Tranquillizing Effect of Passiflora incarnata Extract: Outcome on Behavioral and Physiological Indicators in Weaning Pigs with Intact Tails
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Health Physiological Indicators
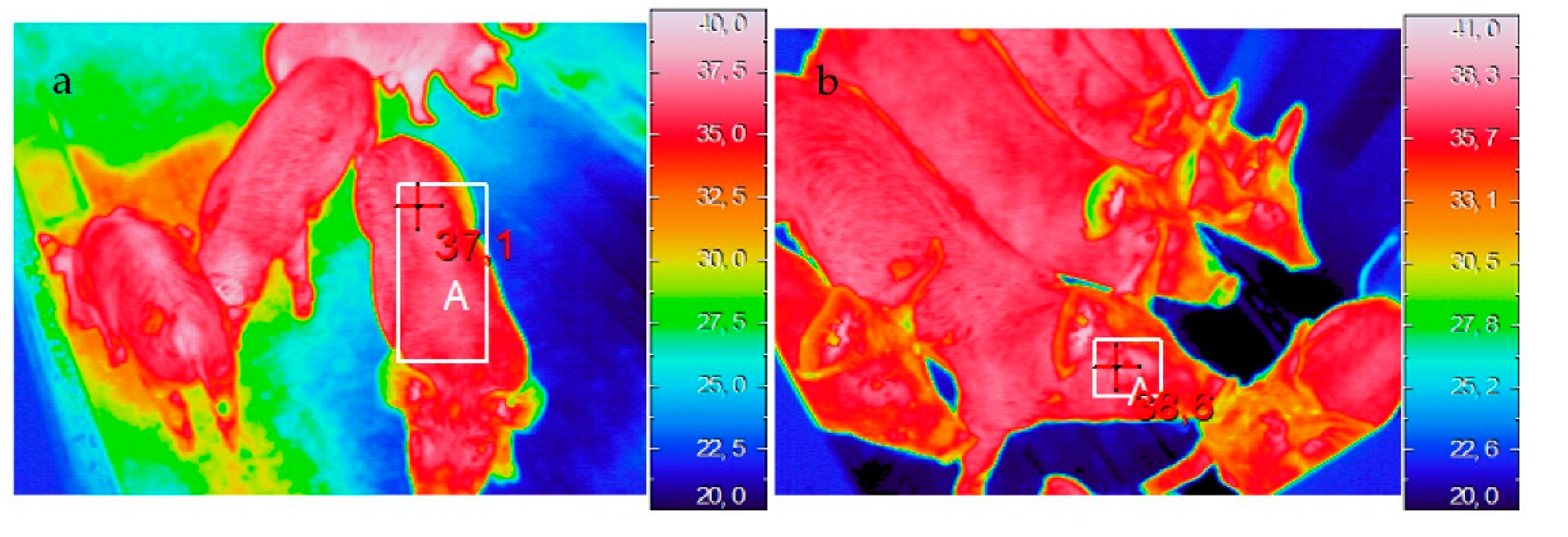
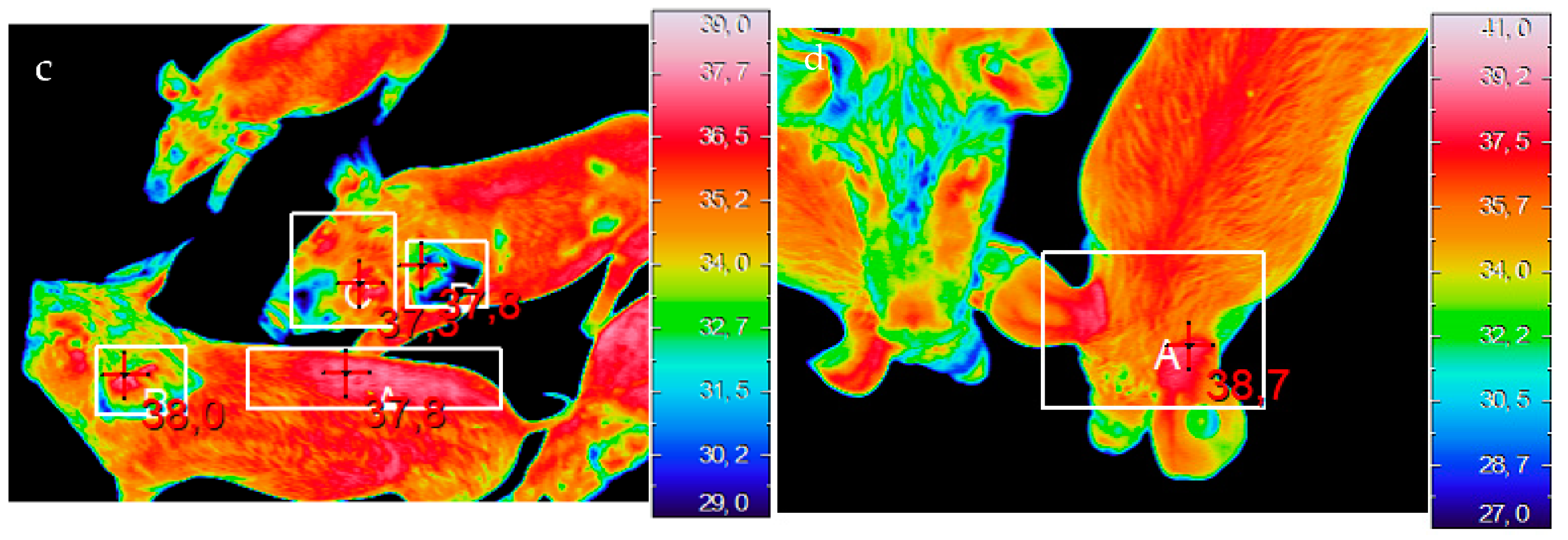
2.2.1. Thermal Imaging
2.2.2. Collection of Saliva Samples
2.2.3. Salivary Analyses: IgA and Cortisol Evaluation
2.2.4. Skin and Tail Lesions
2.3. Behavioral Indicators
2.3.1. Behavioral Observations
2.3.2. Novel Object Test
2.4. Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Health and Physiological Indicators
3.2.1. Thermal Imaging
3.2.2. Salivary IgA
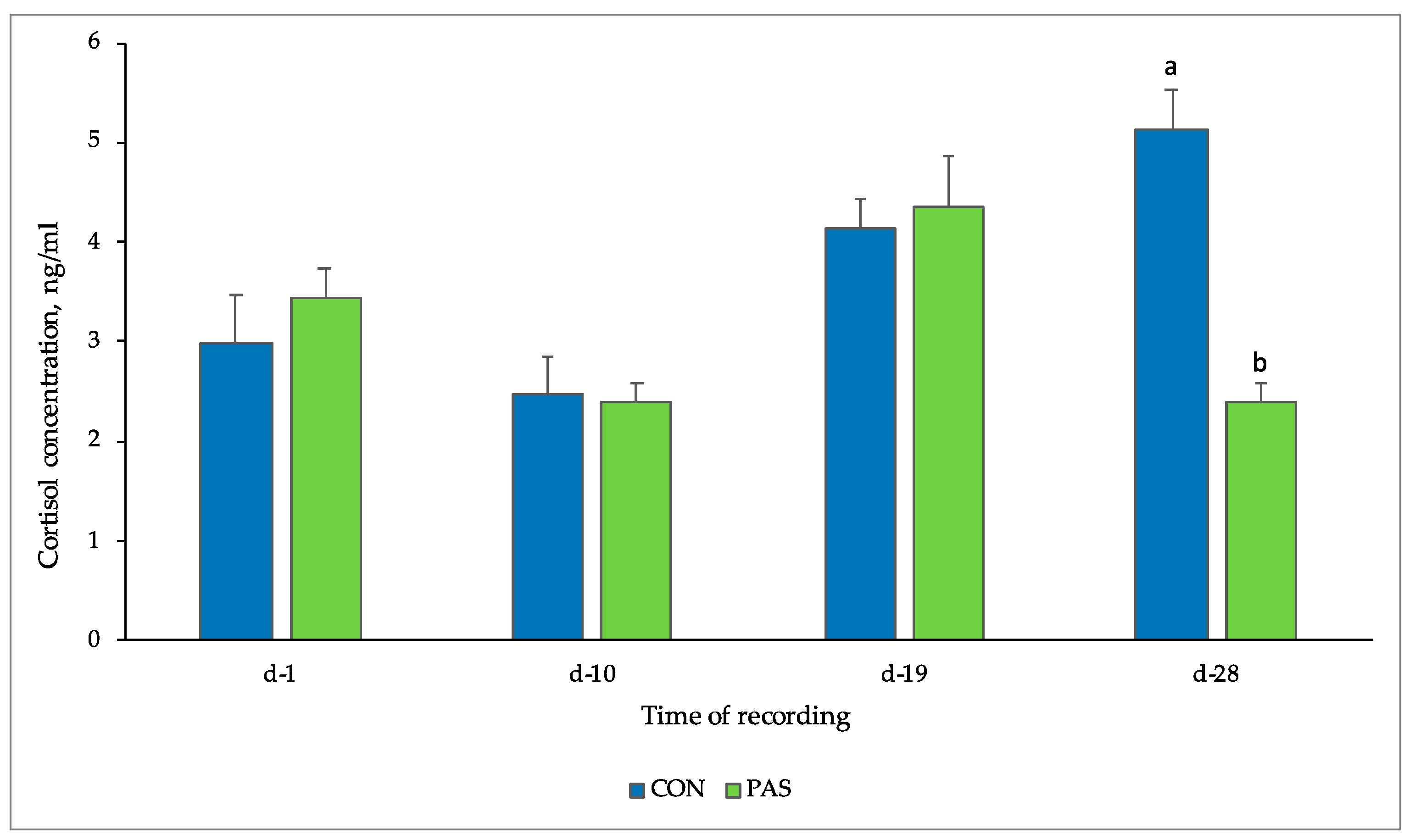
3.2.3. Salivary Cortisol
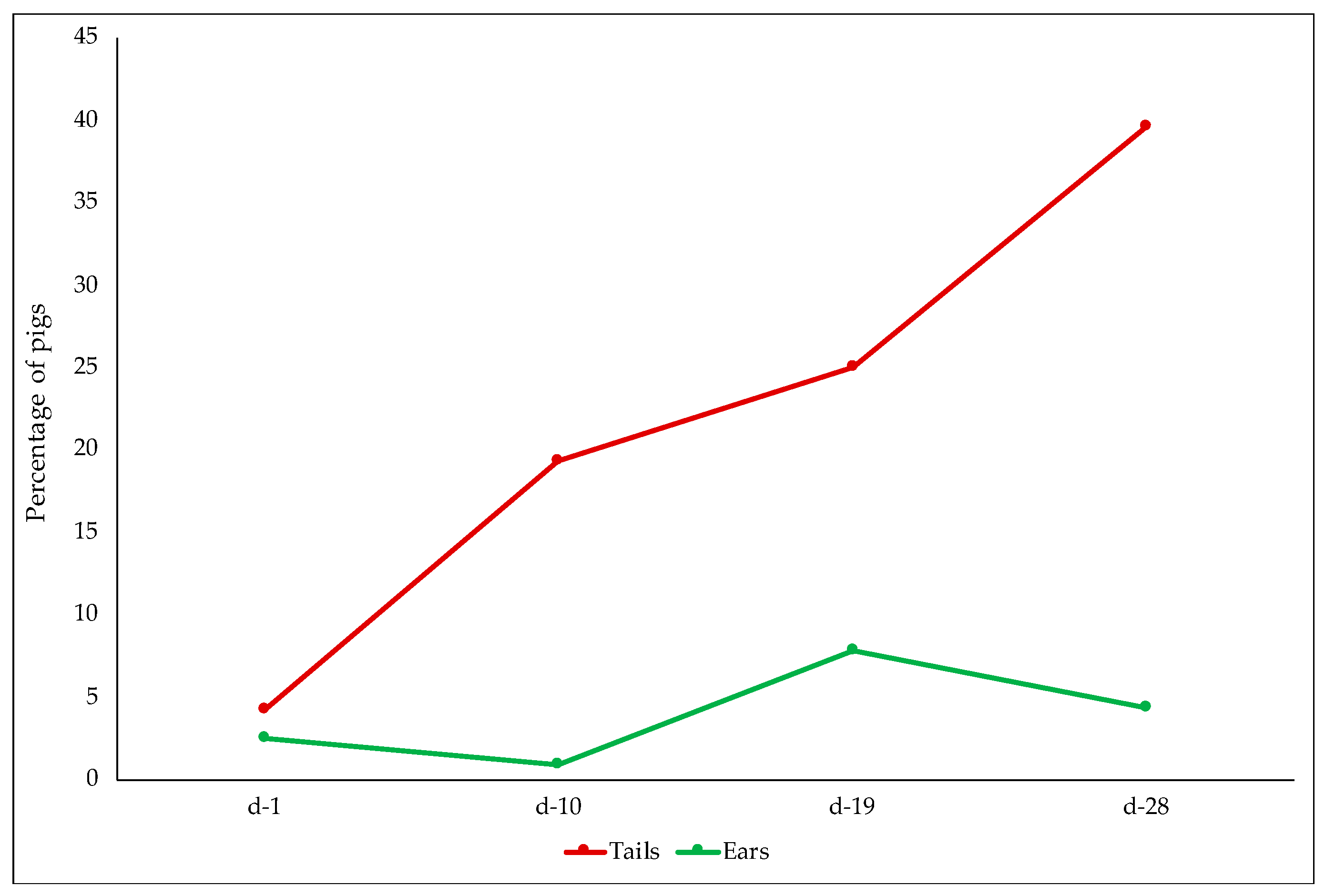
3.2.4. Skin and Tail Lesions
3.3. Behavioral Indicators
3.3.1. Behavioral Observation
3.3.2. Novel Object Test
4. Discussion
4.1. Growth Performance
4.2. Health and Physiological Indicators
4.3. Behavioral Indicators
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunberg, E.I.; Rodenburg, T.; Rydhmer, L.; Kjaer, J.B.; Jensen, P.; Keeling, L.J. Omnivores going astray: A review and new synthesis of abnormal behavior in pigs and laying hens. Front. Vet. Sci. 2016, 3, 57. [Google Scholar] [CrossRef]
- Herskin, M.S.; Di Giminiani, P.; Thodberg, K. Effects of administration of a local anaesthetic and/or an NSAID and of docking length on the behavior of piglets during 5 h after tail docking. Res. Vet. Sci. 2016, 108, 60–67. [Google Scholar] [CrossRef]
- Sandercock, D.A.; Smith, S.H.; Di Giminiani, P.; Edwards, S.A. Histopathological characterization of tail injury and traumatic neuroma development after tail docking in piglets. J. Comp. Pathol. 2016, 155, 40–49. [Google Scholar] [CrossRef]
- European Commission. Council Directive 2008/120/EC of 18 December 2008 Laying Down Minimum Standards for the Protection of Pigs. Off. J. Eur. Union 2008, 52, 5–13. [Google Scholar]
- European Union. Commission Recommendation (EU) 2016/336 on the application of Council Directive 2008/120/EC laying down minimum standards for the protection of pigs as regards measures to reduce the need for tail-docking. Off. J. Eur. Union 2016, L62, 20. [Google Scholar]
