Glycogen Synthase Kinase 3β (GSK3β) Regulates Myogenic Differentiation in Skeletal Muscle Satellite Cells of Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Sheep SMSCs Isolation
2.3. Plasmid Construct and Transfection
2.4. Cell Culture and GSK3β Inhibitor Treatment
2.5. Quantitative PCR (qPCR)
2.6. Western Blotting
3. Results
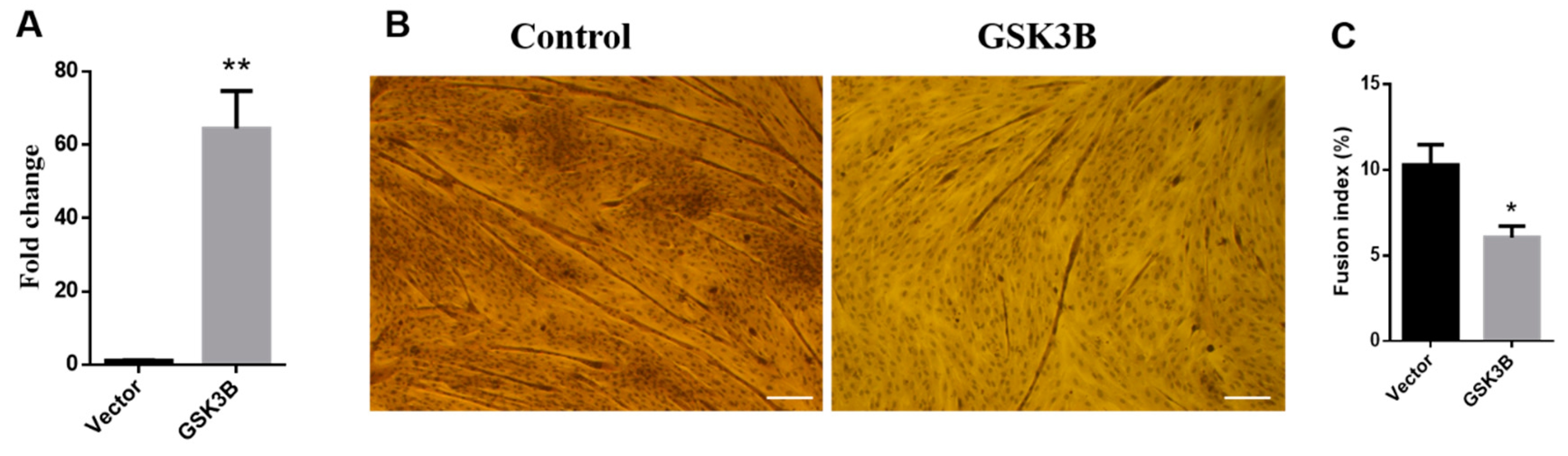
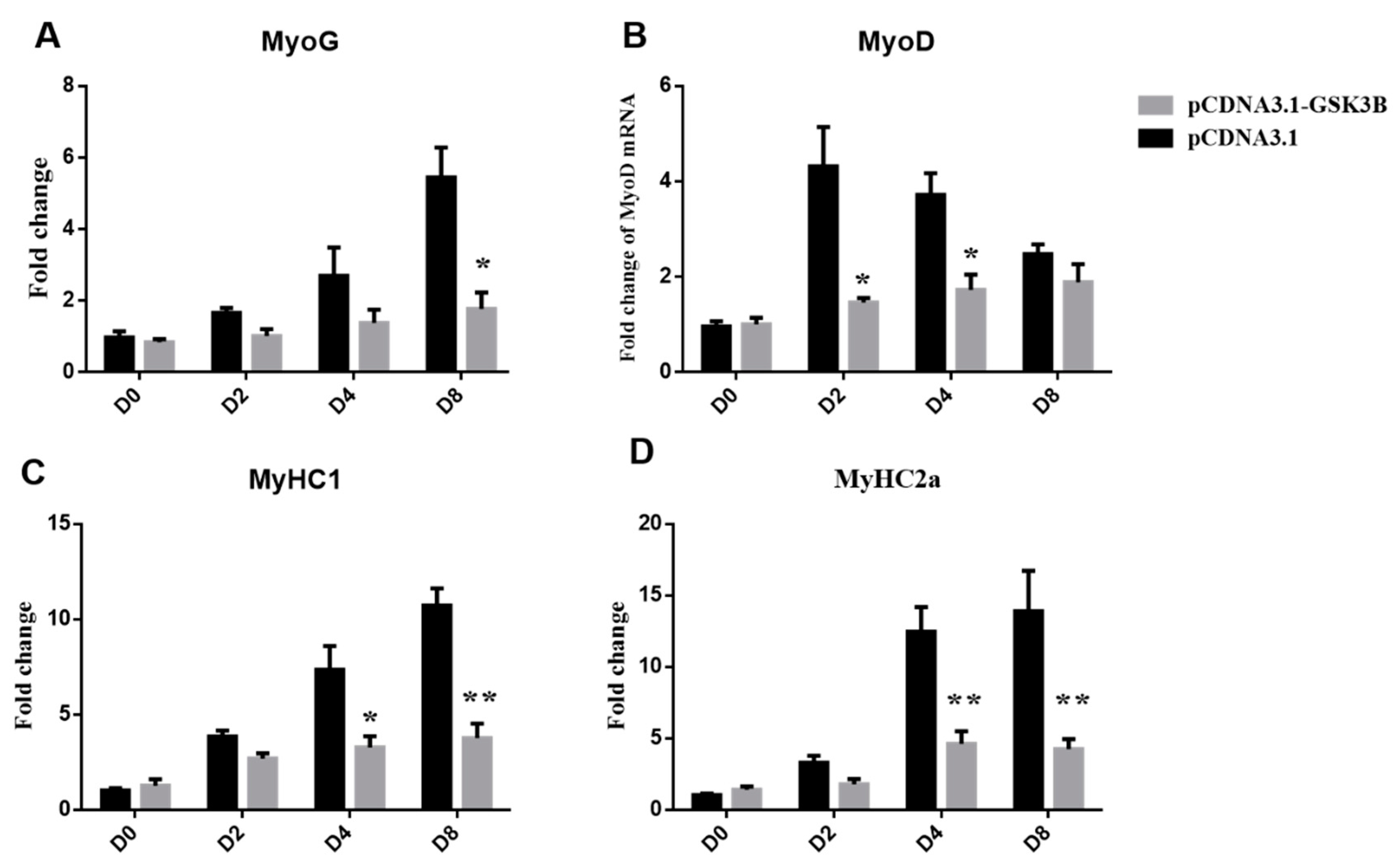
3.1. Overexpression of GSK3β Decreases Differentiation in Sheep SMSCs
3.2. Different Effects of Time-Course SB216763 Treatment on the Phosphorylation Levels of GSK3β in Sheep SMSCs
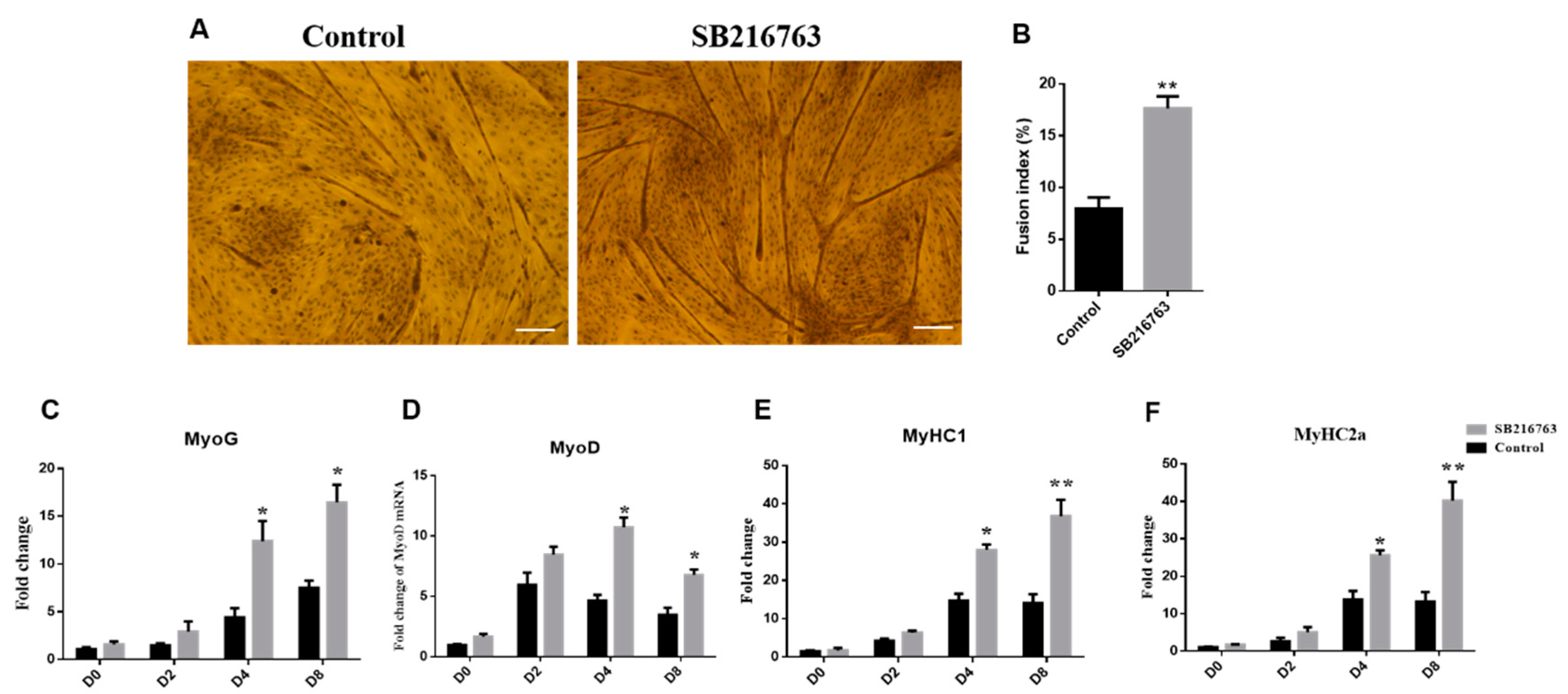
3.3. GSK3β Inhibition Promotes Differentiation of Sheep SMSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Woodgett, J.R.; Cohen, P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim. Biophys. Acta 1984, 788, 339–347. [Google Scholar] [CrossRef]
- Rylatt, D.B.; Aitken, A.; Bilham, T.; Condon, G.D.; Embi, N.; Cohen, P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur. J. Biochem. 1980, 107, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Vieyra, R.; Bravo-Patino, A.; Valdez-Alarcon, J.J.; Juarez, M.C.; Finlay, B.B.; Baizabal-Aguirre, V.M. Role of glycogen synthase kinase-3 beta in the inflammatory response caused by bacterial pathogens. J. Inflamm. 2012, 9, 23. [Google Scholar] [CrossRef]
- Hinds, T.D.; Burns, K.A., Jr.; Hosick, P.A.; McBeth, L.; Nestor-Kalinoski, A.; Drummond, H.A.; AlAmodi, A.A.; Hankins, M.W.; Vanden Heuvel, J.P.; Stec, D.E. Biliverdin Reductase A Attenuates Hepatic Steatosis by Inhibition of Glycogen Synthase Kinase (GSK) 3β Phosphorylation of Serine 73 of Peroxisome Proliferator-activated Receptor (PPAR) α. J. Biol. Chem. 2016, 291, 25179–25191. [Google Scholar] [CrossRef] [PubMed]
- Markussen, L.K.; Winther, S.; Wicksteed, B.; Hansen, J.B. GSK3 is a negative regulator of the thermogenic program in brown adipocytes. Sci. Rep. 2018, 8, 3469. [Google Scholar] [CrossRef] [PubMed]
