Effects of Fat Pre-Emulsification on the Growth Performance, Serum Biochemical Index, Digestive Enzyme Activities, Nutrient Utilization, and Standardized Ileal Digestibility of Amino Acids in Pekin Ducks Fed Diets with Different Fat Sources
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Management
2.2. Sample Collection and Determination
2.3. Assays to Determine the Standardized Ileal Amino Acid Digestibility and Nutrient Utilization of the Diets
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Liver Index and Serum Biochemical Parameters
3.3. Intestinal Digestive Enzyme Activities
3.4. Nutrient Utilization
3.5. Standardized Ileal Digestibility of Amino Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NRC. Nutrient Requirements of Poultry, 9th ed.; National Academic Press: Washington, DC, USA, 1994. [Google Scholar]
- Nayebpor, M.; Hashemi, A.; Farhomand, P. Influence of soybean oil on growth performance, carcass properties, abdominal fat deposition and humoral immune response in male broiler chickens. J. Anim. Vet. Adv. 2007, 6, 1317–1322. [Google Scholar]
- Pesti, G.M.; Bakalli, R.I.; Qiao, M.; Sterling, K.G. A comparison of eight grades of fat as broiler feed ingredients. Poult. Sci. 2002, 81, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Park, J.W.; Park, J.H.; Kim, I.H. Efficacy of 1,3-diacylglycerol as a fat emulsifier in low-density diet for broilers. Poult. Sci. 2017, 96, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Haetinger, V.S.; Dalmoro, Y.K.; Godoy, G.L.; Lang, M.B.; De Souza, O.F.; Aristimunha, P.; Stefanello, C. Optimizing cost, growth performance, and nutrient absorption with a bio-emulsifier based on lysophospholipids for broiler chickens. Poult. Sci. 2021, 100, 101025. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H. Application Research of Substitution of Emulsifier for Partial Oil in the Production of Broilers. Master Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Zampiga, M.; Meluzzi, A.; Sirri, F. Effect of dietary supplementation of lysophospholipids on productive performance, nutrient digestibility and carcass quality traits of broiler chickens. Ital. J. Anim. Sci. 2016, 15, 521–528. [Google Scholar] [CrossRef]
- Rovers, M.; Excentials, O. Saving energy and feed cost with nutritional emulsifier. Intl. Poult. Prod. 2014, 22, 7–8. [Google Scholar]
- Kaczmarek, S.A.; Bochenek, M.; Samuelsson, A.; Rutkowski, A. Effects of glyceryl polyethylene glycol ricinoleate on nutrient utilisation and performance of broiler chickens. Arch. Anim. Nutr. 2015, 69, 285–296. [Google Scholar] [CrossRef]
- Aguilar, Y.M.; Becerra, J.C.; Bertot, R.R.; Peláez, J.C.; Liu, G.; Hurtado, C.B. Growth performance, carcass traits and lipid profile of broiler chicks fed with an exogenous emulsifier and increasing levels of energy provided by palm oil. J. Food. Agric. Environ. 2013, 11, 629–633. [Google Scholar]