- Sonoda, L.T.; Fels, M.; Oczak, M.; Vranken, E.; Ismayilova, G.; Guarino, M.; Viazzi, S.; Bahr, C.; Berckmans, D.; Hartung, J. Tail biting in pigs—Causes and management intervention strategies to reduce the behavioural disorder. A review. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef]
- Turin, L.; Torinesi, R.; Pastorelli, G. Real-time PCR detection of the effect of postweaning on the expression of cytokines and NF-kB in piglets. J. Biol. Regul. Homeost. Agents. 2019, 33, 1737–1745. [Google Scholar] [CrossRef]
- Zupan, M.; Janczak, A.M.; Framstad, T.; Zanella, A.J. The effect of biting tails and having tails bitten in pigs. Physiol. Behav. 2012, 106, 638–644. [Google Scholar] [CrossRef]
- Poletto, R.; Meisel, R.L.; Richert, B.T.; Cheng, H.W.; Marchant-Forde, J.N. Aggression in replacement grower and finisher gilts fed a short-term high-tryptophan diet and the effect of long-term human–animal interaction. Appl. Anim. Behav. Sci. 2010, 122, 98–110. [Google Scholar] [CrossRef]
- Poletto, R.; Meisel, R.L.; Richert, B.T.; Cheng, H.W.; Marchant-Forde, J.N. Behavior and peripheral amine concentrations in relation to ractopamine feeding, sex, and social rank of finishing pigs. J. Anim. Sci. 2010, 88, 1184–1194. [Google Scholar] [CrossRef][Green Version]
- Kuhn, G.; Nowak, A.; Otto, E.; Albrecht, V.; Gassmann, B.; Sandner, E.; Przybilski, H.; Zahn, L. Studies on the control of meat quality by special treatment of swine. 1. Effects of stress and preventative magnesium feeding on selected parameters of carcass value and blood serum. Arch. Tierz. 1981, 24, 217–225. [Google Scholar]
- Peeters, E.; Neyt, A.; Beckers, F.; De Smet, S.; Aubert, A.E.; Geers, R. Influence of supplemental magnesium, tryptophan, vitamin C, and vitamin E on stress responses of pigs to vibration. J. Anim. Sci. 2005, 83, 1568–1580. [Google Scholar] [CrossRef]
- Peeters, E.; Driessen, B.; Geers, R. Influence of supplemental magnesium, tryptophan, vitamin C, vitamin E, and herbs on stress responses and pork quality. J. Anim. Sci. 2006, 84, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, H.S.; Lee, H.H.; Kim, T.H. Role identification of Passiflora incarnata Linnaeus: A mini review. J. Menopausal Med. 2017, 23, 156–159. [Google Scholar] [CrossRef]
- Soulimani, R.; Younos, C.; Jarmouni, S.; Bousta, D.; Misslin, R.; Mortier, F. Behavioural effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. J. Ethnopharmacol. 1997, 57, 11–20. [Google Scholar] [CrossRef]
