna. Sci. Total Environ. 2020, 746, 141254. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Liu, H.; Lv, Y.H.; Chang, W.J.; Wang, C.Y.; Zhen, N.; Wang, J.; Yu, J.R.; Wang, X. Adsorption of antibiotics by microplastics. J. Jilin Inst. Chem. Technol. 2021, 38, 90–94. [Google Scholar]
- Hou, J. Research progress on interaction and environment behavior between microplastics and organic pollutants. J. Hohai Univ. 2020, 48, 22–28. [Google Scholar]
- Sun, J.H.; Xia, S.D.; Ning, Y.; Pan, X.; Qu, J.H.; Xu, Y.J. Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, Hippocampus Kuda Bleeker. Mar. Pollut. Bull. 2019, 149, 110510. [Google Scholar]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef]
- Miranda, T.; Vieira, L.R.; Guilhermino, L. Neurotoxicity, behavior, and lethal effects of cadmium, microplastics, and their mixtures on pomatoschistus microps juveniles from two wild populations exposed under laboratory conditions-implications to environmental and human risk assessment. Int. J. Environ. Res. Public Health 2019, 16, 2857. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.S.; Dong, H.; Wang, Y.; Ren, R.S.; Qin, X.H.; Wang, S.F. Effects of microplastics and their adsorption of cadmium as vectors on the cladoceran Moina monogolica Daday: Implications for plastic-ingesting organisms. J. Hazard. Mater. 2020, 400, 123239. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.X.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, M.; Chen, X.P.; Yang, C.M.; Wu, L.L. Combined toxicity of microplastics and cadmium on the zebrafish embryos (Danio rerio). Sci. Total Environ. 2020, 743, 140638. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.N.; Liu, J.H.; Feng, X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khosraviani, K.; Hosseinpour Delavar, F.; Arghideh, M.; Zavvar, F.; Hoseinifar, S.H.; Van Doan, H.; Zabihi, E.; Reverter, M. Hepatic transcriptomic and histopathological responses of common carp, Cyprinus carpio, to copper and microplastic exposure. Mar. Pollut. Bull. 2022, 175, 113401. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.B.; Wang, D.; Zhu, Q.L.; Zheng, J.L. Combined effects of polystyrene microplastics and cadmium on oxidative stress, apoptosis, and GH/IGF axis in zebrafish early life stages. Sci. Total Environ. 2022, 813, 152514. [Google Scholar] [CrossRef]
- Qin, L.; Duan, Z.H.; Cheng, H.D.; Wang, Y.D.; Zhang, H.H.; Zhu, Z.; Wang, L. Size-dependent impact of polystyrene microplastics on the toxicity of cadmium through altering neutrophil expression and metabolic regulation in zebrafish larvae. Environ. Pollut. 2021, 291, 118169. [Google Scholar] [CrossRef]
- De Tender, C.A.; Devriese, L.I.; Haegeman, A.; Maes, S.; Ruttink, T.; Dawyndt, P. Bacterial Community Profiling of Plastic Litter in the Belgian Part of the North Sea. Environ. Sci. Technol. 2015, 49, 9629–9638. [Google Scholar] [CrossRef]
- Ji, M.R.; Ma, Y.N.; Ji, R. Plastisphere: The vector effects of microplastics on microbial communities. Environ. Prot. 2020, 48, 19–27. [Google Scholar]
- Tender, C.D.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal Dynamics of Bacterial and Fungal Colonization on Plastic Debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Kettner, M.T.; Miki, T.; Grossart, H.P. Microplastics: New substrates for heterotrophic activity contribute to altering organic matter cycles in aquatic ecosystems. Sci. Total Environ. 2018, 635, 1152–1159. [Google Scholar] [CrossRef]