- Øverland, M.; Tokach, M.D.; Cornelius, S.G.; Pettigrew, J.E.; Rust, J.W. Lecithin in swine diets: I. Weanling pigs. J. Anim. Sci. 1993, 71, 1187–1193. [Google Scholar] [CrossRef]
- Øverland, M.; Tokach, M.D.; Cornelius, S.G.; Pettigrew, J.E.; Wilson, M.E. Lecithin in swine diets: II. Growing-finishing pigs. J. Anim. Sci. 1993, 71, 1194–1197. [Google Scholar] [CrossRef]
- Øverland, M.; Mroz, Z.; Sundstøl, F. Effect of lecithin on the apparent ileal and overall digestibility of crude fat and fatty acids in pigs. J. Anim. Sci. 1994, 72, 2022–2028. [Google Scholar] [CrossRef]
- Siyal, F.A.; Babazadeh, D.; Wang, C.; Arain, M.A.; Saeed, M.; Ayasan, T.; Zhang, L.; Wang, T. Emulsifiers in the poultry industry. World’s Poult. Sci. J. 2017, 73, 611–620. [Google Scholar] [CrossRef]
- Mun, S.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Res. Int. 2007, 40, 770–781. [Google Scholar] [CrossRef]
- Garaiova, I.; Guschina, I.A.; Plummer, S.F.; Tang, J.; Wang, D.; Plummer, N.T. A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr. J. 2007, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Haitao, L.; Zhao, D.; Guo, Y.; Barri, A. Effect of fat type and lysophosphatidylcholine addition to broiler diets on performance, apparent digestibility of fatty acids, and apparent metabolizable energy content. Anim. Feed Sci. Technol. 2011, 163, 177–184. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, X.; Luo, Y.; Ding, X.; Bai, S.; Wang, J.; Xuan, Y.; Su, Z.; Liu, Y.; Zhang, K. Effects of a multi-enzyme complex on growth performance, nutrient utilization and bone mineralization of meat duck. J. Anim. Sci. Biotechnol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Wiseman, J.; Salvador, F. The influence of free fatty acid content and degree of saturation on the apparent metabolizable energy value of fats fed to broilers. Poult. Sci. 1991, 70, 573–582. [Google Scholar] [CrossRef]
- Zollitsch, W.; Knaus, W.; Aichinger, F.; Lettner, F. Effects of different dietary fat sources on performance and carcass characteristics of broilers. Anim. Feed Sci. Technol. 1997, 66, 63–73. [Google Scholar] [CrossRef]
- Guerreiro Neto, A.C.; Pezzato, A.C.; Sartori, J.R.; Mori, C.; Cruz, V.C.; Fascina, V.B.; Pinheiro, D.F.; Madeira, L.A.; Gonçalvez, J.C. Emulsifier in broiler diets containing different fat sources. Braz. J. Poult. Sci. 2011, 13, 119–125. [Google Scholar] [CrossRef]
- Polin, D.; Wing, T.L.; Ki, P.; Pell, K.E. The effect of bile acids and lipase on absorption of tallow in young chicks. Poult. Sci. 1980, 59, 2738–2743. [Google Scholar] [CrossRef]
- Dänicke, S. Interaction between cereal identity and fat quality and content in response to feed enzymes in broilers. Enzymes Farm Anim. Nutr. 2001, 199–236. [Google Scholar]