- Speroni, E.; Minghetti, A. Neuropharmacological activity of extracts from Passiflora incarnata. Planta Med. 1988, 54, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Casal, N.; Font-i-Furnols, M.; Gispert, M.; Manteca, X.; Fàbrega, E. Effect of environmental enrichment and herbal compounds-supplemented diet on pig carcass, meat quality traits, and consumers’ acceptability and preference. Animals 2018, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, G.; Faustini, M.; Luzi, F.; Redaelli, V.; Turin, L. Passiflora incarnata powder extract in postweaning piglets feeding slightly improves wellbeing and immune parameters. Livest. Sci. 2020, 235, 104000. [Google Scholar] [CrossRef]
- Statham, P.; Green, L.; Bichard, M.; Mendl, M. Predicting tail-biting from behaviour of pigs prior to outbreaks. Appl. Anim. Behav. Sci. 2009, 121, 157–164. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine: 11th Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 399. [Google Scholar]
- Welfare Quality®. Assessment Protocol for Pigs (Sows and Piglets, Growing and Finishing Pigs); Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Janda, K.; Wojtkowska, K.; Jakubczyk, K.; Antoniewicz, J.; Skonieczna-Żydecka, K. Passiflora incarnata in Neuropsychiatric Disorders—A Systematic Review. Nutrients 2020, 12, 3894. [Google Scholar] [CrossRef] [PubMed]
- Casal-Plana, N.; Manteca, X.; Dalmau, A.; Fàbrega, E. Influence of enrichment material and herbal compounds in the behaviour and performance of growing pigs. Appl. Anim. Behav. Sci. 2017, 195, 38–43. [Google Scholar] [CrossRef]
- Perondi, D.; Moreira, I.; Pozza, P.C.; Carvalho, P.L.O.; Pasquetti, T.J.; Huepa, L.M.D. Passion fruit seed meal at growing and finishing pig (30-90 kg) feeding. Ciên. Agrotec. 2014, 38, 390–400. [Google Scholar] [CrossRef][Green Version]
- Staley, M.; Conners, M.G.; Hall, K.; Miller, L.J. Linking stress and immunity: Immunoglobulin A as a non-invasive physiological biomarker in animal welfare studies. Horm. Behav. 2018, 102, 55–68. [Google Scholar] [CrossRef]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary applications of infrared thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.M.L.; Jørgensen, E.; Dybkjær, L.; Jørgensen, B. The ear skin temperature as an indicator of the thermal comfort of pigs. Appl. Anim. Behav. Sci. 2008, 113, 43–56. [Google Scholar] [CrossRef]
