Effects of the Replacement of Dietary Fish Meal with Defatted Yellow Mealworm (Tenebrio molitor) on Juvenile Large Yellow Croakers (Larimichthys crocea) Growth and Gut Health
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Experimental Diets
2.2. Fish and Feeding Trial
2.3. Sample Collection
2.4. Analytical Methods
2.4.1. Proximate Composition
2.4.2. Digestibility Enzyme
2.4.3. Serum Biochemical Parameters
2.4.4. Intestinal Histology
2.4.5. Gut Microbiota Collection and Analyses
| Ingredients (%) | Experimental Diets | |||
|---|---|---|---|---|
| TM0 | TM15 | TM30 | TM45 | |
| Fish meal | 40.00 | 34.00 | 28.00 | 22.00 |
| Chicken meal a | 13.00 | 13.00 | 13.00 | 13.00 |
| Soybean meal | 10.00 | 10.00 | 10.00 | 10.00 |
| TM b | 0.00 | 6.70 | 13.30 | 20.00 |
| High gluten flour | 23.00 | 21.39 | 19.87 | 18.25 |
| Soybean oil | 1.50 | 1.50 | 1.50 | 1.50 |
| Fish oil | 2.50 | 2.90 | 3.30 | 3.70 |
| Lecithin | 1.50 | 1.50 | 1.50 | 1.50 |
| Stickwater c | 6.00 | 6.00 | 6.00 | 6.00 |
| Vitamin C d | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin premix e | 0.40 | 0.40 | 0.40 | 0.40 |
| Mineral premix f | 0.50 | 0.50 | 0.50 | 0.50 |
| Ca(H2PO4)2 | 1.00 | 1.50 | 2.00 | 2.50 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 |
| Methionine | 0.00 | 0.01 | 0.03 | 0.05 |
| Proximate analysis | ||||
| Dry matter | 98.02 | 98.49 | 98.59 | 98.85 |
| Crude protein | 47.98 | 48.30 | 48.00 | 48.10 |
| Crude lipid | 8.25 | 8.63 | 8.76 | 8.79 |
| Ash | 11.12 | 10.70 | 10.85 | 10.50 |
| Methionine | 2.60 | 2.64 | 2.67 | 2.71 |
| Lysine | 0.93 | 0.93 | 0.93 | 0.94 |
| Total Phosphorus | 1.38 | 1.38 | 1.38 | 1.37 |
2.5. Calculations and Statistical Analysis
3. Results
3.1. Growth and Feed Utilization
3.2. Body Composition
3.3. Digestion Enzyme
3.4. Non-Specific Immunity
3.5. Gut Morphology
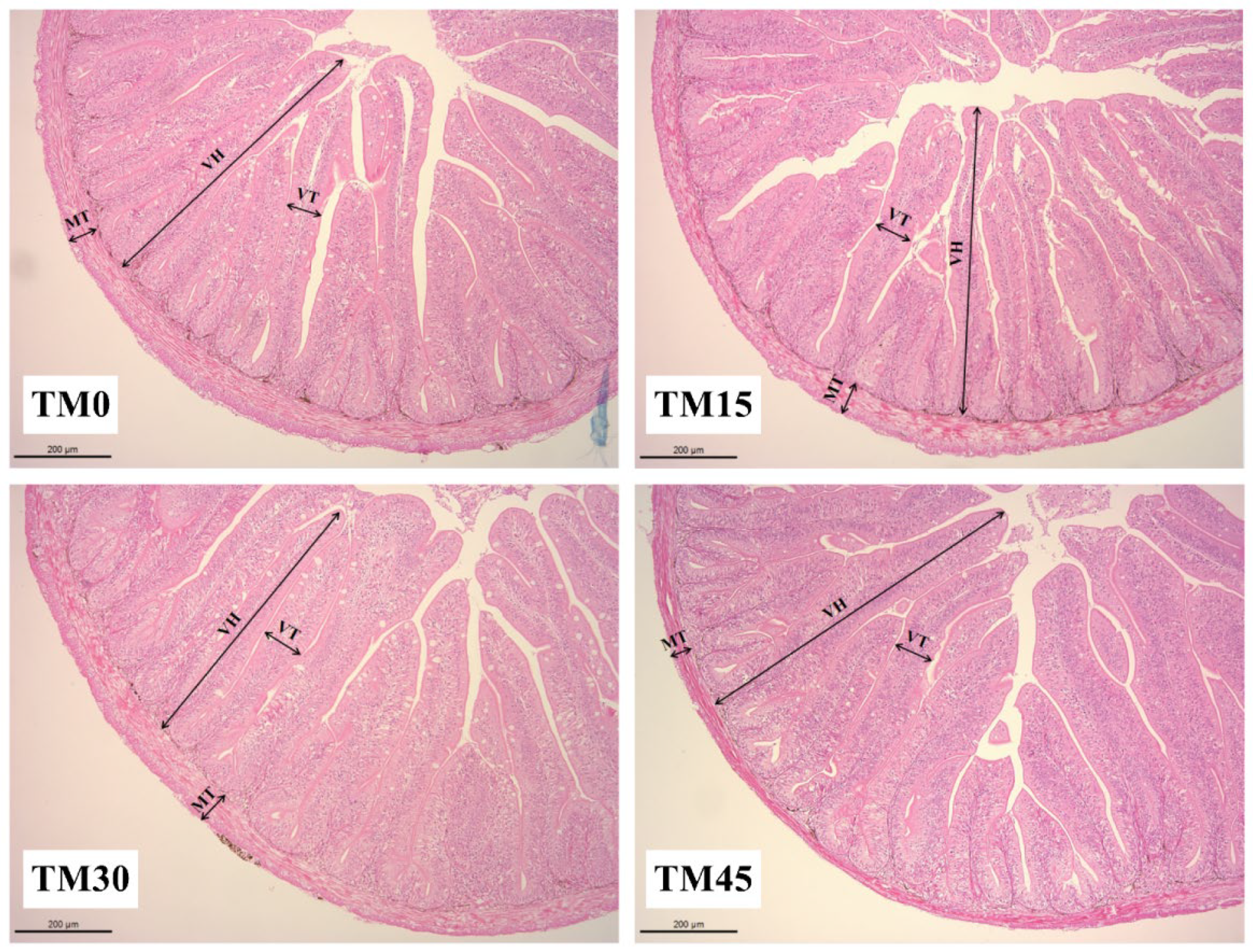
| Index (μm) | Experimental Diets | |||
|---|---|---|---|---|
| TM0 | TM15 | TM30 | TM45 | |
| VH | 588.59 ± 10.57 a | 620.82 ± 5.70 b | 606.52 ± 4.32 ab | 664.46 ± 4.95 c |