- Hu, X.Q.; Wang, W.B.; Liu, L.; Wang, C.; Feng, W.; Luo, Q.P.; Han, R.; Wang, X.D. Effects of fat type and emulsifier in feed on growth performance, slaughter traits, and lipid metabolism of Cherry Valley ducks. Poult. Sci. 2019, 98, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, B.; Guo, Y.; Jiao, P.; Long, F. Effects of dietary lipids and Clostridium butyricum on fat deposition and meat quality of broiler chickens. Poult. Sci. 2010, 89, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Short, F.J.; Gorton, P.; Wiseman, J.; Boorman, K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Office Analysis Chemistry: Gaithersburg, MD, USA, 2007. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Office Analysis Chemistry: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Office Analysis Chemistry: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Qin, S.; Tian, G.; Zhang, K.; Ding, X.; Bai, S.; Wang, J.; Jia, G.; Zeng, Q. Influence of dietary rapeseed meal levels on growth performance, organ health and standardized ileal amino acid digestibility in meat ducks from 15 to 35 days of age. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1297–1306. [Google Scholar] [CrossRef]
- Zhang, K.X.; Zhang, K.Y.; Applegate, T.J.; Bai, S.P.; Ding, X.M.; Wang, J.P.; Peng, H.W.; Xuan, Y.; Su, Z.W.; Zeng, Q.F. Evaluation of the standardized ileal digestibility of amino acids of rapeseed meals varying in protein solubility for Pekin ducks. Poult. Sci. 2020, 99, 1001–1009. [Google Scholar] [CrossRef]
- Han, H.Y.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Luo, Y.H.; Wang, J.P.; Zeng, Q.F. Effect of dietary fiber levels on performance, gizzard development, intestinal morphology, and nutrient utilization in meat ducks from 1 to 21 days of age. Poult. Sci. 2017, 96, 4333–4341. [Google Scholar] [CrossRef]
- Wealleans, A.L.; Buyse, J.; Scholey, D.; Van Campenhout, L.; Burton, E.; Di Benedetto, M.; Pritchard, S.; Nuyens, F.; Jansen, M. Lysolecithin, but not lecithin, improves nutrient digestibility and growth rates in young broilers. Br. Poult. Sci. 2020, 61, 414–423. [Google Scholar] [CrossRef]
- Jiang, S.; Jiang, Z.; Zhou, G.; Chen, Z.; Li, D. Non-phytate phosphorus requirements and efficacy of a genetically engineered yeast phytase in male lingnan yellow broilers from 1 to 21 days of age. J. Anim. Physiol. Anim. Nutr. 2011, 95, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.B.; Araujo, D.N.; Strapazzon, J.V.; Rita, C.; Dilda, A.; Balen, G.; Deolindo, G.L.; Nesi, D.; Furlan, V.J.; Pelisser, G. Use of blend based on an emulsifier, monolaurin, and glycerides of butyric acid in the diet of broilers: Impacts on intestinal health, performance, and meat. An. Acad. Bras. Cienc. 2021, 93. [Google Scholar] [CrossRef]