- Barbieri, S.; Talamonti, Z.; Nannoni, E.; Heinzl, E.U.L.; Minero, M.; Canali, E. Use of thermography in pigs: Relationship between surface and core temperature. Vet. Ital. 2021, 57, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, G.; Serra, V.; Salvatori, G.; Redaelli, V.; Turin, L. Evaluation of Boswellia serrata enriched diet on cytokine gene expression and reactive oxygen metabolites in weaning piglets. J. Anim. Feed Sci. 2021, 30, 119–127. [Google Scholar] [CrossRef]
- Muneta, Y.; Yoshikawa, T.; Minagawa, Y.; Shibahara, T.; Maeda, R.; Omata, Y. Salivary IgA as a useful non-invasive marker for restraint stress in pigs. J. Vet. Med. Sci. 2010, 72, 1295–1300. [Google Scholar] [CrossRef]
- Guhad, F.A.; Hau, J. Salivary IgA as a marker of social stress in rats. Neurosci. Lett. 1996, 216, 137–140. [Google Scholar] [CrossRef]
- Kikkawa, A.; Uchida, Y.; Nakade, T.; Taguchi, K. Salivary secretory IgA concentrations in beagle dogs. J. Vet. Med. Sci. 2003, 65, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Wetherell, M.A.; Hyland, M.E.; Harris, J.E. Secretory immunoglobulin a reactivity to acute and cumulative acute multi-tasking stress: Relationships between reactivity and perceived workload. Biol. Psychol. 2004, 66, 257–270. [Google Scholar] [CrossRef]
- Muneta, Y.; Minagawa, Y.; Nakane, T.; Shibahara, T.; Yoshikawa, T.; Omata, Y. Interleukin-18 expression in pig salivary glands and salivary content changes during acute immobilization stress. Stress 2011, 14, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Escribano, D.; Campos, P.H.R.F.; Gutiérrez, A.M.; Le Floc, N.; Cerón, J.J.; Merlot, E. Effect of repeated administration of lipopolysaccharide on inflammatory and stress markers in saliva of growing pigs. Vet. J. 2014, 200, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Escribano, D.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Serum paraoxonase type-1 activity in pigs: Assay validation and evolution after an induced experimental inflammation. Vet. Immunol. Immunop. 2015, 163, 210–215. [Google Scholar] [CrossRef]
- Prims, S.; Vanden Hole, C.; Van Cruchten, S.; Van Ginneken, C.; Van Ostade, X.; Casteleyn, C. Hair or salivary cortisol analysis to identify chronic stress in piglets? Vet. J. 2019, 252, 105357. [Google Scholar] [CrossRef] [PubMed]
- Colson, V.; Martin, E.; Orgeur, P.; Prunier, A. Influence of housing and social changes on growth, behaviour and cortisol in piglets at weaning. Physiol. Behav. 2012, 107, 59–64. [Google Scholar] [CrossRef]