| VT | 72.56 ± 0.95 a | 93.85 ± 2.25 b | 104.69 ± 2.02 c | 110.06 ± 1.99 c |
| MT | 69.08 ± 0.73 a | 76.65 ± 0.87 b | 73.64 ± 0.84 b | 69.00 ± 0.89a |
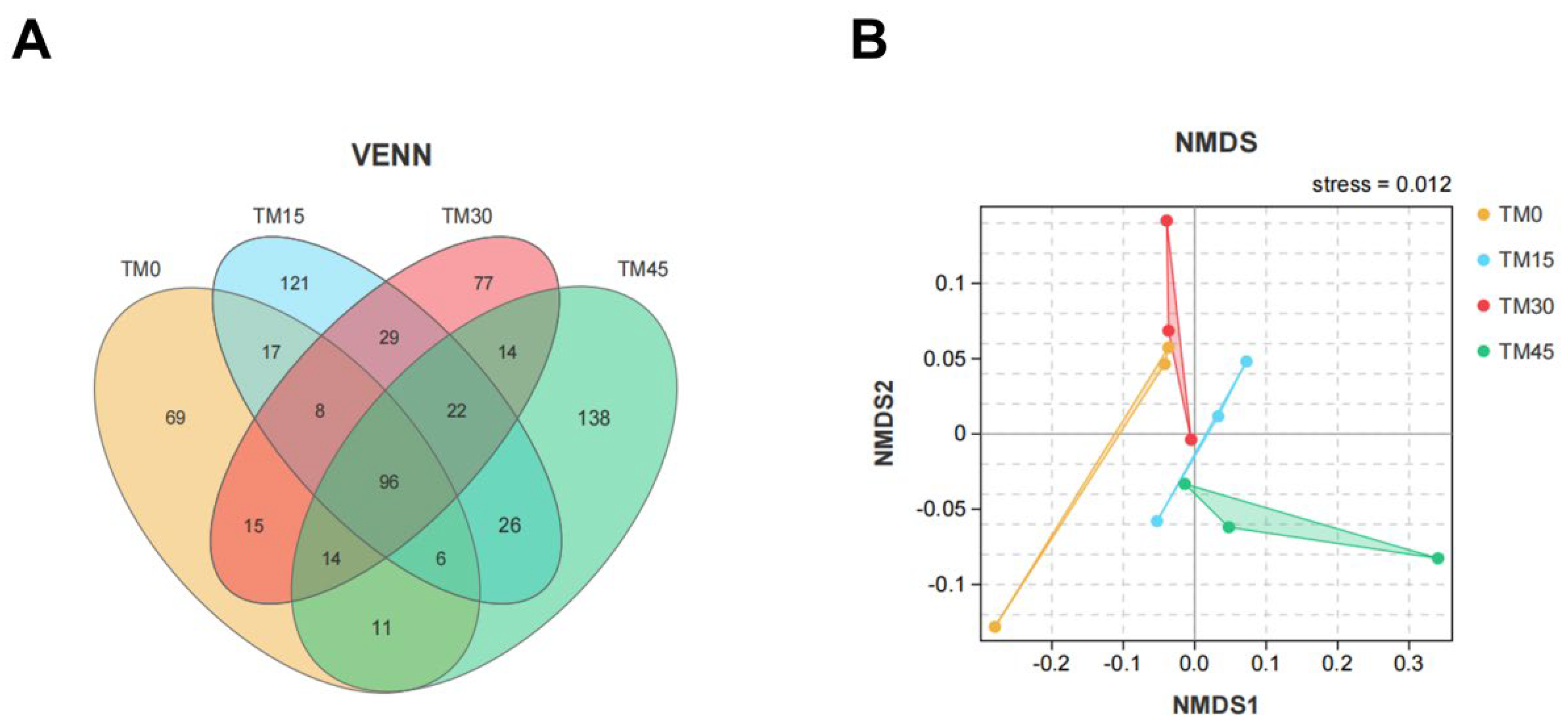
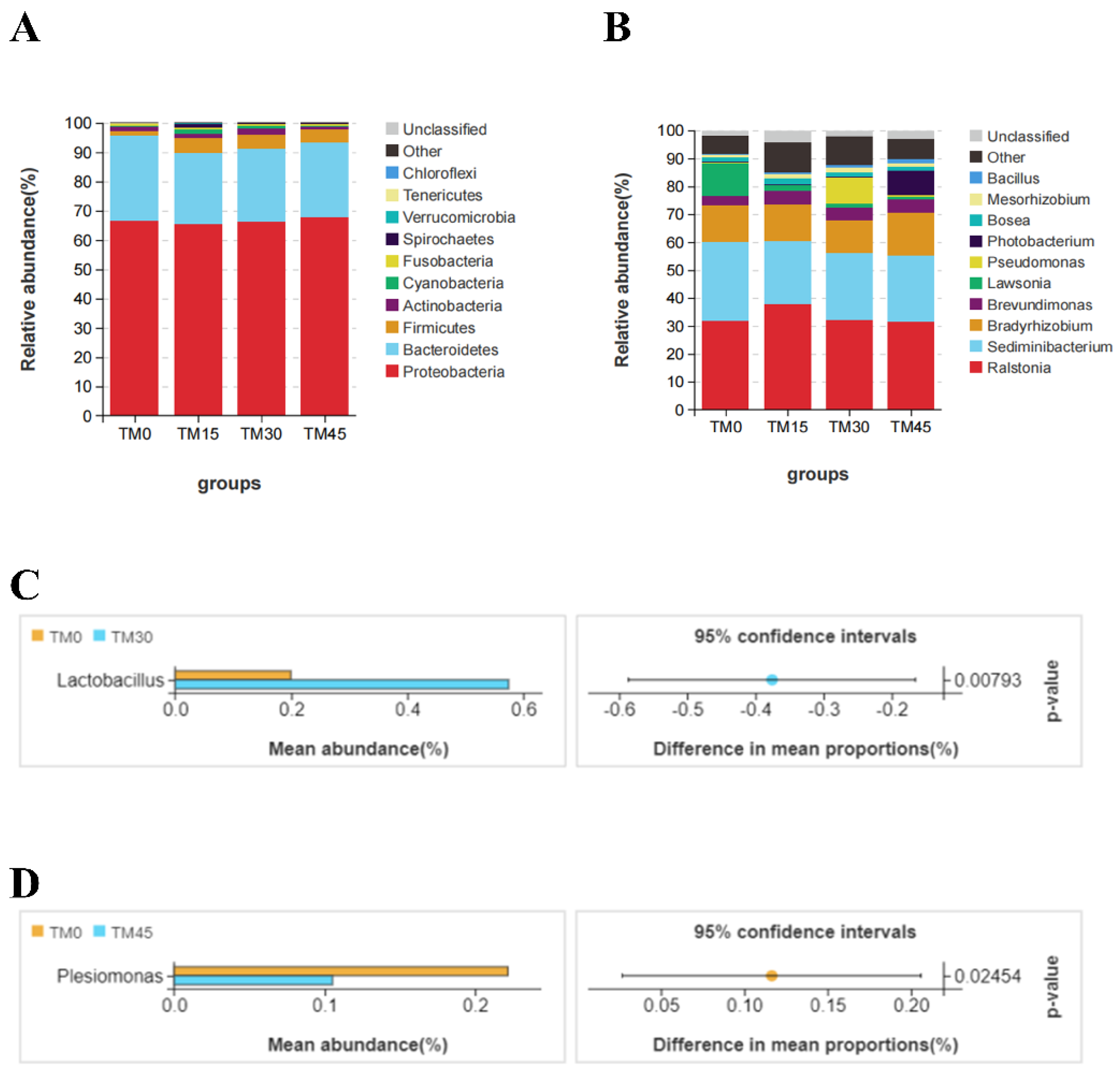
3.6. Gut Microbial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Li, L.; Xia, T.; Wang, L.; Xiao, L.; Ding, N.; Wu, Y.; Lu, K. Oxidative Stress Can Be Attenuated by 4-PBA Caused by High-Fat or Ammonia Nitrogen in Cultured Spotted Seabass: The Mechanism Is Related to Endoplasmic Reticulum Stress. Antioxidants 2022, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- De Wrachien, D.; Schultz, B.; Goli, M.B. Impacts of population growth and climate change on food production and irrigation and drainage needs: A world-wide view*. Irrig. Drain. 2021, 70, 981–995. [Google Scholar] [CrossRef]
- Keating, B.A.; Herrero, M.; Carberry, P.S.; Gardner, J.; Cole, M.B. Food wedges: Framing the global food demand and supply challenge towards 2050. Glob. Food Secur. 2014, 3, 125–132. [Google Scholar] [CrossRef]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.Á.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef]
- Cai, L.; Wang, L.; Song, K.; Lu, K.; Zhang, C.; Rahimnejad, S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 2020, 516, 734615. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.H. Fish meal-nutritive value. J. Anim. Physiol. Anim. Nutr. 2011, 95, 685–692. [Google Scholar] [CrossRef]
- Jia, S.; Li, X.; He, W.; Wu, G. Protein-Sourced Feedstuffs for Aquatic Animals in Nutrition Research and Aquaculture. Adv. Exp. Med. Biol. 2022, 1354, 237–261. [Google Scholar] [CrossRef]
- Lin, Z.; Yoshikawa, S.; Hamasaki, M.; Koyama, T.; Kikuchi, K.; Hosoya, S. Effects of low fishmeal diets on growth performance, blood chemical composition, parasite resistance, and gene expression in the tiger pufferfish, Takifugu rubripes. Aquaculture 2022, 560, 738484. [Google Scholar] [CrossRef]