- Dussud, C.; Meistertzheim, A.L.; Conan, P.; Pujo-Pay, M.; George, M.; Fabre, P.; Coudane, J.; Higgs, P.; Elineau, A.; Pedrotti, M.L.; et al. Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ. Pollut. 2018, 236, 807–816. [Google Scholar] [CrossRef]
- Fu, Q.Q.; Li, D.Z.; Zhang, Y.Q.; Deng, H.; Feng, D.; Zhao, Y.Y.; Yu, H.M.; Wu, X.C.; Ge, C.J. A Study on microbial colonization and communities on microplasticsin urban Mangrove system. Chin. J. Trop. Crops 2021, 42, 3692–3698. [Google Scholar]
- Saygin, H.; Baysal, A. Biofilm formation of clinically important bacteria on bio-based and conventional micro/submicron-sized plastics. Bull. Environ. Contam. Toxicol. 2020, 105, 18–25. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Lu, J.; Wu, J.; Wang, J.H.; Luo, Y.M. Potential risks of microplastics combined with superbugs: Enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicol. Environ. Saf. 2020, 187, 109852. [Google Scholar] [CrossRef]
- Gong, M.T.; Yang, G.Q.; Zhuang, L.; Zeng, E.Y. Microbial biofilm formation and community structure on low-density polyethylene microparticles in lake water microcosms. Environ. Pollut. 2019, 252, 94–102. [Google Scholar] [CrossRef]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Vaseashta, A. The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World J. Microbiol. Biotechnol. 2022, 38, 117. [Google Scholar] [CrossRef] [PubMed]
| Location | Particle Size | Main Types | Abundance | Reference |
|---|---|---|---|---|
| Two large municipal treatment plants (Shanghai, China) | 80–5000 μm | PET, PA, PE, PP | 226.27 ± 83.00 and 171.89 ± 62.98 piece/L, respectively (influent water of WWTP1 and WWTP2) | [10] |
| Two municipal wastewater treatment plants (Harbin, China) | 0.038–0.55 mm | PP, PS, PE | Approx. 1.47 × 106 particles (the daily emission) | [11] |
| Dongting Lake, Hong Lake (China) | 50–5000 μm | PE, PP, PS, PVC | 900–2800 (Dongting Lake) and 1250–4650 (Hong Lake) n/m3 | [12] |
| Three Gorges Reservoir (China) | 112 μm–5 mm | PE, PP, PS | 3407.7 × 103 to 13,617.5 × 103 (in the mainstream) and 192.5 × 103 to 11,889.7 × 103 (the tributaries) items/km2 | [13] |
| Yangtze Estuary System (China) | 0.90 ± 0.74 mm (Yangtze Estuary), 2.01 ± 2.01 mm (East China Sea) | / | 4137.3 ± 2461.5 (estuary) and 0.167 ± 0.138 (sea) n/m3 | [14] |
| Haizhou Bay (China) | 0.08–13.48 mm (surface water), 0.04–14.74 mm (sediment) | Rayon, PET, PP, PE | 2.60 ± 1.40 items/m3 (surface water) and 0.33 ± 0.26 (sediment) items/g | [15] |
| Urban surface waters (Wuhan, China) | <2 mm accounted for >80% | PET, PP, PE, PA, PS | 1660.0 ± 639.1 to 8925 ± 1591 n/m3 | [16] |
| Pearl River Estuary and Guangzhou urban area (China) | 0.05–5 mm | PA, PVC cellophane, PP, PE, VACs | 19,860 items/m3 (urban section), 8902 items/m3 (estuary) | [17] |
| A pond rice-crayfish co-culture water and sediments (Hubei, China) | <1 mm accounted for 42.8 ± 20.8% to 89.1 ± 5.0% (water) and 58.8 ± 16.7% to 97.0 ± 4.8% (sediment) | PP:PE, PE, cellulose, cellophane, PET | 1.3 ± 0.1–2.5 ± 0.1 particles/L (water), 0.03 ± 0.01–0.04 ± 0.02 particles/g (sediment) | [18] |