- Liu, X.; Yoon, S.B.; Kim, I.H. Growth performance, nutrient digestibility, blood profiles, excreta microbial counts, meat quality and organ weight on broilers fed with de-oiled lecithin emulsifier. Animals 2020, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Nutautaitė, M.; Racevičiūtė-Stupelienė, A.; Andalibizadeh, L.; Šašytė, V.; Bliznikas, S.; Pockevičius, A.; Vilienė, V. Improving broiler chickens’ health by using lecithin and lysophosphatidylcholine emulsifiers: A comparative analysis of physiological indicators. Iran. J. Vet. Res. 2021, 22, 33. [Google Scholar] [PubMed]
- Lai, W.; Huang, W.; Dong, B.; Cao, A.; Zhang, W.; Li, J.; Wu, H.; Zhang, L. Effects of dietary supplemental bile acids on performance, carcass characteristics, serum lipid metabolites and intestinal enzyme activities of broiler chickens. Poult. Sci. 2018, 97, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.B.; Nair, R.; Herminghuysen, D.; Gatchair-Rose, A.; Rao, J.; Bagby, G.J.; Prasad, C. Epididymal fat depot lipoprotein lipase activity is lower in animals with high endogenous fat preferences. Life Sci. 1995, 57, 845. [Google Scholar] [CrossRef]
- Svendsen, A. Lipase protein engineering. Biochim. Biophys. Acta. 2000, 1543, 223–238. [Google Scholar] [CrossRef]
- Huang, J.; Yang, D.; Wang, T. Effects of Replacing Soy-oil with Soy-lecithin on Growth Performance, Nutrient Utilization and Serum Parameters of Broilers Fed Corn-based Diets. Asian Austral. J. Anim. 2007, 20, 1880–1886. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Kim, I.H. Effect of diets with different energy and lysophospholipids levels on performance, nutrient metabolism, and body composition in broilers. Poult. Sci. 2017, 96, 1341–1347. [Google Scholar] [CrossRef]
- Ge, X.K.; Wang, A.A.; Ying, Z.X.; Zhang, L.G.; Su, W.P.; Cheng, K.; Feng, C.C.; Zhou, Y.M.; Zhang, L.L.; Wang, T. Effects of diets with different energy and bile acids levels on growth performance and lipid metabolism in broilers. Poult. Sci. 2018, 98, 887–895. [Google Scholar] [CrossRef]
- Hossain, M.E.; Das, G.B. Effect of Crude Soybean Oil Sediment as a Substitute for Refined Soybean Oil in Broiler Diet. Iran. J. Appl. Anim. Sci. 2014, 4, 535–540. [Google Scholar]
- Hulan, H.W.; Proudfoot, F.G.; Nash, D.M. The Effects of Different Dietary Fat Sources on General Performance and Carcass Fatty Acid Composition of Broiler Chickens—ScienceDirect. Poult. Sci. 1984, 63, 324–332. [Google Scholar] [CrossRef]
- Lall, S.P.; Slinger, S.J. The Metabolizable Energy Content of Rapeseed Oils and Rapeseed Oil Foots and the Effect of Blending with Other Fats. Poult. Sci. 1973, 52, 143–151. [Google Scholar] [CrossRef]
