Simple Summary
Antimicrobial resistance (AMR) has become a major health challenge of the 21st century. Several studies confirm the potential role of wildlife as sentinel for pathogens surveillance. Moreover, the presence of AMR bacteria in the wildlife can be considered as a good indicator of anthropization level on the ecosystem. The fast increase in AMR worldwide has been enhanced by several factors as globalization and migration. The study of antimicrobial resistance in wild birds is of great importance, as they can travel hundreds of kilometers and disseminate pathogens and AMR across different regions or even continents. The aim of this study was to compare the level of AMR in three bird species: white stork (Ciconia ciconia), lesser black-backed gull (Larus fuscus) and black-headed gull (Chroicocephalus ridibundus). For the analysis, 17 antibiotics from the most representative classes were tested by disk-diffusion method. Results showed 63.2% of seagulls and 31.6% of white storks as carriers of antimicrobial-resistant Escherichia coli, and from all of them, 38.9% were considered multi-drug resistant. Betalactamics, quinolones and tetracyclines were the antibiotic classes with the highest rate of AMR.
Abstract
The presence of AMR bacteria in the human–animal–environmental interface is a clear example of the One Health medicine. Several studies evidence the presence of resistant bacteria in wildlife, which can be used as a good indicator of anthropization level on the ecosystem. The fast increase in AMR in the environment in the last decade has been led by several factors as globalization and migration. Migratory birds can travel hundreds of kilometers and disseminate pathogens and AMR through different regions or even continents. The aim of this study was to compare the level of AMR in three migratory bird species: Ciconia ciconia, Larus fuscus and Chroicocephalus ridibundus. For this purpose, commensal Escherichia coli has been considered a useful indicator for AMR studies. After E. coli isolation from individual cloacal swabs, antimicrobial susceptibility tests were performed by the disk-diffusion method, including 17 different antibiotics. A total of 63.2% of gulls had resistant strains, in contrast to 31.6% of white storks. Out of all the resistant strains, 38.9% were considered multi-drug resistant (50% of white storks and 30% of seagulls). The antibiotic classes with the highest rate of AMR were betalactamics, quinolones and tetracyclines, the most commonly used antibiotic in human and veterinary medicine in Spain.
1. Introduction
In the recent centuries, the anthropization of ecosystems has forced several animal species to change their biology and adapt to human presence, coexisting today as urban wildlife. Urban areas give them unlimited resources to live, feed and reproduce [1]. Currently, white storks (Ciconia ciconia) are considered migratory birds turned into sedentary wildlife. Similarly, lesser black-backed gulls (Larus fuscus) and black-headed gulls (Chroicocephalus ridibundus) traditionally settle on coastal areas, moving short distances to warmer latitudes in winter. The constant availability of food and other resources in cities has favored the establishment of some populations of the three species as residents, shortening or stopping migration [2,3].
In this sense, landfills constitute an important food source for opportunistic wildlife. However, the nutritional score of this food is poor and incomplete and involve several risks for animal health as the accumulation of contaminants, such as heavy metals, pesticides or drugs (e.g., anti-inflammatories and antimicrobials) [2,4]. The accumulation of organic waste in landfills, plus the inappropriate management of antibiotic residues, have favored the development of antimicrobial resistance (AMR) and the potential acquisition of resistant bacteria by animals who feed at these points [5,6]. One of the key facts on AMR dissemination is the horizontal transmission of antimicrobial resistance genes (ARGs) between bacteria, no matter if they are the same bacterial species or not. Thus, commensal and environmental bacteria can acquire these ARGs and serve as amplifiers that perpetuate the persistence of genes in individuals or even ecosystems [7,8]. Moreover, AMR should be considered a zoonosis as ARGs run between the human–animal–environment interface easily [9]. Now, AMR represents one of the biggest challenges in medicine, as it has been linked to nearly 5 million deaths worldwide in 2019 [10,11]. Escherichia coli is a commensal bacterium in the gastrointestinal tract of a wide range of hosts that has a high survival rate on the environment, which make it a good indicator for AMR studies [12]. Instead of being mainly saprophytic, some serovars can cause disease, called colibacillosis, which is considered relevant to public and animal health as it is the fourth most reported foodborne gastrointestinal infection in humans in the European Union (EU) [13]. Moreover, E. coli has been described as one of the most frequent resistant bacteria in human and animal medicine, which make the treatment of colibacillosis more difficult [14].