- Zaragosi, L.E.; Wdziekonski, B.; Fontaine, C.; Villageois, P.; Peraldi, P.; Dani, C. Effects of GSK3 inhibitors on in vitro expansion and differentiation of human adipose-derived stem cells into adipocytes. BMC Cell Biol. 2008, 9, 11. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, W.; Liang, X.; Rao, Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: Critical roles of GSK-3beta and its upstream regulators. Cell 2005, 120, 123–135. [Google Scholar]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef]
- Chang, N.C.; Rudnicki, M.A. Satellite cells: The architects of skeletal muscle. Curr. Top. Dev. Biol. 2014, 107, 161–181. [Google Scholar] [PubMed]
- Li, J.; Pei, Y.; Zhou, R.; Tang, Z.; Yang, Y. Regulation of RNA N(6)-methyladenosine modification and its emerging roles in skeletal muscle development. Int. J. Biol. Sci. 2021, 17, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F. Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Kerkela, R.; Kockeritz, L.; Macaulay, K.; Zhou, J.; Doble, B.W.; Beahm, C.; Greytak, S.; Woulfe, K.; Trivedi, C.M.; Woodgett, J.R.; et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J. Clin. Investig. 2008, 118, 3609–3618. [Google Scholar] [CrossRef] [PubMed]
- Dionyssiou, M.G.; Nowacki, N.B.; Hashemi, S.; Zhao, J.; Kerr, A.; Tsushima, R.G.; McDermott, J.C. Cross-talk between glycogen synthase kinase 3β (GSK3β) and p38MAPK regulates myocyte enhancer factor 2 (MEF2) activity in skeletal and cardiac muscle. J. Mol. Cell. Cardiol. 2013, 54, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.R.; Spangenburg, E.E.; Abraha, T.W.; Childs, T.E.; Booth, F.W. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am. J. Physiol. Cell Physiol. 2002, 283, C545–C551. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, S.; Lang, C.H.; Vary, T.C. Inhibition of glycogen synthase kinase 3[beta] activity with lithium in vitro attenuates sepsis-induced changes in muscle protein turnover. Shock 2011, 35, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Whitley, K.C.; Hamstra, S.I.; Baranowski, R.W.; Watson, C.J.F.; MacPherson, R.E.K.; MacNeil, A.J.; Roy, B.D.; Vandenboom, R.; Fajardo, V.A. GSK3 inhibition with low dose lithium supplementation augments murine muscle fatigue resistance and specific force production. Physiol. Rep. 2020, 8, e14517. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Liu, X.; Chao, Z.; Wang, Y.; Zhong, T.; Guo, J.; Zhan, S.; Li, L.; Zhang, H. Glycogen synthase kinase 3β (GSK3β) regulates the expression of MyHC2a in goat skeletal muscle satellite cells (SMSCs). Anim. Sci. J. Nihon Chikusan Gakkaiho 2019, 90, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, H.; DiMario, J.X. Control of slow myosin heavy chain 2 gene expression by glycogen synthase kinase activity in skeletal muscle fibers. Cell Tissue Res. 2006, 323, 489–494. [Google Scholar] [CrossRef]
- Verhees, K.J.; Schols, A.M.; Kelders, M.C.; Op den Kamp, C.M.; van der Velden, J.L.; Langen, R.C. Glycogen synthase kinase-3β is required for the induction of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2011, 301, C995–C1007. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.L.; Langen, R.C.; Kelders, M.C.; Willems, J.; Wouters, E.F.; Janssen-Heininger, Y.M.; Schols, A.M. Myogenic differentiation during regrowth of atrophied skeletal muscle is associated with inactivation of GSK-3beta. Am. J. Physiol. Cell Physiol. 2007, 292, C1636–C1644. [Google Scholar] [CrossRef] [PubMed]
- Pansters, N.A.; van der Velden, J.L.; Kelders, M.C.; Laeremans, H.; Schols, A.M.; Langen, R.C. Segregation of myoblast fusion and muscle-specific gene expression by distinct ligand-dependent inactivation of GSK-3β. Cell. Mol. Life Sci. 2011, 68, 523–535. [Google Scholar] [CrossRef]