| Elbe water and sediments (Europe) | 150–5000 μm (water), 125–5000 μm (sediment) | PE, PP, PS ABS, PA, PET, PMMA | 5.57 (water) and 3.35 × 106 (sediment) particles/m3 | [19] |
| Lake Winnipeg (Canada) | <5 mm | / | 193,420 ± 115,567 particles/km2 | [20] |
| The center of the Mediterranean Sea | <3 mm (73%) | PE (69%), PP (24%), PS, PET | 0.01 to 0.66 particles/m2 | [21] |
| Temperate waters north of the Subtropical Front to the Southern Ocean | <5 mm (93%) | PE, PP, PS, PVC, PA, PMMA | 7.7–14.1 (temperate waters), 1.1–2.6 (Southern Ocean) g/km2 | [22] |
| Pacific Northwest | 0.5–1.0 mm (~50%), 1–2.5 mm (29.8%), 2.5–5.0 mm (17.6%) | PE, PP, PA, PVC, PS, rubber, PET | 6.4 × 102 to 4.2 × 104 items/km2 | [23] |
| The mid-west Pacific Ocean | 0.3–2.5 mm | PP, PMMA, PE, PET | 6028–95,335 pieces/km2 | [24] |
| Persian Gulf | 100–5000 μm | PE, PP, PS | 1.5 × 103 to 4.6 × 104 particle/km2 | [25] |
| The lower Chao Phraya (Thailand) | 0.05–1.0 mm (surface water), 0.05–0.3 mm (sediment) | PP, PS, PE | 80 ± 65 items/m3 (surface water), 91 ± 13 items/kg (sediment) | [26] |
| Ems River (Germany) | / | PE | 1.54 ± 1.54 items/m3 | [27] |
| Han River (South Korea) | <1 mm accounted for >90% | Silicone, PE, PS, PTFE, polyester | 7.0 ± 12.9 (surface), 102.0 ± 50.3 (2 m below the surface), 91.1 ± 72.3 (the tributaries) particles/m3 | [28] |
| The nearshore water of South Georgia | 50–100 μm (38.1% in seawater, 45.5% in freshwater, 46.7% in wastewater) | PET | 2.67 ± 3.05 MPs/L (freshwater), 4.67 ± 3.21 MPs/L (precipitation), 1.66 ± 3.00 MPs/L (wastewater) | [29] |
| The northern Baltic Sea (Finland) | 140–2370 μm | / | 16.2 ± 11.2 MPs/m3 | [30] |
| Southern Caspian Sea | 1000–5000 μm | PE, PP, PET | 0.246 ± 0.020 MPs/m3 | [31] |
| Surface water in Rize (Turkey) | / | PA, PET | 1–13 items/L | [32] |
| Rawal Lake (Pakistan) | / | PE, PP, polyester, PET, PVC | 0.142 items/0.1 L (water), 1.04 items/0.01 kg (sediment) | [33] |
| Terengganu estuary and offshore (Malaysia) | / | PA, PE, PP | 1687 particles/m3 (estuary), 1900 particles/m3 (offshore) | [34] |
| Along the Korean coasts | <300 µm | PP, PE, and polyester | 1400 ± 560 n/m3 | [35] |
| Marine Protected Areas of Southern Sri Lanka | 3.14 ± 0.17 and 2.96 ± 0.18 mm (Bundala National Park), 3.46 ± 0.16 and 3.39 ± 0.43 mm (Hikkaduwa Marine National Park) | PE, PP, PS | 0.515 ± 0.054 MPs/m3 (Hikkaduwa Marine National Park), 0.276 ± 0.077 MPs/m3 (Bundala National Park) | [36] |
| Suburban areas, plain countryside and mountainous areas (Zhejiang, China) | >0.1 mm (West Lake District-1), 0.0308–0.1 mm (the rest of areas) | PP, PS, PET, PES, PE, PA | 430–540 items/m3 (suburban countryside) and 1150–2154 items/m3 (plain countryside and mountainous areas) | [37] |
| Species | Particle Size | Shape | Abundance | Reference |
|---|---|---|---|---|
| Procambarus clarkii | 50 to 500 μm | Fiber and fragments | 0.17 ± 0.07–0.92 ± 0.19 particles/individual | [18] |
| Cyrinus carpio, Carassius cuvieri, Lepomis macrochirus, Micropterus salmoides, Silurusasotus, Channa argus | 0.3–0.6 mm | Fragments (>94%) and fiber | 4–48 particles/fish (intestine), 8.3 ± 6.0 particles/fish (gill) | [28] |