However, AMR is not only a human health issue. Several studies have confirmed the presence of AMR in livestock, companion animals and wildlife [6,12,14,15,16]. The prevalence of AMR largely depends on animal species and regions, but resistant bacteria have been detected even in Antarctica [17,18]. The presence of AMR in wildlife is directly related to the pressure of human activity on the ecosystems. Therefore, some wildlife species can be considered sentinels for AMR pressure in the environment, and AMR surveillance in wildlife should be a priority [9,19]. Among bird species, seagulls are considered good sentinels for AMR studies, and high rates of resistant E. coli have been reported in those species [20,21,22,23,24]. There is limited information regarding storks, but recent studies have also described the presence of antimicrobial resistance in E. coli in white storks [5,25,26].
In this context, the aim of this study was to compare the presence of AMR E. coli in white storks and two species of seagulls in central Spain, more than 400 km (≈250 miles) inland.
2. Materials and Methods
2.1. Study Population and Sample Collection
From October 2018 to May 2019, all the white storks (Ciconia ciconia), lesser black-backed gulls (Larus fuscus) and black-headed gulls (Chroicocephalus ridibundus) admitted at the Wildlife Rescue Center (WRC) managed by Grupo de Rehabilitación de la Fauna Autóctona y su Hábitat (GREFA) were examined and sampled. Handling procedures complied to European (Directive 2010/63/EU) and Spanish legislation (Royal Decree 53/2013) [27,28].
This GREFA WRC is located in central Spain (Madrid) and admits almost 7000 wild animals per year, including all kind of birds, mammals, and reptiles of native Iberian fauna. GREFA’s aim is to recover and release them back into the wild. The main reasons of admission to the WRC are related to human activities: hunting, accidents with power lines (electrocution or traumas), windows or cars, among others. Additionally, natural diseases of wildlife are another cause of admission at the WRC. In fact, WRCs can be considered as passive health monitoring centers for wildlife.
During the first examination, a cloacal swab was taken from each animal for E. coli isolation and AMR detection, prior to any treatment. The samples were conserved on a Cary Blair transport medium (Deltalab, Barcelona, Spain) at 4 °C and processed within 24 h from the collection. Information about species, age, area of origin, proximity to landfills and clinical data from each animal was recorded when possible. Moreover, historical information about ringed birds was facilitated by official organisms from the countries where they had been ringed. Animals were classified by age in three groups, based on the feather development and phenotypic changes: nestling (including in this group fledglings), young and adult. According to the origin, five different regions were established to assess potential geographical differences: center, north, south, east, and west of the Community of Madrid (Figure 1).
Figure 1.
Origin of the animals included in the study.
2.2. Microbiological Analysis
Samples were plated onto MacConkey agar (Oxoid Ltd., Basingstoke, United Kingdom) and incubated at 37 ± 1 °C for 24 h. Next, a single colony morphologically compatible with E. coli was subcultured on a Columbia agar plate (Oxoid Ltd., Basingstoke, UK) and incubated again at 37 ± 1 °C for 24 h in order to get a monoclonal culture, which was collected and stored at −20 °C for further analyses. Bacterial identification was confirmed by Gram stain and classical biochemical tests, including catalase, potassium hydroxide (KOH), oxidase, glucose fermentation and motility tests [29]. Additionally, seven random isolates were tested using API (Analytical Profile Index) 20E strips (BioMérieux, Marcy l’Etoile, France). All the confirmed strains were stored in cryovial with nutritive broth and glycerol (80%: 20%, respectively) at −80 °C for further analysis.
Antimicrobial susceptibility test was performed according to the disk diffusion (Kirby–Bauer) method and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [30]. Isolates were recovered from cryovials and spread on Columbia Agar with 5% sheep blood (Becton Dickinson GmbH, Heidelberg, Germany). The inoculum suspension was prepared in sterile 0.8% saline solution to a turbidity 0.5 McFarland. Then, the inoculum was transferred onto Mueller–Hinton agar (Becton, Dickinson Gmb, Heidelberg, Germany) and antimicrobial disks were added to the surface. A total of 17 antimicrobials from nine classes were tested, most of them recommended for indicator commensal E. coli (Decision EU 2020/1729) (Table 1) [31]. After 18–20 h at 36 ± 1 °C, sensitivity or resistance was determined by growth inhibition diameter regarding standardized EUCAST breakpoint tables [32], except for ceftiofur, enrofloxacin and tetracycline that were evaluated according to Markey et al. [29]. Multidrug resistance (MDR) was considered when the isolate was non-susceptible to at least one antimicrobial agent in three or more different classes of antimicrobial [33].

