Complete Blood Count-Derived Inflammatory Markers Changes in Dogs with Chronic Inflammatory Enteropathy Treated with Adipose-Derived Mesenchymal Stem Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation and In Vitro Expansion of Adipose-Derived Mesenchymal Stem Cells (Ad-MSCs)
2.3. Study Protocol
2.4. Statistical Study
3. Results
3.1. Population
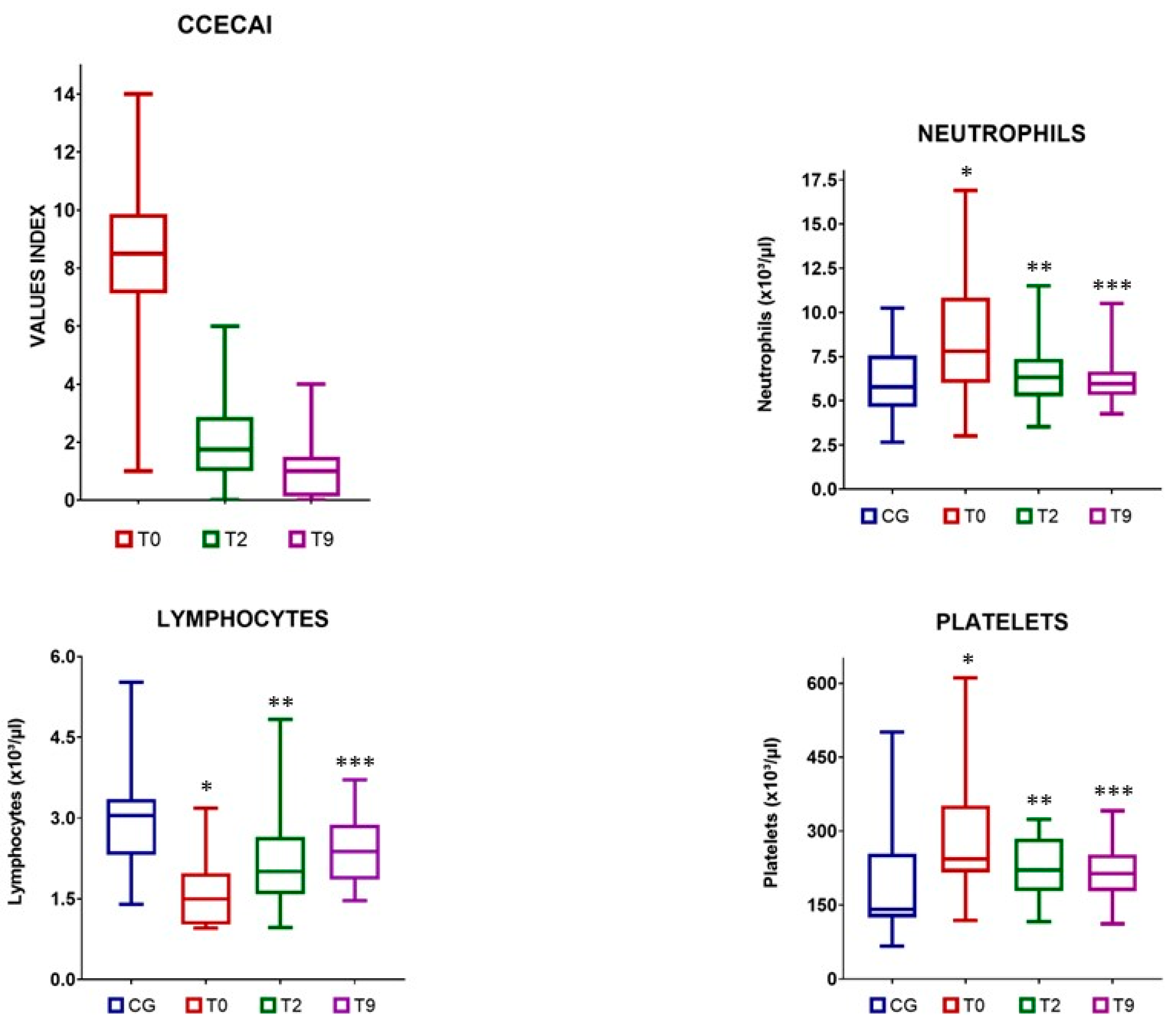
3.2. Clinical Indices
3.3. Changes in the Neutrophil, Lymphocyte, and Platelet Counts
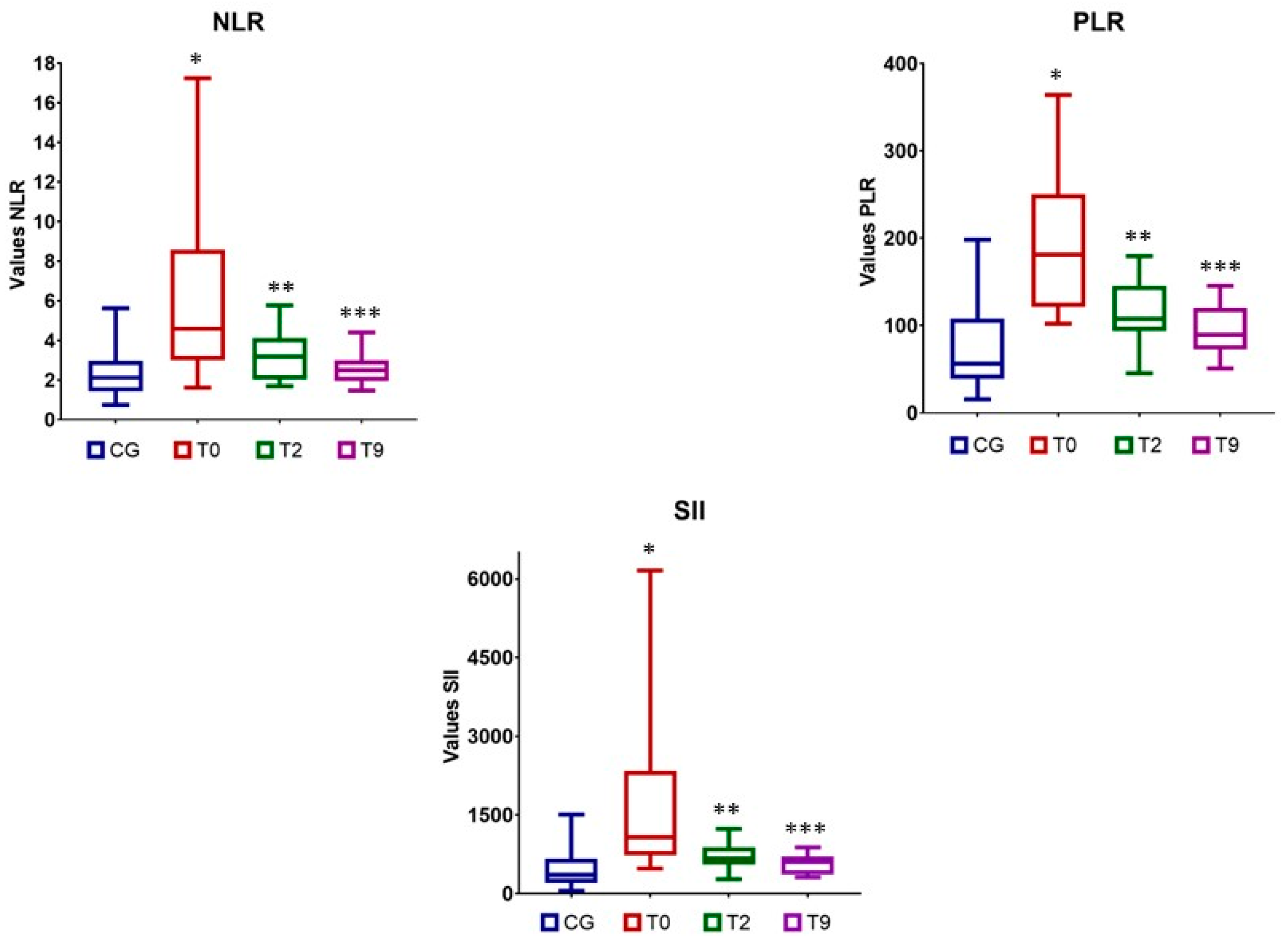
3.4. Blood Inflammatory Indices
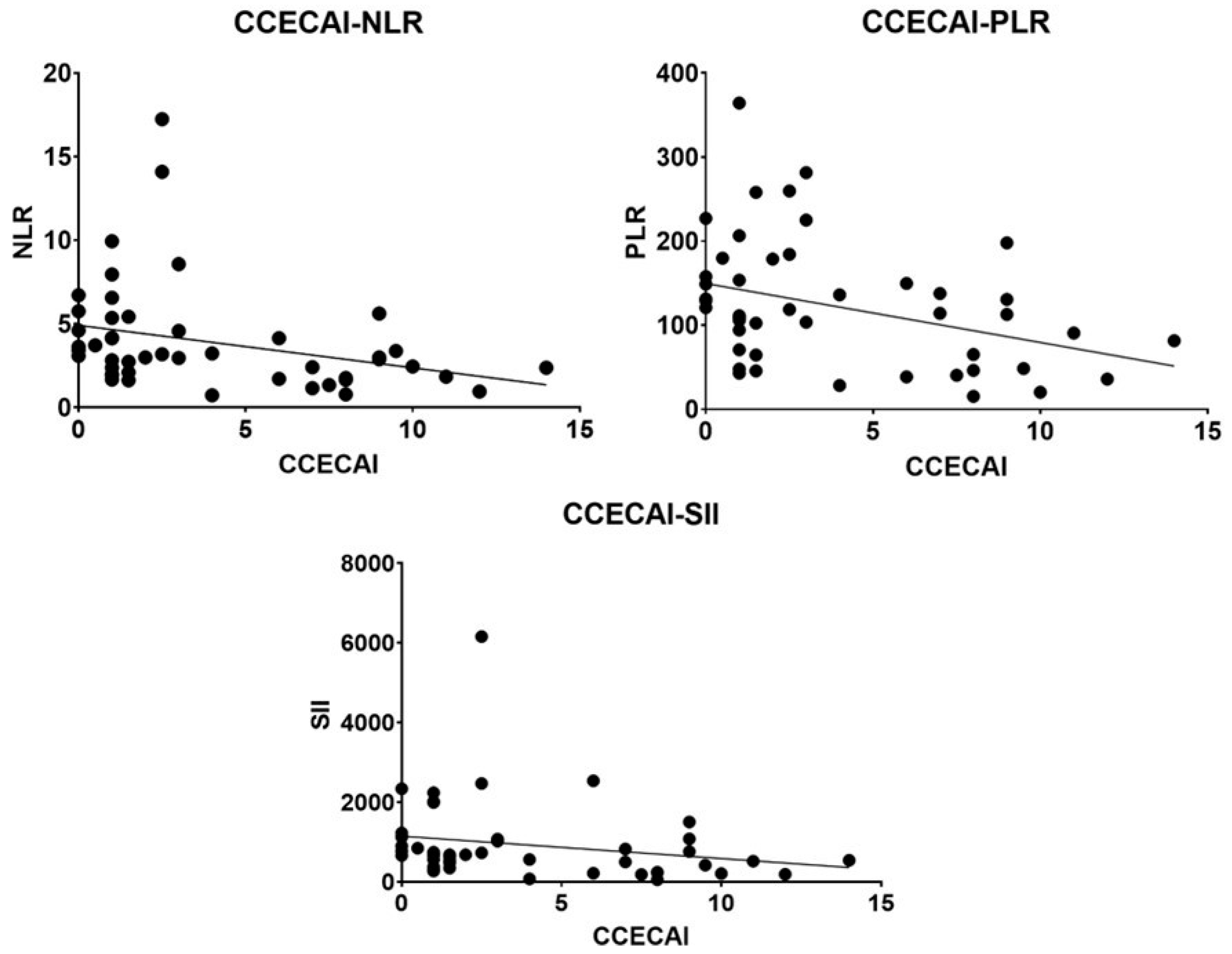
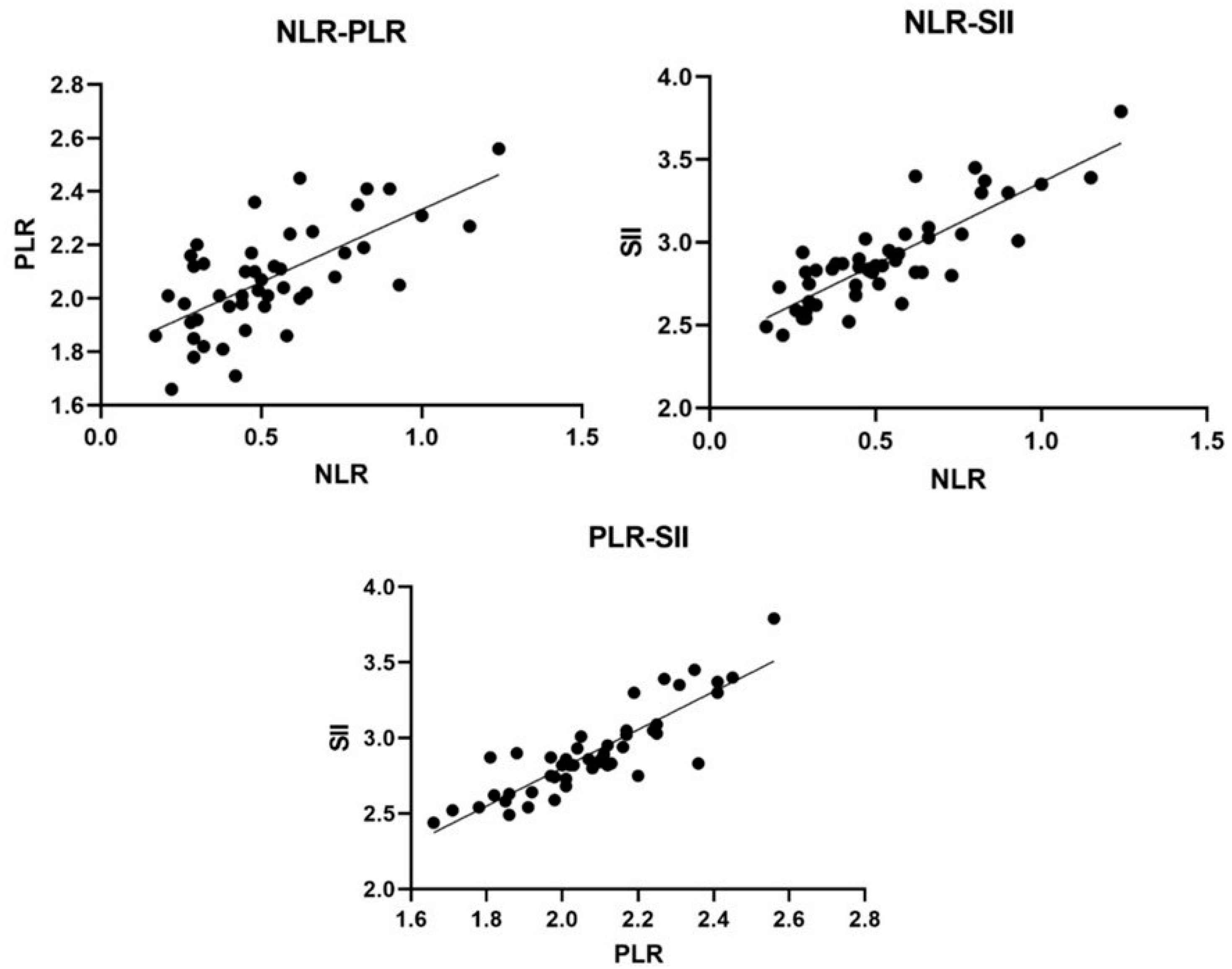
3.5. Association between Inflammatory Indices and the Parameters Analyzed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dandrieux, J.R.S. Inflammatory bowel disease versus chronic enteropathy in dogs: Are they one and the same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Corbee, R.J.; Trabalza-Marinucci, M. Nonpharmacological treatment strategies for the management of canine chronic inflammatory enteropathy-A narrative review. Vet. Sci. 2022, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Diamond, B. Autoimmune diseases. N. Engl. J. Med. 2001, 345, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; Shen, B. Perioperative use of immunosuppressive medications in patients with Crohn’s disease in the new “biological era”. Gastroenterol. Rep. 2017, 5, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Zaragoza-Bayle, C.; Duque-Carrasco, J.