| Three-spined stickleback, Bleak, Perch, Roach | 184 to 2592 μm | / | 51 MPs in 38 fish/424 fish | [30] |
| Calanoida, Cyclopoida, Harpacticoida, Mysids, Decapoda, Cladocera | 68–144 µm and 400.1–500 µm (offshore), 80–99 µm (estuary) | Fiber and fragments | 0.01 ± 0.002 to 0.20 ± 0.14 particles/individual | [34] |
| Oyster, mussel, Manila calm | <300 µm | Fragments | 1.21 ± 0.68 n/individual (Oyster/mussel), 2.19 ± 1.20 n/individual (Manila calm) | [35] |
| Mussel, Ruditapes, philippinarum, Crassostrea gigas, Sinonovacula constricta, Scapharca subcrenata, Meretrix lusoria, Busycon canaliculatu | 983.8 μm (from Qingdao), 1011.2 μm (from Xiamen) | Fiber, granules, film, and fragments | 1.2–4.1 (Qingdao) and 1.3–6.0 (Xiamen) items/individual | [44] |
| Copepoda, Macrura, Brachyuran, Amphipoda, Cumacea, Lernaea, Scaleph, Larval fish, Tunicata | 0.1–1 mm | Fiber (81.53%), fragments (11.71%), line (4.95%), and particles (1.81%) | 0.06–4.55 particles/m3 | [45] |
| Squalius cephalus | 2.41 mm | Fiber and fragments | 18 APs in 60 stomachs and 5% livers contained one or more APs | [46] |
| Hyporhamphus intermedius, Liza haematocheila, Coilia ectenes, Lateolabrax japonicas, Sillago sihama, Larimichthys crocea, Psenopsis anomala, Pampus cinereus, Harpodon nehereus, Mugil cephalus, Muraenesox cinereus, Terapon jarbua, Sebastiscus marmoratus, Photopectoralis bindus, Cynoglossus abbreviates, Thamnaconus septentrionalis, Oxyeleotrix marmorata, Synechogobius ommaturus, Collichthys lucidus, Branchiostegus japonicas, Callionymus planus Cyprinus carpio, Carassius auratus, Hypophthalmichthys molitrix, Pseudorasbora parva, Megalobrama amblycephala, Hemiculter bleekeri | 0.04–5 mm (MPs) and 5.1–24.8 mm (mesoplastics) | Fiber, fragments, and film | 1.1 to 7.2 items/individual (MPs), 0.2 to 3.0 items/individual (mesoplastics) | [47] |
| Dicentrachus labrax, Trachurus trachurus, Scomber colias | 501–1500 µm (gastrointestinal tract) and 151–500 µm (gill) | Fiber (54%), fragments (45%), and pellets (1%) | 1.2 ± 2.0 items/individual (gastrointestinal tract), 0.7 ± 1.2 items/individual (gill) and 0.054 ± 0.099 items/g (dorsal muscle) | [48] |
| Amoya chlorostigmatoides, Acanthopagrus latus, Arius leiotetocephalus, Acentrogobius viridipunctatus, Cynoglossus puncticeps, Callionymus richardsoni, Dendrophysa russelii, Epinephelus akaara, Gerreomorpha decacantha, Gerres filamentosus, Gerres lucidus, Muraenesox cinereus, Oreochromis niloticus, Odontamblyopus rubicundus, Pisodonophis boro, Platycephalus indicus, Periophthalmus modestus, Solea ovate, Sillago sihama, Terapon jarbua, Takifugu niphobles, Zebrias zebra, Atherina bleekeri, Alepes djedaba, Caranx malam, Konosirus punctatus, Leiognathus brevirostris, Osteomugil ophuyseni, Osteomugil stronylophalus, Stolephorus commersonnii, Tylosurus melanotus, Thryssa vitrirostris | 0.02–5 mm | Fiber (70%), fragments (18%), film (9%), and pellets (3%) | 0.6 to 8.0 items/individual | [49] |
| Boops boops, Dentex macrophthalmus, Dentex maroccanus, Lepidotrigla cavillone, Mullus barbatus, Saurida lessepsianus, Trigla lucerna, Upeneus moluccensis, Upeneus pori, Pagellus erythrinus, Etrumeus golanii, Sardina pilchardus, Trachurus mediterraneus | 1.26 ± 1.38 mm | Film, fragments, pellets, and rubber | 1.3 MPs/individual | [50] |