Table 1.
Antimicrobial disks used in this study.
2.3. Statistical Analysis
Statistical analysis was done using a commercially available software application (SPSS 21.0 software package: SPSS Inc., Chicago, IL, USA, 2002). For the presentation of mean results, 95% confidence values were calculated. Different statistical tests were performed to assess the relationship between the presence of antimicrobial resistances and different variables (species, age, origin and pathology). Chi-square and Fisher’s exact test were employed to study parametric variables, and Mann–Whitney U test for non-parametric variables. A two-tailed p-value ≤ 0.05 was considered to indicate a statistically significant difference.
3. Results
A total of 40 animals were included in the study: 20 white storks, 16 lesser black-backed gulls and 4 black-headed gulls. Because of the small number of black-headed gulls included on the study, they have been grouped with lesser black-backed gulls for the statistical analysis (seagulls from now on). Animals were admitted at GREFA WRC because of different conditions, mainly trauma for white storks and botulism or trauma for seagulls. Classification by age showed that 20% were nestlings (8/40), 20% young (8/40) and 60% adults (24/40).
Individuals included in the study were firstly found and collected in areas with human activity, where some resident populations have been established and feed regularly in landfills. However, some animals maintain recent migratory habits, as detected by leg rings. Regarding their geographical origin, 20% were from the Center of the Community of Madrid (8/40), 10% from the south (4/40), 20% from the west (8/40), 20% from the north (8/40) and 30% from the east (12/40).
E. coli was isolated from 38 animals: 19 storks and 19 seagulls. Overall, 47.4% (45.9–48.9%) of E. coli isolates were resistant to at least one of the 17 antimicrobials tested (18/38). The higher percentage of resistance was found in (in decreasing order): ampicillin (13/18), ticarcillin (13/18), nalidixic acid (9/18), tetracycline (8/18) and enrofloxacin (7/18). The resistance to ampicillin was always related to resistance to ticarcillin. Only one isolate was resistant to aztreonam. All isolates were susceptible to cefoxitin, ceftiofur, cefotaxime, imipenem and amikacin.
The percentage of AMR observed for each antimicrobial and the phenotypic profiles are detailed in Table 2.

Table 2.
Phenotypic profiles of antimicrobial resistance in Escherichia coli by species and overall prevalence of resistant or intermediate results.
From all the resistant E. coli isolates, 38.9% (35.16–42.64%) were considered MDR (7/18). The distribution of non-susceptible isolates to one or several antimicrobial classes is detailed in Figure 2.
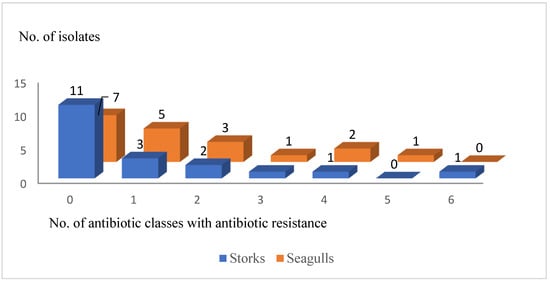
Figure 2.
Number of Escherichia coli isolates showing sensitivity (0) or phenotypic resistance to one or more antibiotic classes (one to six) according to bird species.
Regarding bird species, 31.6% (28.8–34.4%) of E. coli isolates from the white storks (6/19) were resistant to at least to one antimicrobial, and three of these isolates were considered MDR. The six isolates were resistant to penicillins and one also showed resistance to aztreonam. Additional resistances were found in four isolates. Two MDR isolates showed resistance to four and six antimicrobial classes, combining (in addition to penicillins) quinolones, tetracycline, trimethoprim-sulfamethoxazole and gentamicin or chloramphenicol (Table 2).
Among seagulls, 63.2% (60.5–65.8%) of E. coli were resistant (12/19), and four of them were considered MDR. The E. coli isolated from two of the four black-headed gulls showed resistance: one to tetracyclines and the other one had an MDR pattern (AMP-TIC-CAZ-TET). Patterns showed more diversity than those observed in white storks. Resistance to penicillins was found in seven isolates, resistance to quinolones in seven isolates and to tetracycline in six isolates. The four MDR isolates showed resistance against three, four or five categories, combining penicillins and/or quinolones, with trimethoprim-sulfamethoxazole, tetracycline, chloramphenicol or gentamicin (Table 2).