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Barrera-Chacon, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Clinical and laboratory outcomes. Vet. J. 2015, 206, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.L.; Webb, C.B. Stem cell therapy in cats with chronic enteropathy: A proof-of-concept study. J. Feline Med. Surg. 2015, 17, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, J.I.; Duque, F.J.; Usón-Casaús, J.M.; Ruiz, P.; López, E.; Pérez-Merino, E.M. Effects of allogeneic mesenchymal stem cell transplantation in dogs with inflammatory bowel disease treated with and without corticosteroids. Animals 2021, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Kang, K.S.; Koo, H.C.; Park, J.R.; Choi, E.W.; Park, Y.H. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008, 17, 681–693. [Google Scholar] [CrossRef]
- Matthay, M.A.; Thompson, B.T.; Read, E.J.; McKenna, D.H.; Liu, K.D.; Calfee, C.S.; Lee, J.W. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 2010, 138, 965–972. [Google Scholar] [CrossRef]
- Song, J.I.; Volz, S.; Liodaki, M.E.; Mailänder, P.; Kalousis, K. Stem cells therapy: The future in the management of systemic sclerosis? A case report. Hell. J. Nucl. Med. 2017, 20, 164. [Google Scholar] [PubMed]
- Regmi, S.; Pathak, S.; Kim, J.O.; Yong, C.S.; Jeong, J.H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019, 98, 151041. [Google Scholar] [CrossRef] [PubMed]
- Sacoor, C.; Barros, L.M.; Montezinho, L. What are the potential biomarkers that should be considered in diagnosing and managing canine chronic inflammatory enteropathies? Open Vet. J 2021, 10, 412–430. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Rossari, F.; Barberio, B.; Demarzo, M.G.; Tapete, G.; Albano, E.; Baiano Svizzero, G.; Ceccarelli, L.; Mumolo, M.G.; Brombin, C.; et al. Novel prognostic biomarkers of mucosal healing in ulcerative colitis patients treated with