- Schmidt, V.; Amaral-Zettler, L.; Davidson, J.; Summerfelt, S.; Good, C. Influence of Fishmeal-Free Diets on Microbial Communities in Atlantic Salmon (Salmo salar) Recirculation Aquaculture Systems. Appl. Environ. Microbiol. 2016, 82, 4470–4481. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Bautista-Teruel, M.N.; Fermin, A.C.; Koshio, S.S. Diet development and evaluation for juvenile abalone, Haliotis asinina: Animal and plant protein sources. Aquaculture 2003, 219, 645–653. [Google Scholar] [CrossRef]
- Rhodes, M.A.; Zhou, Y.; Salze, G.P.; Hanson, T.R.; Davis, D.A. Development of plant-based diets and the evaluation of dietary attractants for juvenile Florida pompano, Trachinotus carolinus L. Aquac. Nutr. 2017, 23, 1065–1075. [Google Scholar] [CrossRef]
- Jensen, H.; Elleby, C.; Domínguez, I.P.; Chatzopoulos, T.; Charlebois, P. Insect-based protein feed: From fork to farm. J. Insects Food Feed 2021, 7, 1219–1233. [Google Scholar] [CrossRef]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2016, 100, 70–79. [Google Scholar] [CrossRef]
- Niyonsaba, H.H.; Höhler, J.; Kooistra, J.; Van der Fels-Klerx, H.J.; Meuwissen, M.P.M. Profitability of insect farms. J. Insects Food Feed 2021, 7, 923–934. [Google Scholar] [CrossRef]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Belghit, I.; Lock, E.; Krogdahl, Å. Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar). Aquaculture 2020, 520, 734967. [Google Scholar] [CrossRef]
- Hashizume, A.; Ido, A.; Ohta, T.; Thiaw, S.T.; Morita, R.; Nishikawa, M.; Takahashi, T.; Miura, C.; Miura, T. Housefly (Musca domestica) Larvae Preparations after Removing the Hydrophobic Fraction Are Effective Alternatives to Fish Meal in Aquaculture Feed for Red Seabream (Pagrus major). Fishes 2019, 4, 38. [Google Scholar] [CrossRef]