No significant differences were found between the presence of AMR and the species (p = 0.11), age (p = 0.59), origin (p = 0.47) and pathology (p = 0.24); neither between the existence of MDR and the same variables (p = 0.78, p = 0.15, p = 0.11 and p = 0.46, respectively).
4. Discussion
This study shows a high proportion of AMR Escherichia coli isolates (47.4%) from white storks, lesser black-backed gulls, and black-headed gulls, three urban bird species that have modified their feeding habits and migratory behavior in recent years. Despite the fact AMR should be expected in a lower proportion in wild birds, urban birds have a closer contact with human garbage, increasing the risk of acquiring AMR [24,34,35]. Free-living wildlife is never supposed to have received antibiotic treatment; thus, the main source of this resistance may be the interaction with human wastes and sewage. Several publications highlight the development of AMR in bacteria present in landfills and water treatment plants [36,37]. The trend of white storks, black-backed gulls and black-headed gulls wintering in Spain to feed on landfills observed in recent years would fit the hypotheses of this exposure [3,38].
It is known that antibiotic consumption promotes the development of AMR in animals [39]. In the EU, the most employed antibiotics in human medicine are betalactamics, macrolides and quinolones, while in veterinary it is betalactamics and tetracyclines [40]. Betalactamics and quinolones were shown to be the antimicrobial classes with the highest rate of resistance in the present study. According to the Spanish Agency for Drugs and Medical Devices (AEMPS), the antibiotic with the highest percentage of AMR in E. coli in Spain in 2017 was ampicillin in humans and livestock, with rates of 65% and 72.81%, respectively [39]. The extended use of this antibiotic agrees with the high percentage of ampicillin-resistant strains detected in both white storks and seagulls.
The prevalence of AMR found in the seagulls included in the present study (63.2%) is higher compared to those published in EU countries. Among the antimicrobials tested by other authors in samples from seagulls from the Mediterranean countries, the present study showed similar results: antimicrobial classes with higher resistance were penicillins, tetracyclines and quinolones [9,15,20,22,41,42]. Previously, Stedt et al. assessed the AMR presence in seagulls from the Spanish Mediterranean coast among other regions and the presence of AMR is similar between both studies (61.2% Stedt vs. 63.2%) [22]. It is interesting to note that, despite the high level of AMR, all the isolates were susceptible to cephalosporins (except one to ceftazidime) and carbapenems. Resistance to these antimicrobials has been raised in seagulls worldwide [9,43,44,45] and has also been described in the Iberian Peninsula [21,46,47]. It is tempting to speculate if resistances to cephalosporins found in coastline birds are related to seawater contaminated with human sewage, and thus not affecting inland wild birds.
Regarding white storks, there is less information about the burden of AMR in E. coli in this species. Our results agree to those reported by Skarzynska et al. [48]: a high proportion of resistance to penicillins, quinolones and trimethoprim-sulfamethoxazole. However, Camacho et al. evaluated the AMR in white storks from central south Spain and found a higher level for gentamicin (44.8–46.7%) and enrofloxacin (40.2–41.4%), compared to our results (10.5%, both) [5]. Additionally, cefotaxime-resistant strains were detected in a high proportion (22–37.9%) while all our isolates were sensitive. A recent study that has analyzed the AMR on E. coli isolated from storks, including 12 antimicrobials [26], found a higher proportion of resistant isolates, compared to our panel, specifically to ampicillin (100%), nalidixic acid and ciprofloxacin (80%), tetracycline (67%) and gentamicin (33%) [26].
In addition, wild birds may harbor other relevant bacterial species, such as Salmonella and Staphylococcus aureus. Salmonella spp. isolates from white storks feeding on the same area of our study showed resistance to quinolones and ampicillin, which is in accordance with our results, but none was resistant to gentamicin and chloramphenicol [6]. Additionally, methicillin-resistant S. aureus has been isolated in tracheal samples from white storks (3.3%) [49]. In these studies, AMR carriage has been attributed to human residues exposure [5,6,26,49].
The rate of MDR in white storks (50%) is higher than that obtained in previous studies [5]. However, the proportion of MDR observed in seagulls (25%) agrees with those published by Stedt et al. (28.6–45.3%) [22].
Some species of wildlife had been suggested as sentinels for AMR surveillance in the ecosystems. However, sampling wildlife requires considerable logistical efforts and/or the handling of animals without interfering in their welfare. In this context, WRCs can be valuable resources [5]. One advantage is the success of the microbiological analysis, as the time between the sample collection and the arrival at the laboratory is shortened. E. coli was recovered from a higher proportion of sampled animals than in other studies [5,23,50].