Anti-TNF: Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Inflamm. Bowel Dis. 2020, 26, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhuang, T.; Ping, Y.; Zhang, Y.; Wang, X.; Yu, P.; Duan, X. Elevated systemic immune inflammation index level is associated with disease activity in ulcerative colitis patients. Clin. Chim. Acta 2021, 517, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Wang, H.; Wang, H.G.; Wen, X.; Yang, X.Z. Effective immune-inflammation index for ulcerative colitis and activity assessments. World J. Clin. Cases 2021, 9, 334–343. [Google Scholar] [CrossRef]
- Benvenuti, E.; Pierini, A.; Gori, E.; Lucarelli, C.; Lubas, G.; Marchetti, V. Neutrophil-to-lymphocyte ratio (NLR) in canine inflammatory bowel disease (IBD). Vet. Sci. 2020, 7, 141. [Google Scholar] [CrossRef]
- Becher, A.; Suchodolski, J.S.; Steiner, J.M.; Heilmann, R.M. Blood neutrophil-to-lymphocyte ratio (NLR) as a diagnostic marker in dogs with chronic enteropathy. J. Vet. Diagn. Investig. 2021, 33, 516–527. [Google Scholar] [CrossRef]
- Pierini, A.; Esposito, G.; Gori, E.; Benvenuti, E.; Ruggiero, P.; Lubas, G.; Marchetti, V. Platelet abnormalities and platelet-to-lymphocyte ratios in canine immunosuppressant-responsive and non-responsive enteropathy: A retrospective study in 41 dogs. J. Vet. Med. Sci. 2021, 83, 248–253. [Google Scholar] [CrossRef]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, X.; Zhang, W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: Interaction effect with disease severity-a retrospective study. BMJ Open 2019, 9, e022896. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Han, X.; Zha, H.; Tao, H.; Li, X.; Yuan, F.; Chen, G.; Wang, L.; Ma, J.; Hu, Y. Systemic Immune-Inflammation Index and Changes of Neutrophil-Lymphocyte Ratio as Prognostic Biomarkers for Patients With Pancreatic Cancer Treated With Immune Checkpoint Blockade. Front. Oncol. 