- Prache, S.; Schreurs, N.; Guillier, L. Review: Factors affecting sheep carcass and meat quality attributes. Anim. Int. J. Anim. Biosci. 2022, 16 (Suppl. S1), 100330. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, Y.; Wang, Y.; Zhong, T.; Li, L.; Zhang, H.; Wang, L. Multiple alternative splicing and differential expression pattern of the glycogen synthase kinase-3β (GSK3β) gene in goat (Capra hircus). PLoS ONE 2014, 9, e109555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Li, X.; Chao, Z.; Zhong, T.; Guo, J.; Wang, Y.; Li, L.; Zhang, H. Transcriptional Regulation of NAMPT Gene by Glycogen Synthase Kinase 3β in Goat Adipocytes. DNA Cell Biol. 2019, 38, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zuo, B.; Xu, D.; Ren, Z.; Zhang, H.; Li, X.; Lei, M.; Xiong, Y. Alternative splicing of the porcine glycogen synthase kinase 3beta (GSK-3beta) gene with differential expression patterns and regulatory functions. PLoS ONE 2012, 7, e40250. [Google Scholar]
- Ring, D.B.; Johnson, K.W.; Henriksen, E.J.; Nuss, J.M.; Goff, D.; Kinnick, T.R.; Ma, S.T.; Reeder, J.W.; Samuels, I.; Slabiak, T.; et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes 2003, 52, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Nikoulina, S.E.; Ciaraldi, T.P.; Mudaliar, S.; Carter, L.; Johnson, K.; Henry, R.R. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes 2002, 51, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Shinoda, K.; Ohyama, K.; Sharp, L.Z.; Kajimura, S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 2013, 504, 163–167. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Zhan, S.; Guo, J.; Yang, S.; Zhong, T.; Li, L.; Zhang, H.; Wang, Y. Inhibition of GSK3β Reduces Ectopic Lipid Accumulation and Induces Autophagy by the AMPK Pathway in Goat Muscle Satellite Cells. Cells 2019, 8, 1378. [Google Scholar] [CrossRef]
- Pansters, N.A.; Schols, A.M.; Verhees, K.J.; de Theije, C.C.; Snepvangers, F.J.; Kelders, M.C.; Ubags, N.D.; Haegens, A.; Langen, R.C. Muscle-specific GSK-3β ablation accelerates regeneration of disuse-atrophied skeletal muscle. Biochim. Biophys. Acta 2015, 1852, 490–506. [Google Scholar] [CrossRef]