Finally, assigning the source and dissemination of AMR is not easy and the role of wildlife as reservoirs of AMR for humans would require extended studies [19,50,51]. Two issues should be highlighted regarding the wildlife species studied here. First, some of these birds are migratory. In fact, two white storks and two lesser black-backed gulls sampled in this study had a leg ring from Belgium (stork no. 28) and Germany (stork no. 12, seagulls no. 11 and no. 16), respectively. Interestingly, stork no. 12 and seagull no. 11 carried MDR (penicillins, quinolones, tetracyclines, sulfonamides) and AMR-resistant E. coli isolates, respectively. It has been described that migratory birds can carry more AMRs than non-migratory [52], as the migratory patterns of wild birds can exponentially magnify the dissemination and acquisition of AMRs, even in remote regions [53]. Second, most of the animals sampled in this study had been brought into the WRC by citizens who found the animals, which implies a direct contact in the human–wildlife interface. Although the spillover of enteric pathogens from wild birds to humans is controversial [54], a close contact by handling an injured animal could represent a transference route in both directions for citizens and WRC staff [51].
Once a health issue is introduced into a wild population, its control is difficult to achieve. Thus, a key priority would be a prudent management of anthropogenic wastes and sewage to avoid the access of wild animals and prevent the transmission of AMR to wildlife.
5. Conclusions
In conclusion, our study describes the profiles of phenotypic AMR detected in E. coli isolated from white storks and seagulls in central Spain. These bird species can be considered suitable sentinels for AMR and MDR surveillance. The proportion of AMR and MDR detected in the present study is higher than the rates published by other authors, which could be in concordance with the upward trend of AMRs detection worldwide. In this context, an adequate management of antibiotic residues and urban waste should be a priority to prevent further AMR dissemination into wildlife.
Author Contributions
Conceptualization, B.M.-M. and A.A.; methodology, B.M.-M., A.A., P.R.-A., N.P., L.S. and V.M.; statistical analysis, B.M.-M. and A.F.-N.; formal analysis, A.A. and B.M.-M.; resources, F.G. and A.A.; data curation, A.A.; writing—original draft preparation, P.R.-A., B.M.-M. and A.A.; writing—review and editing, A.A., F.G., I.L. and L.S.; visualization, B.M.-M.; supervision, B.M.-M. and A.A.; funding acquisition, A.F.-N., F.G. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Ecological Transition (MITECO) of Spain and the Complutense University of Madrid (Spain).
Institutional Review Board Statement
Ethical review and approval were waived for this study because in the standard protocol for the sanitary status analysis of animals admitted to the GREFA Wildlife Hospital. Therefore, no extra handling of the animals was necessary to collect the samples, and no extra samples were collected outside the hospital standard work protocol. For this reason, according to the current legislation at the time of the research (Directive 2010/63/EU), it is not mandatory to have the approval of an Ethics Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (A.A.).
Acknowledgments
We wish to thank GEMAS Research Group (Grupo de Estudio de Medicina y Conservación de Animales Salvajes) and all the GREFA volunteers for their technical support.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have influenced the work reported in this paper.