- Pol, F.; Courboulay, V.; Cotte, J.P.; Martrenchar, A.; Hay, M.; Mormède, P. Urinary cortisol as an additional tool to assess the welfare of pregnant sows kept in two types of housing. Vet. Res. 2002, 33, 13–22. [Google Scholar] [CrossRef]
- Escribano, D.; Fuentes-Rubio, M.; Cerón, J.J. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J. Vet. Diagn. Investig. 2012, 24, 918–923. [Google Scholar] [CrossRef]
- Brandt, Y.; Einarsson, S.; Ljung, A.; Lundeheim, N.; Rodríguez-Martínez, H.; Madej, A. Effects of continuous elevated cortisol concentrations during oestrus on concentrations and patterns of progesterone, oestradiol and LH in the sow. Anim. Reprod. Sci. 2009, 110, 172–185. [Google Scholar] [CrossRef]
- Jarvis, S.; Moinard, C.; Robson, S.K.; Sumner, B.E.H.; Douglas, A.J.; Seckl, J.R.; Russell, J.A.; Lawrence, A.B. Effects of weaning age on the behavioural and neuroendocrine development of piglets. Appl. Anim. Behav. Sci. 2008, 110, 166–181. [Google Scholar] [CrossRef]
- Yang, C.H.; Ko, H.L.; Salazar, L.C.; Llonch, L.; Manteca, X.; Camerlink, I.; Llonch, P. Pre-weaning environmental enrichment increases piglets’ object play behaviour on a large scale commercial pig farm. Appl. Anim. Behav. Sci. 2018, 202, 7–12. [Google Scholar] [CrossRef]
- Smulders, D.; Verbeke, G.; Mormede, P.; Geers, R. Validation of a behavioral observation tool to assess pig welfare. Physiol. Behav. 2006, 89, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Schönreiter, S.; Zanella, A.J. Assessment of cortisol in swine by saliva: New methodological approaches. Arch. Tierz. 2000, 43, 165–170. [Google Scholar]
- Merlot, E.; Mounier, A.M.; Prunier, A. Endocrine response of gilts to various common stressors: A comparison of indicators and methods of analysis. Physiol. Behav. 2011, 102, 259–265. [Google Scholar] [CrossRef]
- Barnett, J.L.; Cronin, G.M.; McCallum, T.H.; Newman, E.A.; Hennessy, D.P. Effects of grouping unfamiliar adult pigs after dark, after treatment with amperozide and by using pens with stalls, on aggression, skin lesions and plasma cortisol concentrations. Appl. Anim. Behav. Sci. 1996, 50, 121–133. [Google Scholar] [CrossRef]