- Leeson, J.; Atteh, O. Utilization of fats and fatty acids by turkey poults. Poult. Sci. 1995, 74, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Smits, C.H.; Moughan, P.J.; Beynen, A.C. The inhibitory effect of a highly viscous carboxymethylcellulose on dietary fat digestibility in the growing chicken is dependent on the type of fat. J. Anim. Physiol. Anim. Nutr. 2000, 83, 231–238. [Google Scholar] [CrossRef]
- Austic, R.E.; Nesheim, M.C.; Nesheim, M.C. Poultry Production, 13th ed.; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]

| Duck Fat | Mixed Fat | |||
|---|---|---|---|---|
| Variables | TD | DP | TM | MP |
| Ingredients | ||||
| Corn | 28.20 | 28.20 | 28.20 | 28.20 |
| Wheat | 24.10 | 24.10 | 24.10 | 24.10 |
| Soybean meal | 20.94 | 20.94 | 20.94 | 20.94 |
| Wheat bran | 5.10 | 5.10 | 5.10 | 5.10 |
| Rice bran meal | 12.00 | 12.00 | 12.00 | 12.00 |
| Duck fat/Mixed fat | 5.40 | 5.40 | 5.40 | 5.40 |
| Bentonite | 0.50 | 0.40 | 0.50 | 0.40 |
| Emulsifier | — | 0.10 | — | 0.10 |
| Dicalcium phosphate | 1.47 | 1.47 | 1.47 | 1.47 |
| Calcium carbonate | 1.13 | 1.13 | 1.13 | 1.13 |
| L-Lysine. HCl (98.5%) | 0.05 | 0.05 | 0.05 | 0.05 |
| DL-Methionine (99%) | 0.14 | 0.14 | 0.14 | 0.14 |
| Sodium chloride | 0.30 | 0.30 | 0.30 | 0.30 |
| Choline chloride (50%) | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin premix | 0.03 | 0.03 | 0.03 | 0.03 |
| Mineral premix | 0.50 | 0.50 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutrients, % | ||||
| ME, MJ/kg | 12.14 | 12.14 | 12.14 | 12.14 |
| Crude protein | 17.50 | 17.50 | 17.50 | 17.50 |
| Calcium | 0.86 | 0.86 | 0.86 | 0.86 |
| Ether extract | 7.53 | 7.53 | 7.53 | 7.53 |
| Total phosphorus | 0.80 | 0.79 | 0.80 | 0.79 |
| Available phosphorus | 0.41 | 0.41 | 0.41 | 0.41 |
| Total lysine | 0.86 | 0.86 | 0.86 | 0.86 |
| Total methionine | 0.40 | 0.40 | 0.40 | 0.40 |
| Total threonine | 0.62 | 0.62 | 0.62 | 0.62 |
| Total tryptophan | 0.20 | 0.20 | 0.20 | 0.20 |
| Analyzed nutrient levels, % | ||||
| Crude protein | 17.05 | 17.06 | 17.03 | 17.08 |
| Ether extract | 6.79 | 6.83 | 6.71 | 6.83 |
| Calcium | 1.01 | 0.96 | 0.97 | 0.98 |
| Total phosphorus | 0.78 | 0.73 | 0.74 | 0.70 |
| Item | Duck Fat | Mixed Fat |
|---|---|---|
| Content | ||
| Lauric acid (C12:0) | 0.20 | 0.10 |
| Myristic acid (C14:0) | 0.82 | 0.68 |
| Palmitic acid (C16:0) | 22.37 | 22.34 |
| Palmitoleic acid (C16:1) | 2.28 | 3.06 |
| Margaric acid (C17:0) | 0.39 | 0.34 |
| Stearic acid (C18:0) | 6.78 | 6.22 |
| Elaidic acid (C18:1n9t) | 0.29 | 0.24 |
| Oleic acid (C18:1n9c) | 42.36 | 41.46 |
| Linoleic acid (C18:2n6) | 21.49 | 22.97 |
| γ-linolenic acid (C18:3n6) | 0.11 | 0.14 |
| α-linolenic acid (C18:3n3) | 1.37 | 1.33 |
| Arachidic acid (C20:0) | 0.14 | 0.11 |
| cis-11, 14-Eicosadienoic acid (C20:2) | 0.39 | 0.25 |
| cis-8, 11, 14-Eicosadienoic acid (C20:3n6) | 0.16 | 0.11 |
| Behenic acid (C22:0) | 0.12 | 0.08 |