References
- Miranda, A.C. Mechanisms of behavioural change in urban animals: The role of microevolution and phenotypic plasticity. In Ecology and Conservation of Birds in Urban Enironments; Murgui, E., Hedblom, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 113–132. [Google Scholar] [CrossRef]
- Molina, B. Gaviota sombría. In Atlas de las Aves en Invierno en España 2007–2010; Del Moral, J.C., Molina, B., Bermejo, A., Palomino, D., Eds.; Ministerio de Agricultura, Alimentación y Medio Ambiente-SEO/BirdLife: Madrid, Spain, 2012; pp. 294–296. [Google Scholar]
- Bécares, J.; Blas, J.; López-López, P.; Schulz, H.; Torres-Medina, F.; Flack, A.; Enggist, P.; Höfle, U.; Bermejo, A.; de la Puente, J. Migración y Ecología Espacial de la Cigüeña Blanca en España; Monografía no. 5 del Programa Migra; SEO/BirdLife: Madrid, Spain, 2019. [Google Scholar] [CrossRef]
- Plaza, P.I.; Lambertucci, S.A. How are garbage dumps impacting vertebrate demography, health, and conservation? Glob. Ecol. Conserv. 2017, 12, 9–20. [Google Scholar] [CrossRef]
- Camacho, M.; Hernández, J.M.; Lima-Barbero, J.F.; Höfle, U. Use of wildlife rehabilitation centres in pathogen surveillance: A case study in white storks (Ciconia ciconia). Prev. Vet. Med. 2016, 130, 106–111. [Google Scholar] [CrossRef]
- Martín-Maldonado, B.; Vega, S.; Mencía-Gutiérrez, A.; Lorenzo-Rebenaque, L.; de Frutos, C.; González, F.; Revuelta, L.; Marin, C. Urban birds: An important source of antimicrobial resistant Salmonella strains in Central Spain. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101519. [Google Scholar] [CrossRef]
- Esperón, F.; Sacristán, C.; Carballo, M.; de la Torre, A. Antimicrobial resistance genes in animal manure, manure-amended and non-anthropogenically impacted soils in Spain. Adv. Biosci. Biotechnol. 2018, 9, 469–480. [Google Scholar] [CrossRef]
- Kunhikannan, S.; Thomas, C.J.; Franks, A.E.; Mahadevaiah, S.; Kumar, S.; Petrovski, S. Environmental hotspots for antibiotic resistance genes. Microbiologyopen 2021, 10, e1197. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.P.; Pace, A.; Varriale, L.; Borrelli, L.; Gargiulo, A.; Pompameo, M.; Fioretti, A.; Dipineto, L. Prevalence and antimicrobial resistance of enteropathogenic bacteria in yellow-legged gulls (Larus michahellis) in Southern Italy. Animals 2021, 11, 275. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; HM Government: London, UK, 2016; Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 27 February 2021).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Naghavi, M. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2021, 19, e06490. [Google Scholar] [CrossRef]
- Giacopello, C.; Foti, M.; Mascetti, A.; Grosso, F.; Ricciardi, D.; Fisichella, V.; Piccolo, F.L. Antimicrobial resistance patterns of Enterobacteriaceae in European wild bird species admitted in a wildlife rescue centre. Vet. Ital. 2016, 52, 139–144. [Google Scholar] [CrossRef]
- Carter, D.L.; Docherty, K.M.; Gill, S.A.; Baker, K.; Teachout, J.; Vonhof, M.J. Antibiotic resistant bacteria are widespread in songbirds across rural and urban environments. Sci. Total Environ. 2018, 627, 1234–1241. [Google Scholar] [CrossRef]
- Rabbia, V.; Bello-Toledo, H.; Jiménez, S.; Quezada, M.; Domínguez, M.; Vergara, L.; Gómez-Fuentes, C.; Calisto-Ulloa, N.; González-Acuña, D.; López, J.; et al. Antibiotic resistance in Escherichia coli strains isolated from Antarctic bird feces, water from inside a wastewater treatment plant, and seawater samples collected in the Antarctic Treaty area. Polar Sci. 2016, 10, 123–131. [Google Scholar] [CrossRef]
- Hwengwere, K.; Paramel Nair, H.; Hughes, K.A.; Peck, L.S.; Clark, M.S.; Walker, C.A. Antimicrobial resistance in Antarctica: Is it still a pristine environment? Microbiome 2022, 10, 71. [Google Scholar] [CrossRef]
- White, A.; Hughes, J.M. Critical importance of a One Health approach to antimicrobial resistance. EcoHealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- Bonnedahl, J.; Drobni, M.; Gauthier-Clerc, M.; Hernandez, J.; Granholm, A.; Kayser, Y.; Melhus, Å.; Kahlmeter, G.; Waldenström, J.; Johansson, A.; et al. Dissemination of Escherichia coli with CTX-M Type ESBL between humans and yellow-legged gulls in the South of France. PLoS ONE 2009, 4, e5958. [Google Scholar] [CrossRef]
- Alves, M.S.; Pereira, A.; Araújo, S.M.; Castro, B.B.; Correia, A.C.M.; Henriques, I. Seawater is a reservoir of multi-resistant Escherichia coli, including strains hosting plasmid-mediated quinolones resistance and extended-spectrum beta-lactamases genes. Front. Microbiol. 2014, 5, 426. [Google Scholar] [CrossRef]