- Perera, A.D.; Bhujel, R.C. Field cricket (Gryllus bimaculatus) meal (FCM) to replace fishmeal in the diets for sex reversal and nursing of Nile tilapia (Oreochromis niloticus) fry. Aquac. Res. 2021, 52, 4946–4958. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former Foodstuff Products in Tenebrio Molitor Rearing: Effects on Growth, Chemical Composition, Microbiological Load, and Antioxidant Status. Animals 2019, 9, 484. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poult. Sci. 2019, 99, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yu, J.S.; Parker, B.L.; Skinner, M.; Je, Y.H.; Kim, J.S. Production of antibacterial Bombyx mori cecropin A in mealworm-pathogenic Beauveria bassiana ERL1170. J. Ind. Microbiol. Biotechnol. 2014, 42, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Motte, C.; Rios, A.; Lefebvre, T.; Do, H.; Henry, M.; Jintasataporn, O. Replacing Fish Meal with Defatted Insect Meal (Yellow Mealworm Tenebrio molitor) Improves the Growth and Immunity of Pacific White Shrimp (Litopenaeus vannamei). Animals 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Ido, A.; Hashizume, A.; Ohta, T.; Takahashi, T.; Miura, C.; Miura, T. Replacement of Fish Meal by Defatted Yellow Mealworm (Tenebrio molitor) Larvae in Diet Improves Growth Performance and Disease Resistance in Red Seabream (Pargus major). Animals 2019, 9, 100. [Google Scholar] [CrossRef]
- Ge, C.; Cheng, H.; Li, J.; Wang, H.; Ma, S.; Qin, Y.; Xue, M. Effects of defatted yellow mealworm (Tenebrio molitor) on the feed qualities and the growth performance of largemouth bass (Micropterus salmoides). J. Insects Food Feed 2022, 1, 15. [Google Scholar] [CrossRef]
- Lan, W.; Zhao, X.; Wang, M.; Xie, J. Effects of chitosan and apple polyphenol coating on quality and microbial composition of large yellow croaker (Pseudosciaena crocea) during ice storage. J. Sci. Food Agric. 2021, 102, 3099–3106. [Google Scholar] [CrossRef]
- Duan, Q.; Mai, K.; Zhong, H.; Si, L.; Wang, X. Studies on the nutrition of the large yellow croaker, Pseudosciaena crocea R. I: Growth response to graded levels of dietary protein and lipid. Aquac. Res. 2001, 32, 46–52. [Google Scholar] [CrossRef]