| Cyprinus carpio | 452 ± 161 µm | Fragments (65%), film (25%), pellets (7%), and fiber (3%) | 57 MPs/150 fish | [51] |
| Anchovy | / | / | 50–100 mg/kg | [52] |
| Six batches of fish meal produced from the United States, Denmark, Myanmar, Mauritania, Mexico, Chile, Peru, Panama, China, and Russia purchased from Huangyan Ensor Feed Corporation (China) | 500–1000 µm | Fiber | 5.5 ± 1.6 items/g | [53] |
| Two kinds of fish meal comprised of fish mainly Rastrelliger kanagurta, and one kinds of fish meal comprised of fish waste | 855.82 ± 1082.90 µm | Fragments (78.2%), filaments (13.4%), and film (8.4%) | 216 particles/336 particles (64.3%) | [54] |
| Twenty-six kinds of fish meal produced from Antarctica, Chile, China, Denmark, India, Morocco, Mauritania, Norway, Peru, South Africa, South Korea, and Turkey | 4.2 ± 0.3 mm | Fiber, film, and fragments | 8.9 ± 1.0 particles/50 g | [55] |
| Sixteen commercially available angling baits products (six groundbaits, six boilies, and four pellets) | 700 µm–5 mm | Fragments | 17.4 MPs/kg (groundbaits), 6.78 mg/kg (boilies), not detected (pellets) | [56] |
| Oreochromis niloticus, Prochilodus magdalenae, Pimelodus grosskopfii | / | Fragments, film, and fiber | 2.1 ± 1.26 items/individual | [57] |
| Sparus aurata, Cyprinus carpio | 0.24 to 8.86 mm (S. aurata), 0.07 to 2.23 mm (C. carpio) | Fiber (S. aurata), fiber and fragments (C. carpio) | 0.48 items/individual (fry and adult S. aurata), 0.11 items/individual (fry and adult C. carpio) | [58] |
| Model Animal | Particle Size | MP Concentration | Time | Toxicological Effects | Reference |
|---|---|---|---|---|---|
| Sparus aurata | / | 3.33 g/kg of feed | 45 d | Did not induce stress, pathology, accumulate in the gastrointestinal tract and alter the growth rate | [58] |
| Danio rerio | PA, PE, PP, PVC: ~70 μm and PS: 0.1, 1.0, 5.0 μm | 0.001–10.0 mg/L | 10 d | Enhanced mortality and histopathology | [59] |
| Scophthalmus Maximus | 20 μm | 0, 100, 200 and 300 particles/L | 46 d | Enhanced mortality | [60] |
| Carassius auratus | 70 nm and 50 μm | 10, 100 and 1000 μg/L | 7 d | Bioaccumulation, oxidative stress, tissue (intestine, liver and gill) damage, inhibiting growth and swimming speed | [61] |
| Sebastes schlegelii | 0.5 and 15 μm | 190 μg/L | 14 d | Histopathology, altered behavior, energy reserve and nutritional quality, enhanced mortality, respiration and metabolism stress | [62] |
| Adult Danio rerio | 8 μm | 10 μg/L and 1 mg/L | 21 d | Caused microbiota dysbiosis and inflammation | [63] |
| Eriocheir sinensis | 5 μm | 0, 0.04, 0.4, 4 and 40 mg/L | 21 d | Affected non-specific immune responses and intestinal microflora | [64] |
| Danio rerio | 5 and 50 μm | 100 and 1000 μg/L | 7 d | Intestinal flora, oxidative stress, immune response, neurodevelopment, swimming behavior, hepatic metabolism, growth and metabolism | [65] |
| Danio rerio | 5 μm | 50 and 500 μg/L | 21 d | Inflammation, oxidative stress, altered in gut metabolome and microbiome | [66] |
| Larimichthys crocea | 100 nm | 0, 10, 104 and 106 items/L | 14 d | Enhanced mortality, reduced immune and digestive enzyme activities and caused microbiota dysbiosis | [67] |