- Stedt, J.; Bonnedahl, J.; Hernandez, J.; McMahon, B.J.; Hasan, B.; Olsen, B.; Drobni, M.; Waldenström, J. Antibiotic resistance patterns in Escherichia coli from gulls in nine European countries. Infect. Ecol. Epidemiol. 2014, 4, 21565. [Google Scholar] [CrossRef]
- Carroll, D.; Wang, J.; Fanning, S.; McMahon, B.J. Antimicrobial resistance in wildlife: Implications for Public Health. Zoonoses Public Health 2015, 62, 534–542. [Google Scholar] [CrossRef]
- Atterby, C.; Ramey, A.M.; Hall, G.G.; Järhult, J.; Börjesson, S.; Bonnedahl, J. Increased prevalence of antibiotic-resistant E. coli in gulls sampled in Southcentral Alaska is associated with urban environments. Infect. Ecol. Epidemiol. 2016, 6, 32334. [Google Scholar] [CrossRef][Green Version]
- Bouaziz, A.; Loucif, L.; Ayachi, A.; Guehaz, K.; Bendjama, E.; Rolain, E.M. Migratory white stork (Ciconia ciconia): A potential vector of the OXA-48-producing Escherichia coli ST38 clone in Algeria. Microb. Drug Resist. 2018, 24, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Höfle, U.; Gonzalez-Lopez, J.J.; Camacho, M.C.; Solà-Ginés, M.; Moreno-Mingorance, A.; Hernández, J.M.; de la Puente, J.; Pineda-Pampliega, J.; Aguirre, J.I.; Torres-Medina, F.; et al. Foraging at solid urban waste disposal sites as risk factor for cephalosporin and colistin resistant Escherichia coli carriage in white storks (Ciconia ciconia). Front. Microbiol. 2020, 11, 1397. [Google Scholar] [CrossRef]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. 2010. OJ L 276. pp. 33–79. Available online: https://eur-lex.europa.eu/oj/direct-access.html (accessed on 17 September 2022).
- BOE. Boletín Oficial del Estado, Real Decreto 53/2013, de 1 de Febrero, por el que se Establecen las Normas Básicas Aplicables para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia; BOE: Madrid, Spain, 2013. [Google Scholar]
- Markey, B.; Leonard, F.; Archambault, M.; Cullinane, A.; Maguire, D. Clinical Veterinary Microbiology, 2nd ed.; Mosby Elsevier Ltd.: Dublin, Ireland, 2013. [Google Scholar]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria and Repealing Implementing Decision 2013/652/EU. 2020. OJ L 387. pp. 8–21. Available online: https://eur-lex.europa.eu/eli/dec_impl/2020/1729/oj (accessed on 17 September 2022).
- EUCAST. European Committee on Antimicrobial Susceptibility Testing (EUCAST), Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 11. 2021. Available online: http://www.eucast.org (accessed on 15 March 2021).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.; Drum, D.J.V.; Stallknecht, D.E.; White, D.G.; Lee, M.D.; Ayers, S.; Sobsey, M.; Maurer, J.J. Free-living Canada geese and antimicrobial resistance. Emerg. Infect. Dis. 2005, 11, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Jones, S.H.; Edwards, C.; Ellis, J.C. Characterization of Escherichia coli populations from gulls, landfill trash, and wastewater using ribotyping. Dis. Aquat. Org. 2008, 81, 53–63. [Google Scholar] [CrossRef]
- Chen, Q.L.; Li, H.; Zhou, X.Y.; Zhao, Y.; Su, J.Q.; Zhang, X.; Huang, F.Y. An underappreciated hotspot of antibiotic resistance: The groundwater near the municipal solid waste landfill. Sci. Total Environ. 2017, 609, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, K.; Semerád, J.; Petráčková, D.; Novotný, Č. Antibiotic resistance in Czech urban wastewater treatment plants: Microbial and molecular genetic characterization. Microb. Drug Resist. 2018, 24, 830–838. [Google Scholar] [CrossRef]
- Gilbert, N.I.; Correia, R.A.; Silva, J.P.; Pacheco, C.; Catry, I.; Atkinson, P.W.; Gill, J.A.; Franco, A.M. Are white storks addicted to junk food? Impacts of landfill use on the movement and behaviour of resident white storks (Ciconia ciconia) from a partially migratory population. Mov. Ecol. 2016, 4, 7. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Informe JIACRA España. Primer Análisis Integrado del Consumo de Antibióticos y su Relación con la Aparición de Resistencia [Joint Interagency Antimicrobial Consumption and Resistance Analysis]. 2018. Available online: https://resistenciaantibioticos.es/es/publicaciones/informe-jiacra-espana (accessed on 15 March 2021).
- European Medicines Agency (EMA). European Surveillance of Veterinary Antimicrobial Consumption, 2020. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018 (EMA/24309/2020). 2018. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/european-surveillance-veterinary-antimicrobial-consumption-esvac (accessed on 27 February 2021).