- O’Driscoll, K.; O’Gorman, D.M.; Taylor, S.; Boyle, L.A. The influence of a magnesium-rich marine extract on behaviour, salivary cortisol levels and skin lesions in growing pigs. Animal 2013, 7, 1017–1027. [Google Scholar] [CrossRef]
- Ruis, M.A.W.; Te Brake, J.H.A.; Engel, B.; Ekkel, E.D.; Buist, W.G.; Blokhuis, H.J.; Koolhaa, J.M. The circadian rhythm of salivary cortisol in growing pigs: Effects of age, gender, and stress. Physiol. Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). The risks associated with tail-biting in pigs and possible means to reduce the need for tail-docking considering the different housing and husbandry systems. EFSA J. 2007, 611, 1–13. [Google Scholar]
- Van Putten, G. An investigation into tail-biting among fattening pigs. Br. Vet. J. 1969, 125, 511–517. [Google Scholar] [CrossRef]
- Schrøder-Petersen, D.L.; Simonsen, H.B.; Lawson, L.G. Tail-in-mouth behaviour among weaner pigs in relation to age, gender and group composition regarding gender. Acta Agric. Scand. Sect. A—Anim. Sci. 2003, 53, 29–34. [Google Scholar] [CrossRef]
- Calderón Díaz, J.A.; Manzanilla, E.G.; Diana, A.; Boyle, L. Cross-fostering implication for pig mortality, welfare and performance. Front. Vet. Sci. 2018, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Peden, R.S.E.; Turner, S.P.; Boyle, L.A.; Camerlink, I. The translation of animal welfare research into practice: The case of mixing aggression between pigs. Appl. Anim. Behav. Sci. 2018, 204, 1–9. [Google Scholar] [CrossRef]
- McGlone, J.J. A quantitative ethogram of aggressive and submissive behaviours in recently regrouped pigs. J. Anim. Sci. 2005, 61, 559–565. [Google Scholar] [CrossRef]
- Arey, D.S.; Franklin, M.F. Effects of straw and unfamiliarity on fighting between newly mixed growing pigs. Appl. Anim. Behav. Sci. 1995, 45, 23–30. [Google Scholar] [CrossRef]
- Averós, X.; Brossard, L.; Dourmad, J.Y.; de Greef, K.H.; Edge, H.L.; Edwards, S.A.; Meunier-Salaün, M.C. A meta-analysis of the combined effect of housing and environmental enrichment characteristics on the behaviour and performance of pigs. Appl. Anim. Behav. Sci. 2010, 127, 73–85. [Google Scholar] [CrossRef]
- Taylor, N.R.; Main, D.C.J.; Mendl, M.; Edwards, S.A. Tail-biting: A new perspective. Vet. J. 2010, 186, 137–147. [Google Scholar] [CrossRef]