- Litwiniuk, A.; Pijet, B.; Pijet-Kucicka, M.; Gajewska, M.; Pająk, B.; Orzechowski, A. FOXO1 and GSK-3β Are Main Targets of Insulin-Mediated Myogenesis in C2C12 Muscle Cells. PLoS ONE 2016, 11, e0146726. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.L.; Langen, R.C.; Kelders, M.C.; Wouters, E.F.; Janssen-Heininger, Y.M.; Schols, A.M. Inhibition of glycogen synthase kinase-3beta activity is sufficient to stimulate myogenic differentiation. Am. J. Physiol.-Cell Physiol. 2006, 290, C453–C462. [Google Scholar] [CrossRef]
- Agley, C.C.; Lewis, F.C.; Jaka, O.; Lazarus, N.R.; Velloso, C.; Francis-West, P.; Ellison-Hughes, G.M.; Harridge, S.D.R. Active GSK3β and an intact β-catenin TCF complex are essential for the differentiation of human myogenic progenitor cells. Sci. Rep. 2017, 7, 13189. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Chang, M.W.; Pandey, P.R.; Tsitsipatis, D.; Yang, X.; Martindale, J.L.; Munk, R.; De, S.; Abdelmohsen, K.; Gorospe, M. Interaction of OIP5-AS1 with MEF2C mRNA promotes myogenic gene expression. Nucleic Acids Res. 2020, 48, 12943–12956. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Wang, Y. GSK3beta inhibition attenuates LPS-induced IL-6 expression in porcine adipocytes. Sci. Rep. 2018, 8, 15967. [Google Scholar] [CrossRef] [PubMed]
- Westmark, P.R.; Garrone, B.; Ombrato, R.; Milanese, C.; Di Giorgio, F.P.; Westmark, C.J. Testing Fmr1 (KO) Phenotypes in Response to GSK3 Inhibitors: SB216763 versus AFC03127. Front. Mol. Neurosci. 2021, 14, 751307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Ding, X.J.; Dai, H.Y.; Peng, W.S.; Guo, N.F.; Zhang, Y.; Zhou, Q.L.; Chen, X.L. SB-216763, a GSK-3β inhibitor, protects against aldosterone-induced cardiac, and renal injury by activating autophagy. J. Cell. Biochem. 2018, 119, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Schulz, L.; Pries, R.; Lanka, A.S.; Drenckhan, M.; Rades, D.; Wollenberg, B. Inhibition of GSK3α/β impairs the progression of HNSCC. Oncotarget 2018, 9, 27630–27644. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Y.; Xiong, Z.; Kong, J.; Fu, X.; Shen, F.; Huang, Z. Inhibition of glycogen synthase kinase 3beta ameliorates triptolide-induced acute cardiac injury by desensitizing mitochondrial permeability transition. Toxicol. Appl. Pharmacol. 2016, 313, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Torres, O.C.; Szczesna, K.; Roa, L.; Casal, C.; Gonzalez-Somermeyer, L.; Soler, M.; Velasco, C.D.; Martínez-San Segundo, P.; Petazzi, P.; Sáez, M.A.; et al. Inhibition of Gsk3b Reduces Nfkb1 Signaling and Rescues Synaptic Activity to Improve the Rett Syndrome Phenotype in Mecp2-Knockout Mice. Cell Rep. 2018, 23, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Baghaban Eslaminejad, M.; Karimi, N.; Shahhoseini, M. Enhancement of Glycosaminoglycan-Rich Matrix Production in Human Marrow-Derived Mesenchymal Stem Cell Chondrogenic Culture by Lithium Chloride and SB216763 Treatment. Cell J. 2011, 13, 117–126. [Google Scholar]
- Romagnoli, C.; Iantomasi, T.; Brandi, M.L. Available In Vitro Models for Human Satellite Cells from Skeletal Muscle. Int. J. Mol. Sci. 2021, 22, 13221. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Yang, H.; Wang, L.; Zhou, P. Glycogen Synthase Kinase 3β (GSK3β) Regulates Myogenic Differentiation in Skeletal Muscle Satellite Cells of Sheep. Animals 2022, 12, 2789. https://doi.org/10.3390/ani12202789
Yang J, Yang H, Wang L, Zhou P. Glycogen Synthase Kinase 3β (GSK3β) Regulates Myogenic Differentiation in Skeletal Muscle Satellite Cells of Sheep. Animals. 2022; 12(20):2789. https://doi.org/10.3390/ani12202789
Chicago/Turabian StyleYang, Jingquan, Haosen Yang, Linjie Wang, and Ping Zhou. 2022. "Glycogen Synthase Kinase 3β (GSK3β) Regulates Myogenic Differentiation in Skeletal Muscle Satellite Cells of Sheep" Animals 12, no. 20: 2789. https://doi.org/10.3390/ani12202789
APA StyleYang, J., Yang, H., Wang, L., & Zhou, P. (2022). Glycogen Synthase Kinase 3β (GSK3β) Regulates Myogenic Differentiation in Skeletal Muscle Satellite Cells of Sheep. Animals, 12(20), 2789. https://doi.org/10.3390/ani12202789