| Dicentrarchus labrax L. | 104.1 ± 36.2 µm (PVC), 77.5 ± 18.3 µm (PE) | 100 or 500 mg PVC or PE kg−1 diet | 3 w | Oxidative stress, immune response, and histopathology | [68] |
| Poecilia reticulata | 33–40 μm | 100 and 1000 μg/L | 28 d | Impaired digestive performance, stimulating immune response, and inducing microbiota dysbiosis | [69] |
| Danio rerio | 0.5 and 10 μm | 0.1, 1.0, 10, 100, 200 and 500 mg/L | 3 d | Enhanced mortality and embryo hatching rates | [70] |
| Danio rerio | 70 nm, 5 μm and 20 μm | 20, 200 and 2000 μg/L | 7 d | Inflammation, altered of metabolic profiles, oxidative stress, lipid accumulation | [71] |
| Yellow River Carp | 100–200 μm | 10%, 20%, 30%, 40% PVC in diets | 60 d | Gonadal development, oxidative stress, and immune function | [72] |
| Danio rerio | 5 and 50 μm | 100 and 1000 μg/L | 7 d | Oxidative stress, altered of metabolic profiles, glycolysis-related and lipid metabolism-related genes | [73] |
| Red Crucian Carp | 124 μm | 150, 300 and 600 μg/L | 96 h | Oxidative stress | [74] |
| Oryzias melastigma | 10 μm | 2, 20 and 200 μg/L | 60 d | Oxidative stress, histopathology, decreased fecundity, altered the HPG | [75] |
| Danio rerio | 15 μm | 20 mg/L | 24 h | Histopathology, inflammation, metabolism disruption, gut microbiota dysbiosis and bacteria alterations | [76] |
| Sediment-dwelling invertebrate | 1–4, 10–27, 43–54 and 100–126 μm | 500 per kg sediments | 10 d | Enhanced mortality, body length, head capsule and emergence | [77] |
| Artemia salina | 10 μm | 1, 10, 100, 1000, and 10,000 MPs/mL | 7 d | Affected feeding behavior and life cycle | [78] |
| Artemia salina | 11.86–44.62 μm | 1, 25, 50, 75 and 100 μg/mL | 48 h | Accumulation, oxidative stress, changed swimming behavior, histopathology | [79] |
| Brine shrimp | 5 μm | 1, 25, 50, 75 and 100 mg/L | 14 d | Generation of ROS, histopathology, and transcriptome | [80] |
| Danio rerio | ~32.50 μm | 1, 10, 20 mg/L | 2 hpf–10 dpf | Growth inhibition, intestine injury, and lipid malabsorption | [81] |
| Perca flavescens | 100–125 μm | 0, 1, 2, 4 and 8 g MPs/100 g dry diet | 9 w | Changed nutrient metabolism, decreased nutritional fish quality, disrupted intestinal histopathology and microbiota diversity | [82] |
| Oreochromis urolepis | 38–45 μm | 1, 10, 100 MPs/mL | 65 d | Growth inhibition, intestine damage, impaired digestion and nutrient absorption functions | [83] |
| Oryzias latipes | 100 (larvae) and 400 (juvenile) μm | 0.5, 1.5, 3 and 6 MPs/fish/day | 21 d | Influenced digestive gene expression | [84] |
| Danio rerio | 10–20, 45–53, 250–300 μm | 3 × 104 particles/L | 12 h, 4 w | The elimination and distribution of MPs presented size-effect and time-effect relationships | [85] |
| Gobiocypris rarus larvae | 0.1, 1, 10 μm | 0.055, 0.55, 5.5, 55 and 550 μg/L | 7 d | No observed effect | [86] |
| Tigriopus japonicus | 10 μm | 1 × 103 particle/mL | 0, 3, 6, 9, 12, 24, 48 h | Accumulation | [87] |
| Xenopus laevis | 3 μm | 0.125, 1.25 and 12.5 μg/mL | Stage 36 to stage 46 | Neither body growth nor swimming activity were affected | [88] |
| Oreochromis niloticus | 0.1 μm | 1, 10 and 100 μg/L | 14 d | Neurotoxicity and oxidative stress | [89] |
| Cyprinus carpio var. | / | 10%, 20%, 30% MPs in diets | 60 d | Oxidative stress, antioxidant-related gene, growth, and histopathology | [90] |