- Ursinus, W.W.; Van Reenen, C.G.; Reimert, I.; Bolhuis, J.E. Tail biting in pigs: Blood serotonin and fearfulness as pieces of the puzzle? PLoS ONE 2014, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Brunberg, E.; Wallenbeck, A.; Keeling, L.J. Tail biting in fattening pigs: Associations between frequency of tail biting and other abnormal behaviours. Appl. Anim. Behav. Sci. 2011, 133, 18–25. [Google Scholar] [CrossRef]

| Ingredients | % |
|---|---|
| Corn meal | 29 |
| Barley meal | 25 |
| Whey powder | 12.5 |
| Hulled barley | 12.5 |
| Soybean meal 48% | 11.8 |
| Wheat bran | 6.0 |
| Coconut oil | 1.0 |
| Dextrose monohydrate | 1 |
| Vitamin-Mineral premix 1 | 0.40 |
| L-Lysine | 0.50 |
| Sodium chloride | 0.20 |
| DL-Methionine | 0.18 |
| L-Threonine | 0.15 |
| L-Tryptophan | 0.07 |
| Chemical composition 2 | |
| Crude protein, | 17.19 |
| Ether extract | 4.88 |
| Crude fiber | 3.16 |
| Starch | 40.90 |
| Lactose | 3.77 |
| Lysine | 1.24 |
| Calcium | 0.66 |
| P dig | 0.44 |
| Net Energy (NE), kcal/kg | 2462.44 |
| n | CON | PAS | SEM | p-Value | |
|---|---|---|---|---|---|
| Initial BW, kg | 60 | 9.13 | 9.01 | 0.21 | 0.779 |
| Final BW, kg | 56 | 22.48 | 22.49 | 0.43 | 0.989 |
| ADG, g/day | 56 | 0.475 | 0.485 | 0.01 | 0.654 |
| FCR, kg/kg | - | 2.48 | 2.44 | 0.027 | 0.418 |
| CON | PAS | Average | p-Value | ||
|---|---|---|---|---|---|
| Diet | Time | ||||
| T (°C) eye | |||||
| d-1 | 37.72 ± 0.10 | 37.71 ± 0.08 | 37.71 | ||
| d-10 | 37.82 ± 0.10 | 37.87 ± 0.08 | 37.84 | ||
| d-19 | 38.48 ± 0.08 | 38.07 ± 0.09 | 38.27 | ||
| d-28 | 38.04 ± 0.14 | 38.05 ± 0.07 | 38.04 | ||
| ns | p < 0.01 | ||||
| T (°C) ear back | |||||
| d-1 | 38.23 ± 0.08 | 38.52 ± 0.10 | 38.37 | ||
| d-10 | 38.29 ± 0.09 | 38.61 ± 0.09 | 38.45 | ||
| d-19 | 39.08 ± 0.10 | 38.62 ± 0.07 | 38.85 | ||
| d-28 | 38.74 ± 0.17 | 38.48 ± 0.11 | 38.61 | ||
| ns | p < 0.01 | ||||
| T (°C) ear front | |||||
| d-1 | 38.19 ± 0.09 | 38.38 ± 0.15 | 38.28 | ||
| d-10 | 38.42 ± 0.10 | 38.71 ± 0.07 | 38.56 | ||
| d-19 | 39.22 ± 0.12 | 38.77 ± 0.15 | 38.99 | ||
| d-28 | 38.88 ± 0.11 | 38.94 ± 0.11 | 38.91 | ||
| ns | p < 0.01 | ||||
| T (°C) dorsal | |||||
| d-1 | 37.05 ± 0.10 | 37.09 ± 0.12 | 37.07 | ||
| d-10 | 37.04 ± 0.13 | 37.38 ± 0.11 | 37.21 | ||
| d-19 | 38.20 ± 0.09 | 37.75 ± 0.09 | 37.97 | ||
| d-28 | 37.65 ± 0.16 | 37.71 ± 0.10 | 37.68 | ||
| ns | p < 0.01 | ||||
| CON | PAS | p-Value | |||
|---|---|---|---|---|---|
| Diet | Time | Diet × Time | |||
| day 1 | 1.47 | 1.80 | |||
| day 10 | 1.52 | 1.56 | |||
| day 19 | 1.53 | 1.55 | |||