| Saturated fatty acid (SFA) | 31.11 | 30.09 |
| Monounsaturated fatty acid (MUFA) | 45.23 | 45.02 |
| Polyunsaturated fatty acid (PUFA) | 23.65 | 24.89 |
| Unsaturated fatty acid to SFA (U:S ratio) | 2.21 | 2.32 |
| Fat Sources | Fat Pre-Emulsification | 10 d BW/g | 34 d BW/g | 11–34 d BWG/g | 11–34 d F: G | 11–34 d FI/g |
|---|---|---|---|---|---|---|
| Duck fat | − | 414 | 2181 | 1769 | 2.05 | 3614 |
| + | 402 | 2268 | 1867 | 1.95 | 3670 | |
| Mixed fat | − | 410 | 2196 | 1788 | 2.02 | 3577 |
| + | 411 | 2238 | 1827 | 1.99 | 3634 | |
| SEM | 4.87 | 19.05 | 19.34 | 0.03 | 52.74 | |
| Main effects | ||||||
| Duck fat | 408 | 2228 | 1821 | 2.00 | 3642 | |
| Mixed fat | 411 | 2218 | 1809 | 2.00 | 3605 | |
| − | 412 | 2189 b | 1779 b | 2.03 a | 3595 | |
| + | 407 | 2253 a | 1847 a | 1.97 b | 3652 | |
| p-Value | ||||||
| Fat sources | 0.541 | 0.687 | 0.590 | 0.863 | 0.494 | |
| Pre-emulsification | 0.257 | 0.002 | 0.002 | 0.021 | 0.293 | |
| Fat sources × Pre-emulsification | 0.170 | 0.259 | 0.145 | 0.183 | 0.988 | |
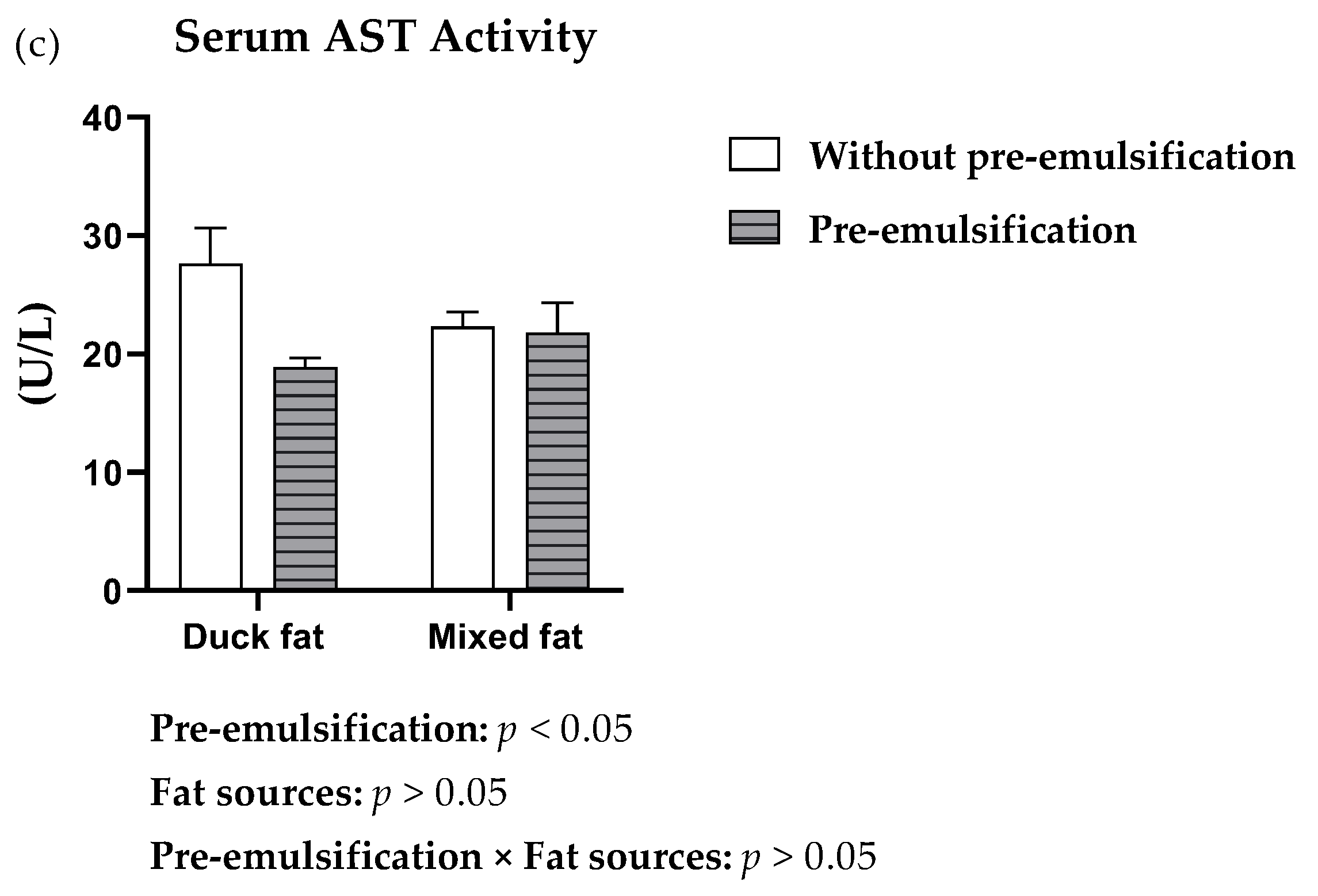
| Fat Sources | Fat Pre-Emulsification | TBA | TC | TG | HDL-C | LDL-C | VLDL-C |
|---|---|---|---|---|---|---|---|
| (µmol/L) | (mmol/L) | (mmol/L) | (mmol/L) | (mmol/L) | (mmol/L) | ||
| Duck fat | − | 31.18 | 4.99 a | 0.85 | 2.68 | 1.61 | 0.47 |
| + | 19.03 | 4.25 b | 0.79 | 2.37 | 1.44 | 0.44 | |
| Mixed fat | − | 20.10 | 4.27 b | 1.05 | 2.19 | 1.34 | 0.64 |
| + | 21.74 | 4.25 b | 0.97 | 2.44 | 1.51 | 0.38 | |
| SEM | 4.70 | 0.19 | 0.11 | 0.15 | 0.13 | 0.09 | |
| Main effects | |||||||
| Duck fat | 25.11 | 4.59 a | 0.82 | 2.52 | 1.53 | 0.46 | |
| Mixed fat | 20.87 | 4.26 b | 1.01 | 2.31 | 1.41 | 0.53 | |
| − | 24.85 | 4.55 a | 0.96 | 2.42 | 1.46 | 0.57 | |
| + | 20.49 | 4.25 b | 0.89 | 2.40 | 1.47 | 0.41 | |
| p-Value | |||||||
| Fat sources | 0.383 | 0.046 | 0.085 | 0.163 | 0.416 | 0.539 | |
| Pre-emulsification | 0.276 | 0.037 | 0.490 | 0.826 | 0.983 | 0.110 | |
| Fat sources × Pre-emulsification | 0.157 | 0.047 | 0.936 | 0.065 | 0.198 | 0.201 | |
| Fat Sources | Fat Pre-Emulsification | Jejunum | Duodenum | ||
|---|---|---|---|---|---|
| Trypsin (U/mg prot) | Lipase (U/mg prot) | Trypsin (U/mg prot) | Lipase (U/mg prot) | ||
| Duck fat | − | 72,254 | 71.37 | 57,887 | 52.72 |
| + | 58,857 | 39.87 | 30,027 | 24.74 | |
| Mixed fat | − | 81,381 | 63.32 | 58,902 | 57.6 |
| + | 81,291 | 76.88 | 55,062 | 50.51 | |
| SEM | 6932 | 12.86 | 6209 | 9.16 | |
| Main effects | |||||
| Duck fat | 65,555 b | 55.62 | 44,885 b | 37.79 | |
| Mixed fat | 81,336 a | 70.10 | 56,982 a | 54.29 | |
| − | 76,818 | 67.35 | 58,394 a | 55.32 | |
| + | 70,074 | 58.37 | 43,379 b | 36.76 | |
| p-Value | |||||
| Fat sources | 0.031 | 0.270 | 0.046 | 0.106 | |
| Pre-emulsification | 0.339 | 0.491 | 0.017 | 0.067 | |