- Alcalá, L.; Alonso, C.A.; Simón, C.; González-Esteban, C.; Orós, J.; Rezusta, A.; Ortega, C.; Torres, C. Wild birds, frequent carriers of extended-spectrum β-lactamase (ESBL) producing Escherichia coli of CTX-M and SHV-12 types. Microb. Ecol. 2016, 72, 861–869. [Google Scholar] [CrossRef]
- Barguigua, A.; Idrissi, H.R.; Timinouni, M. Virulence and antibiotic resistance patterns in E. coli, Morocco. EcoHealth 2019, 16, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Potron, A.; De La Cuesta, C.; Cleary, T.; Nordmann, P.; Munoz-Price, L.S. Wild coastline birds as reservoirs of broad-spectrum-β-Lactamase-producing Enterobacteriaceae in Miami Beach, Florida. Antimicrob. Agents Chemother. 2012, 56, 2756–2758. [Google Scholar] [CrossRef] [PubMed]
- Bonnedahl, J.; Järhult, J.D. Antibiotic resistance in wild birds. Upsala J. Med. Sci. 2014, 119, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Merkeviciene, L.; Klimiene, I.; Siugzdiniene, R.; Virgailis, M.; Mockeliunas, R.; Ruzauskas, M. Prevalence and molecular characteristics of multi-resistant Escherichia coli in wild birds. Acta Vet. Brno 2018, 87, 9–17. [Google Scholar] [CrossRef]
- Simões, R.R.; Poirel, L.; Martins Da Costa, P.; Nordmann, P. Seagulls and beaches as reservoirs for multidrug-resistant Escherichia coli. Emerg. Infect. Dis. 2010, 16, 110–112. [Google Scholar] [CrossRef]
- Vergara, A.; Pitart, C.; Montalvo, T.; Roca, I.; Sabaté, S.; Hurtado, J.C.; Planell, R.; Marco, F.; Ramírez, B.; Peracho, V.; et al. Prevalence of extended-spectrum-β-lactamase- and/or carbapenemase-producing Escherichia coli isolated from yellow-legged gulls from Barcelona, Spain. Antimicrob. Agents Chemother. 2017, 61, e02071-16. [Google Scholar] [CrossRef]
- Skarżyńska, M.; Zajac, M.; Bomba, A.; Bocian, Ł.; Kozdruń, W.; Polak, M.; Wiacek, J.; Wasyl, D. Antimicrobial Resistance Glides in the Sky—Free-Living Birds as a Reservoir of Resistant Escherichia coli with Zoonotic Potential. Front. Microbiol. 2021, 12, 656223. [Google Scholar] [CrossRef]
- Gómez, P.; Lozano, C.; Camacho, M.C.; Lima-Barbero, J.F.; Hernández, J.M.; Zarazaga, M.; Höfle, U.; Torres, C. Detection of MRSA ST3061-t843-mecC and ST398-t011-mecA in white stork nestlings exposed to human residues. J. Antimicrob. Chemother. 2016, 71, 53–57. [Google Scholar] [CrossRef]
- Swift, B.M.C.; Bennett, M.; Waller, K.; Dodd, C.; Murray, A.; Gomes, R.L.; Humphreys, B.; Hobman, J.L.; Jones, M.A.; Whitlock, S.E.; et al. Anthropogenic environmental drivers of antimicrobial resistance in wildlife. Sci. Total Environ. 2019, 649, 12–20. [Google Scholar] [CrossRef]
- Dolejska, M.; Literak, I. Wildlife is overlooked in the epidemiology of medically important antibiotic-resistant bacteria. Antimicrob. Agents Chemother. 2019, 63, e01167-19. [Google Scholar] [CrossRef]
- Shobrak, M.Y.; Abo-Amer, A.E. Role of wild birds as carriers of multi-drug resistant Escherichia coli and Escherichia vulneris. Braz. J. Microbiol. 2014, 45, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Sjölund, M.; Bonnedahl, J.; Hernandez, J.; Bengtsson, S.; Cederbrant, G.; Pinhassi, J.; Kahlmeter, G.; Olsen, B. Dissemination of multidrug-resistant bacteria into the Arctic. Emerg. Infect. Dis. 2008, 14, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.M.; Snyder, W.E.; Owen, J.P. Are we overestimating risk of enteric pathogen spillover from wild birds to humans? Biol. Rev. 2020, 95, 652–679. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).