- Ma, H.; Cahu, C.; Zambonino, J.; Yu, H.; Duan, Q.; Le Gall, M.; Mai, K. Activities of selected digestive enzymes during larval development of large yellow croaker (Pseudosciaena crocea). Aquaculture 2005, 245, 239–248. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Q.; Feng, J.; He, J.; Lou, Y.; Zhu, J. Effect of dietary fermented soybean meal on growth, intestinal morphology and microbiota in juvenile large yellow croaker, Larimichthys crocea. Aquac. Res. 2019, 50, 748–757. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, J.; Feng, J.; He, J.; Lou, Y.; Zhou, Q. Effects of dietary soy protein concentrate meal on growth, immunity, enzyme activity and protein metabolism in relation to gene expression in large yellow croaker Larimichthys crocea. Aquaculture 2017, 477, 15–22. [Google Scholar] [CrossRef]
- Mai, K.; Yu, H.; Ma, H.; Duan, Q.; Gisbert, E.; Infante, J.L.Z.; Cahu, C.L. A histological study on the development of the digestive system of Pseudosciaena crocea larvae and juveniles. J. Fish Biol. 2005, 67, 1094–1106. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Y.; Xu, N.; Ding, T.; Cui, K.; Chen, Q.; Zhang, J.; Fang, W.; Mai, K.; Ai, Q. Effects of dietary Astragalus polysaccharides (APS) on survival, growth performance, activities of digestive enzyme, antioxidant responses and intestinal development of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2020, 517, 734752. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Analysis of Offical Analytical Chemists International, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2002. [Google Scholar]
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Syedbasha, M.; Linnik, J.; Santer, D.; O’Shea, D.; Barakat, K.; Joyce, M.; Khanna, N.; Tyrrell, D.L.; Houghton, M.; Egli, A. An ELISA Based Binding and Competition Method to Rapidly Determine Ligand-receptor Interactions. J. Vis. Exp. 2016, 109, 53575. [Google Scholar] [CrossRef]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 2018, 45, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, K.; Wang, L.; Song, K.; Zhang, L.; Li, P. Apparent digestibility coefficients and amino acid availability of common protein ingredients in the diets of bullfrog, Rana (Lithobates) catesbeiana. Aquaculture 2015, 437, 38–45. [Google Scholar] [CrossRef]