2021, 11, 585271. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Bilzer, T.; Mansell, J.; Wilcock, B.; Hall, E.J.; Jergens, A.; Minami, T.; Willard, M.; Washabau, R. World Small Animal Veterinary Association Gastrointestinal Standardization Group. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 2008, 138 (Suppl. 1), S1–S43. [Google Scholar] [PubMed]
- Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W. WSAVA International Gastrointestinal Standardization Group. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar] [PubMed]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Blackwood, L. Disorders of leukocytes. In BSAVA Manual of Canine and Feline Clinical Pathology, 3rd ed.; Villiers, E., Ristić, J., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2016; pp. 67–93. [Google Scholar]
- Marchetti, V.; Lubas, G.; Lombardo, A.; Corazza, M.; Guidi, G.; Cardini, G. Evaluation of erythrocytes, platelets, and serum iron profile in dogs with chronic enteropathy. Vet. Med. Int. 2010, 2010, 716040. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Cassinotti, A.; Onida, F.; Trabattoni, D.; Annaloro, C.; Della Volpe, A.; Rainone, V.; Lissoni, F.; Duca, P.; Sampietro, G.; et al. Immunomodulatory effects of unselected haematopoietic stem cells autotransplantation in refractory Crohn’s disease. Dig. Liver Dis. 2011, 43, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Wang, Z.H.; Cai, S.X. Mesenchymal stem cell attenuates neutrophil-predominant inflammation and acute lung injury in an in vivo rat model of ventilator-induced lung injury. Chin. Med. J. 2015, 128, 361–367. [Google Scholar] [CrossRef]