| Fat sources × Pre-emulsification | 0.345 | 0.091 | 0.064 | 0.265 | |
| Fat Sources | Fat Pre-Emulsification | Dry Matter (%) | EE (%) | Energy (%) | AME (kcal/kg) | Crude Protein (%) | TP (%) | Ca (%) |
|---|---|---|---|---|---|---|---|---|
| Duck fat | − | 70.20 | 86.79 | 74.56 | 2918 | 60.49 | 33.70 | 37.34 |
| + | 71.72 | 89.50 | 75.89 | 2968 | 64.90 | 40.93 | 38.56 | |
| Mixed fat | − | 69.01 | 82.20 | 73.44 | 2875 | 61.76 | 28.28 | 41.14 |
| + | 71.42 | 87.45 | 75.59 | 2960 | 66.87 | 38.70 | 40.79 | |
| SEM | 0.79 | 1.43 | 0.67 | 26.15 | 2.84 | 2.74 | 4.30 | |
| Main effects | ||||||||
| Duck fat | 71.06 | 88.33 a | 75.27 | 2945 | 62.84 | 37.56 | 37.99 | |
| Mixed fat | 70.21 | 85.00 b | 74.51 | 2917 | 64.32 | 33.84 | 40.97 | |
| − | 69.52 b | 84.31 b | 73.92 b | 2893 b | 61.17 | 30.99 b | 39.37 | |
| + | 71.57 a | 88.47 a | 75.73 a | 2964 a | 65.89 | 39.82 a | 39.68 | |
| p-Value | ||||||||
| Fat sources | 0.360 | 0.029 | 0.298 | 0.333 | 0.573 | 0.175 | 0.489 | |
| Pre-emulsification | 0.020 | 0.010 | 0.015 | 0.016 | 0.105 | 0.003 | 0.920 | |
| Fat sources × Pre-emulsification | 0.581 | 0.383 | 0.541 | 0.504 | 0.902 | 0.567 | 0.856 | |
| Fat Sources | Fat Pre-Emulsification | Asp | Ser | Glu | Gly | Ala | Cys | Tyr | Pro | Total NEAA |
|---|---|---|---|---|---|---|---|---|---|---|
| Duck fat | − | 59.06 | 65.36 | 76.64 | 55.58 | 55.80 | 87.95 | 59.75 | 74.70 | 67.91 |
| + | 58.10 | 69.60 | 74.15 | 52.72 | 55.35 | 86.42 | 60.61 | 72.46 | 66.86 | |
| Mixed fat | − | 62.98 | 66.22 | 77.37 | 56.35 | 57.54 | 79.78 | 68.30 | 74.33 | 69.60 |
| + | 63.91 | 68.27 | 78.31 | 58.51 | 60.56 | 84.87 | 67.73 | 72.48 | 70.92 | |
| SEM | 1.96 | 1.78 | 1.19 | 2.33 | 2.25 | 2.22 | 2.47 | 1.40 | 1.60 | |
| Main effects | ||||||||||
| Duck fat | 58.61b | 67.34 | 75.47 b | 54.25 | 55.59 | 87.19 a | 60.15 b | 73.58 | 67.41 | |
| Mixed fat | 63.45a | 67.25 | 77.84 a | 57.50 | 59.15 | 82.32 b | 68.01 a | 73.41 | 70.26 | |
| − | 60.89 | 65.76 | 76.98 | 55.94 | 56.61 | 83.86 | 63.74 | 74.51 | 68.70 | |
| + | 61.01 | 68.93 | 76.23 | 55.81 | 58.13 | 85.64 | 64.17 | 72.47 | 68.89 | |
| p-Value | ||||||||||
| Fat sources | 0.020 | 0.898 | 0.050 | 0.173 | 0.136 | 0.038 | 0.004 | 0.903 | 0.083 | |
| Pre-emulsification | 0.996 | 0.090 | 0.524 | 0.882 | 0.571 | 0.430 | 0.955 | 0.157 | 0.933 | |
| Fat sources × Pre-emulsification | 0.634 | 0.543 | 0.161 | 0.292 | 0.449 | 0.150 | 0.775 | 0.889 | 0.465 | |
| Fat Sources | Fat Pre-Emulsification | Thr | Val | Met | Ile | Leu | Phe | Lys | His | Arg | Total EAA | Total AA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duck fat | − | 50.85 | 57.26 | 59.38 | 58.56 | 59.88 | 65.13 | 52.89 | 63.01 | 68.50 | 59.88 | 64.57 |
| + | 55.90 | 54.45 | 60.44 | 56.24 | 59.53 | 64.23 | 51.34 | 61.03 | 69.47 | 59.36 | 63.69 | |