| Carassius auratus | 0.7–5.0 mm (fiber), 2.5–3.0 mm (fragments), 4.9–5.0 mm (pellets) | 0.96%, 1.36%, 1.94%, 3.81% (g(food + MPs)/g fish) | 6 w | Observed sub-lethal effects, weight loss, histopathology, and inflammation | [91] |
| Sebastes schlegelii | 15 μm | 1 × 106 particle/L | 14 d | Weakened feeding activity and hunting behavior, histopathology, and influenced the energy reserve and nutritional quality | [92] |
| Model Animal | Concentration | Time | Toxicological Effects | Reference |
|---|---|---|---|---|
| Hippocampus kuda | MPs (15–80 μm): 0.1 g/3 L, copper: 0.05 mg/L, Cd: 0.01 mg/L, lead: 0.05 mg/L | 45 d | Growth, enhanced mortality, and oxidative stress | [107] |
| Cyprinus carpio | MPs: 250 and 500 µg/L, Cd: 100 and 200 µg/L | 30 d | Altered biochemical and immunological parameters | [108] |
| Pomatoschistus microps | MPs (1–5 μm): 0.18 mg/L, Cd: 3, 6, 13, 25 and 50 mg/L | 96 h | Observed sub-lethal effects, especially neurotoxicity | [109] |
| Moina monogolica Daday | MPs (2–4 μm): 300 µg/L, Cd: 5 and 10 µg/L | 21 d | Enhanced mortality, poor nutritional status in progeny, MPs with adsorbed Cd showed greater adverse dose-dependent effects | [110] |
| Danio rerio | MPs: 20 mg/L, Cd: 100 mg/L | 3 w | Oxidative stress, inflammation, histopathological, affected functional gene expression, and increased accumulation of Cd | [111] |
| Danio rerio | PS: 0.05, 0.1, 1, 5, 10 mg/L, Cd: 0.01 mg/L | 96 h | Negative impacts on survival and heart rate, observed lethal and sublethal effects | [112] |
| Symphysodon aequifasciatus | MPs (32–40 μm): 50 and 500 µg/L, Cd: 25 and 50 µg/L | 30 d | Reduced Cd accumulation, oxidative stress, stimulated innate immunity | [113] |
| Cyprinus carpio | MPs: 0.5 mg/L, copper: 0.25 mg/L | 14 d | Facilitated copper accumulation, induced significant hepatic stress and inflammation | [114] |
| Danio rerio | MPs: 500 µg/L, Cd: 5 µg/L | 30 d | Negative effects on growth, oxidative stress, and apoptosis | [115] |
| Danio rerio | MPs: 20 mg/L, Cd: 1 mg/L | 4 hpf–120 hpf | Oxidative stress promoting taurine metabolism and unsaturated fatty biosynthesis | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Yue, Y.; Bao, X.; Yu, H.; Tan, Y.; Tong, B.; Kumkhong, S.; Yu, Y. Microplastics as Contaminants in Water Bodies and Their Threat to the Aquatic Animals: A Mini-Review. Animals 2022, 12, 2864. https://doi.org/10.3390/ani12202864
Chen M, Yue Y, Bao X, Yu H, Tan Y, Tong B, Kumkhong S, Yu Y. Microplastics as Contaminants in Water Bodies and Their Threat to the Aquatic Animals: A Mini-Review. Animals. 2022; 12(20):2864. https://doi.org/10.3390/ani12202864
Chicago/Turabian StyleChen, Mingshi, Yuhua Yue, Xiaoxue Bao, Hui Yu, Yuansheng Tan, Binbin Tong, Suksan Kumkhong, and Yingying Yu. 2022. "Microplastics as Contaminants in Water Bodies and Their Threat to the Aquatic Animals: A Mini-Review" Animals 12, no. 20: 2864. https://doi.org/10.3390/ani12202864
APA StyleChen, M., Yue, Y., Bao, X., Yu, H., Tan, Y., Tong, B., Kumkhong, S., & Yu, Y. (2022). Microplastics as Contaminants in Water Bodies and Their Threat to the Aquatic Animals: A Mini-Review. Animals, 12(20), 2864. https://doi.org/10.3390/ani12202864