- Neumann, S. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in dogs and cats with acute pancreatitis. Vet. Clin. Pathol. 2021, 50, 45–51, Erratum in: Vet. Clin. Pathol. 2021, 50, 469. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.S.; Fernandes, P.C.; Silva, M.J.; Lima, V.C.; Fontes, W.; Freitas Jr., R.; Eterovic, A.K.; Forget, P. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 2016, 10, 702. [Google Scholar]
- Xing, L.; Lijiang, G.; Yuhang, C.; Yue, C.; Xinyang, W.; Peng, G.; Dalin, H. Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: A systematic review and meta-analysis. Ann. Med. 2021, 53, 1827–1838. [Google Scholar]
- Simadibrata, D.M.; Pandhita, B.A.W.; Ananta, M.E.; Tango, T. Platelet-to-lymphocyte ratio, a novel biomarker to predict the severity of COVID-19 patients: A systematic review and meta-analysis. J. Intensive Care Soc. 2022, 23, 20–26. [Google Scholar] [CrossRef]
- Okba, A.M.; Amin, M.M.; Abdelmoaty, A.S.; Ebada, H.E.; Kamel, A.H.; Allam, A.S.; Sobhy, O.M. Neutrophil/lymphocyte ratio and lymphocyte/monocyte ratio in ulcerative colitis as non-invasive biomarkers of disease activity and severity. Auto. Immun. Highlights 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Jeon, S.R.; Kim, H.G.; Moon, J.R.; Lee, T.H.; Jang, J.Y.; Cho, J.H.; Park, J.S.; Park, H.; Lee, K.H.; et al. The role of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in ulcerative colitis. Intest. Res. 2021, 19, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Yagi, S.; Shiraishi, K.; Mori, K.; Ninomiya, T.; Kawasaki, K.; Mizukami, Y.; Suzuki, S.; Uraoka, M.; Shibata, N.; et al. Association between platelet count and mucosal healing in Japanese patients with ulcerative colitis: A cross-sectional study. BMC Gastroenterol. 2020, 20, 384. [Google Scholar] [CrossRef]