| day 28 | 1.61 | 1.46 | |||
| ns | ns | p < 0.001 |
| CON | PASS | p-Value | |||
|---|---|---|---|---|---|
| Diet | Time | Diet × Time | |||
| Aggression | |||||
| days 7–9 | 1.815 | 1.582 | |||
| days 13–15 | 1.814 | 1.466 | |||
| days 20–22 | 1.418 | 0.677 | |||
| days 25–27 | 1.178 | 0.695 | |||
| p < 0.001 | p < 0.001 | p < 0.05 | |||
| Exploration of enrichment | |||||
| days 7–9 | 0.304 | 1.895 | |||
| days 13–15 | 0.824 | 1.489 | |||
| days 20–22 | 0.618 | 1.417 | |||
| days 25–27 | 0.410 | 1.092 | |||
| p < 0.001 | p < 0.05 | p < 0.001 | |||
| Exploration of pen mates | |||||
| days 7–9 | 1.727 | 1.923 | |||
| days 13–15 | 1.921 | 1.890 | |||
| days 20–22 | 1.864 | 1.873 | |||
| days 25–27 | 1.947 | 1.926 | |||
| Ns | ns | ns | |||
| Exploration of tail | |||||
| days 7–9 | 1.624 | 1.221 | |||
| days 13–15 | 1.380 | 0.797 | |||
| days 20–22 | 1.317 | 0.664 | |||
| days 25–27 | 1.142 | 0.752 | |||
| p < 0.001 | p < 0.001 | ns | |||
| Exploration of ear | |||||
| days 7–9 | 1.965 | 1.823 | |||
| days 13–15 | 1.979 | 1.716 | |||
| days 20–22 | 1.948 | 1.794 | |||
| days 25–27 | 1.995 | 1.884 | |||
| p < 0.001 | p < 0.05 | p < 0.05 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorelli, G.; Serra, V.; Turin, L.; Redaelli, V.; Luzi, F.; Barbieri, S. Tranquillizing Effect of Passiflora incarnata Extract: Outcome on Behavioral and Physiological Indicators in Weaning Pigs with Intact Tails. Animals 2022, 12, 203. https://doi.org/10.3390/ani12020203
Pastorelli G, Serra V, Turin L, Redaelli V, Luzi F, Barbieri S. Tranquillizing Effect of Passiflora incarnata Extract: Outcome on Behavioral and Physiological Indicators in Weaning Pigs with Intact Tails. Animals. 2022; 12(2):203. https://doi.org/10.3390/ani12020203
Chicago/Turabian StylePastorelli, Grazia, Valentina Serra, Lauretta Turin, Veronica Redaelli, Fabio Luzi, and Sara Barbieri. 2022. "Tranquillizing Effect of Passiflora incarnata Extract: Outcome on Behavioral and Physiological Indicators in Weaning Pigs with Intact Tails" Animals 12, no. 2: 203. https://doi.org/10.3390/ani12020203
APA StylePastorelli, G., Serra, V., Turin, L., Redaelli, V., Luzi, F., & Barbieri, S. (2022). Tranquillizing Effect of Passiflora incarnata Extract: Outcome on Behavioral and Physiological Indicators in Weaning Pigs with Intact Tails. Animals, 12(2), 203. https://doi.org/10.3390/ani12020203