| Mixed fat | − | 54.52 | 62.09 | 55.69 | 64.30 | 64.23 | 69.09 | 59.63 | 66.69 | 70.03 | 63.79 | 67.15 |
| + | 55.70 | 61.59 | 59.14 | 63.35 | 63.99 | 69.85 | 60.66 | 67.73 | 71.90 | 65.31 | 68.56 | |
| SEM | 2.54 | 2.25 | 3.13 | 2.47 | 2.17 | 1.73 | 2.27 | 1.90 | 1.87 | 2.06 | 1.78 | |
| Main effects | ||||||||||||
| Duck fat | 53.20 | 55.95b | 59.88 | 57.48 b | 59.71 | 64.71 b | 52.23 b | 62.08 b | 68.95 | 59.64 b | 64.16 b | |
| Mixed fat | 55.15 | 61.82a | 57.53 | 63.83 a | 64.11 | 69.47 a | 60.18 a | 67.21 a | 70.97 | 64.55 a | 67.86 a | |
| − | 52.56 | 59.51 | 57.66 | 61.24 | 61.91 | 66.97 | 56.03 | 64.73 | 69.21 | 61.70 | 65.77 | |
| + | 55.79 | 58.26 | 59.75 | 59.80 | 61.76 | 67.04 | 56.67 | 64.38 | 70.69 | 62.34 | 66.13 | |
| p-Value | ||||||||||||
| Fat sources | 0.500 | 0.013 | 0.432 | 0.016 | 0.053 | 0.011 | 0.002 | 0.011 | 0.299 | 0.025 | 0.047 | |
| Pre-emulsification | 0.232 | 0.468 | 0.478 | 0.516 | 0.893 | 0.969 | 0.910 | 0.807 | 0.452 | 0.810 | 0.882 | |
| Fat sources × Pre-emulsification | 0.452 | 0.612 | 0.706 | 0.785 | 0.982 | 0.637 | 0.575 | 0.435 | 0.811 | 0.625 | 0.528 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Zhang, K.; Tian, G.; Ding, X.; Bai, S.; Wang, J.; Lv, L.; Liao, Y.; Xuan, Y.; Zeng, Q. Effects of Fat Pre-Emulsification on the Growth Performance, Serum Biochemical Index, Digestive Enzyme Activities, Nutrient Utilization, and Standardized Ileal Digestibility of Amino Acids in Pekin Ducks Fed Diets with Different Fat Sources. Animals 2022, 12, 2729. https://doi.org/10.3390/ani12202729
Zeng X, Zhang K, Tian G, Ding X, Bai S, Wang J, Lv L, Liao Y, Xuan Y, Zeng Q. Effects of Fat Pre-Emulsification on the Growth Performance, Serum Biochemical Index, Digestive Enzyme Activities, Nutrient Utilization, and Standardized Ileal Digestibility of Amino Acids in Pekin Ducks Fed Diets with Different Fat Sources. Animals. 2022; 12(20):2729. https://doi.org/10.3390/ani12202729
Chicago/Turabian StyleZeng, Xiangyi, Keying Zhang, Gang Tian, Xuemei Ding, Shiping Bai, Jianping Wang, Li Lv, Yupeng Liao, Yue Xuan, and Qiufeng Zeng. 2022. "Effects of Fat Pre-Emulsification on the Growth Performance, Serum Biochemical Index, Digestive Enzyme Activities, Nutrient Utilization, and Standardized Ileal Digestibility of Amino Acids in Pekin Ducks Fed Diets with Different Fat Sources" Animals 12, no. 20: 2729. https://doi.org/10.3390/ani12202729
APA StyleZeng, X., Zhang, K., Tian, G., Ding, X., Bai, S., Wang, J., Lv, L., Liao, Y., Xuan, Y., & Zeng, Q. (2022). Effects of Fat Pre-Emulsification on the Growth Performance, Serum Biochemical Index, Digestive Enzyme Activities, Nutrient Utilization, and Standardized Ileal Digestibility of Amino Acids in Pekin Ducks Fed Diets with Different Fat Sources. Animals, 12(20), 2729. https://doi.org/10.3390/ani12202729