- Djaballah-Ider, F.; Touil-Boukoffa, C. Effect of combined colchicine-corticosteroid treatment on neutrophil/lymphocyte ratio: A predictive marker in Behçet disease activity. Inflammopharmacology 2020, 28, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Sgrignoli, M.R.; Silva, D.A.; Nascimento, F.F.; Sgrignoli, D.A.M.; Nai, G.A.; da Silva, M.G.; de Barros, M.A.; Bittencourt, M.K.W.; de Morais, B.P.; Dinallo, H.R.; et al. Reduction in the inflammatory markers CD4, IL-1, IL-6 and TNFα in dogs with keratoconjunctivitis sicca treated topically with mesenchymal stem cells. Stem Cell Res. 2019, 39, 101525. [Google Scholar] [CrossRef]
- Muir, P.; Hans, E.C.; Racette, M.; Volstad, N.; Sample, S.J.; Heaton, C.; Holzman, G.; Schaefer, S.L.; Bloom, D.D.; Bleedorn, J.A.; et al. Autologous Bone Marrow-Derived Mesenchymal Stem Cells Modulate Molecular Markers of Inflammation in Dogs with Cruciate Ligament Rupture. PLoS ONE 2016, 11, e0159095. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal stem cells: A friend or foe in immune-mediated diseases. Stem Cell Rev. Rep. 2015, 11, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, F.; Krampera, M. Mesenchymal stem cells and autoimmune diseases. Best Pract. Res. Clin. Haematol. 2011, 24, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Ricci, E.; Migliorati, G.; Gentili, M.; Riccardi, C. How Glucocorticoids Affect the Neutrophil Life. Int. J. Mol. Sci. 2018, 19, 4090. [Google Scholar] [CrossRef]