- Ng, W.; Liew, F.; Ang, L.; Wong, K. Potential of mealworm (Tenebrio molitor) as an alternative protein source in practical diets for African catfish, Clarias gariepinus. Aquac. Res. 2001, 32, 273–280. [Google Scholar] [CrossRef]
- Hung, Q.T.; Markéta, P.; Mahyar, Z.; Jan, M.; Ilario, F.; Laura, G.; Vlastimil, S. Production performance, nutrient digestibility, serum biochemistry, fillet composition, intestinal microbiota and environmental impacts of European perch (Perca fluviatilis) fed defatted mealworm (Tenebrio molitor). Aquaculture 2021, 547, 737499. [Google Scholar] [CrossRef]
- Song, Y.; Kim, M.; Moon, C.; Seo, D.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.; Kim, S.; Kim, Y.W.; et al. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.; de Haro, C.; Sanz, A.; Trenzado, C.E.; Villareces, S.; Barroso, F.G. Nutritional evaluation of Tenebrio molitor meal as fishmeal substitute for tilapia (Oreochromis niloticus) diet. Aquac. Nutr. 2015, 22, 943–955. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Xi, L.; Lu, Q.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Xie, S. The effect of dietary Tenebrio molitor meal inclusion on growth performance and liver health of largemouth bass (Micropterus salmoides). J. Insects Food Feed 2022, 1–14. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Kalhor, N.; Dawood, M.A.O.; Ahmadifar, M.; Shahriari Moghadam, M.; Yousefi, M. Effects of dietary p-coumaric acid on the growth performance, digestive enzyme activity, humoral immunity and immune-related gene expression in common carp, Cyprinus carpio. Aquac. Nutr. 2021, 27, 747–756. [Google Scholar] [CrossRef]