- Waldner, M.; Zhang, W.; James, I.B.; Allbright, K.; Havis, E.; Bliley, J.M.; Almadori, A.; Schweizer, R.; Plock, J.A.; Washington, K.M.; et al. Characteristics and Immunomodulating Functions of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells Across Defined Human Leukocyte Antigen Barriers. Front. Immunol. 2018, 9, 1642. [Google Scholar] [CrossRef]
| Reference Value | T0 | T2 | T9 | p | |
|---|---|---|---|---|---|
| CCECAI | 8.5 (1–14) a | 1.75 (0–6) b | 1 (0–4) c | <0.0001 | |
| Effect size (95% CI) | - | 2.83 (1.79; 3.73) | 3.42 (2.26; 4.40) | ||
| Neutrophils (×103/µL) | 5.78 (2.65–10.24) | 7.8 (3–16.9) a | 6.25 (3.53–7.9) b | 5.88 (4.26–7.12) b | 0.003 |
| Effect size (95% CI) | 0.85 (0.11; 1.55) | 0.96 (0.21; 1.67) | |||
| Lymphocytes (×103/µL) | 3.05 (1.40–5.52) | 1.49 (0.95–3.18) a | 2 (0.96–4.83) a | 2.38 (1.46–3.71) b | 0.039 |
| Effect size (95% CI) | −0.76 (−1.46; −0.03) | −1.25 (−1.97; −0.46) | |||
| Platelets (×103/µL) | 141 (67–501) | 243.5 (119–611) a | 221 (116–324) a | 214 (112–341) b | 0.019 |
| Effect size (95% CI) | 0.60 (−0.12; 1.29) | 0.76 (0.02; 1.46) | |||
| NLR | 2.11 (0.74–5.62) | 4.58 (1.62–17.24) a | 3.24 (1.68–5.76) b | 2.35 (1.47–4.39) c | 0.002 |
| Effect size (95% CI) | 0.96 (0.20; 1.66) | 1.21 (0.43; 1.93) | |||
| PLR | 56.41 (15.34–198.02) | 181.42 (102.35–364.29) a | 107.54 (45.53–179.73) b | 89.37 (50.80–145.40) c | |
| Effect size (95% CI) | 1.28 (0.49; 2.01) | 1.72 (0.87; 2.48) | |||
| SII (×103) | 360.98 (52.93–1503) | 1071.76 (475.94–6156.43) a | 662.13 (273.18–1227.55) b | 547.66 (308.08–878.23) c | |
| Effect size (95% CI) | 0.99 (0.23; 1.69) | 1.16 (0.39; 1.88) |
| NLR | PLR | SII | ||||
|---|---|---|---|---|---|---|
| Spearman ρ | p Value | Spearman ρ | p Value | Spearman ρ | p Value | |
| Neutrophils | 0.71 | <0.0001 | 0.38 | 0.01 | 0.73 | <0.0001 |
| Lymphocytes | −0.82 | <0.0001 | −0.67 | <0.0001 | −0.59 | <0.0001 |
| Platelets | 0.003 | 0.99 | 0.54 | <0.0001 | 0.54 | <0.0001 |
| NLR | - | - | 0.62 | <0.0001 | 0.77 | <0.0001 |
| PLR | 0.63 | <0.0001 | - | - | 0.87 | <0.0001 |
| SII | 0.77 | <0.0001 | 0.87 | <0.0001 | - | - |
| CCECAI | 0.52 | <0.0001 | 0.53 | <0.0001 | 0.495 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristóbal, J.I.; Duque, F.J.; Usón-Casaús, J.; Barrera, R.; López, E.; Pérez-Merino, E.M. Complete Blood Count-Derived Inflammatory Markers Changes in Dogs with Chronic Inflammatory Enteropathy Treated with Adipose-Derived Mesenchymal Stem Cells. Animals 2022, 12, 2798. https://doi.org/10.3390/ani12202798
Cristóbal JI, Duque FJ, Usón-Casaús J, Barrera R, López E, Pérez-Merino EM. Complete Blood Count-Derived Inflammatory Markers Changes in Dogs with Chronic Inflammatory Enteropathy Treated with Adipose-Derived Mesenchymal Stem Cells. Animals. 2022; 12(20):2798. https://doi.org/10.3390/ani12202798
Chicago/Turabian StyleCristóbal, José Ignacio, Francisco Javier Duque, Jesús Usón-Casaús, Rafael Barrera, Esther López, and Eva María Pérez-Merino. 2022. "Complete Blood Count-Derived Inflammatory Markers Changes in Dogs with Chronic Inflammatory Enteropathy Treated with Adipose-Derived Mesenchymal Stem Cells" Animals 12, no. 20: 2798. https://doi.org/10.3390/ani12202798
APA StyleCristóbal, J. I., Duque, F. J., Usón-Casaús, J., Barrera, R., López, E., & Pérez-Merino, E. M. (2022). Complete Blood Count-Derived Inflammatory Markers Changes in Dogs with Chronic Inflammatory Enteropathy Treated with Adipose-Derived Mesenchymal Stem Cells. Animals, 12(20), 2798. https://doi.org/10.3390/ani12202798