- Lallès, J. Biology, environmental and nutritional modulation of skin mucus alkaline phosphatase in fish: A review. Fish Shellfish Immunol. 2019, 89, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Silva, M.J.; Da Costa, F.F.B.; Leme, F.P.; Takata, R.; Costa, D.C.; Mattioli, C.C.; Luz, R.K.; Miranda-Filho, K.C. Biological responses of Neotropical freshwater fish Lophiosilurus alexandri exposed to ammonia and nitrite. Sci. Total Environ. 2017, 616–617, 1566–1575. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Copenhaver, M.; Yu, C.; Hoffman, R.P. Complement Components, C3 and C4, and the Metabolic Syndrome. Curr. Diabetes Rev. 2018, 15, 44–48. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Serum complement C3 and C4 and COVID-19 severity and mortality: A systematic review and meta-analysis with meta-regression. Front. Immunol. 2021, 12, 696085. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, M.; Wang, L.; Lu, K.; Song, K.; Zhang, C. Bacillus subtilis LCBS1 supplementation and replacement of fish meal with fermented soybean meal in bullfrog (Lithobates catesbeianus) diets: Effects on growth performance, feed digestibility and gut health. Aquaculture 2021, 545, 737217. [Google Scholar] [CrossRef]
- Li, Y.; Hu, H.; Liu, J.; Yang, P.; Zhang, Y.; Ai, Q.; Xu, W.; Zhang, W.; Mai, K. Dietary soya allergen β-conglycinin induces intestinal inflammatory reactions, serum-specific antibody response and growth reduction in a carnivorous fish species, turbot Scophthalmus maximus L. Aquac. Res. 2016, 48, 4022–4037. [Google Scholar] [CrossRef]
- Pereira, S.A.; Jesus, G.F.A.; Cardoso, L.; Silva, B.C.; Ferrarezi, J.V.S.; Ferreira, T.H.; Sterzelecki, F.C.; Sugai, J.K.; Martins, M.L.; Mouriño, J.L.P. The intestinal health of silver catfish Rhamdia quelen can be changed by organic acid salts, independent of the chelating minerals. Aquaculture 2019, 505, 118–126. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Shao, L.; Ling, Z. Alterations of the Predominant Fecal Microbiota and Disruption of the Gut Mucosal Barrier in Patients with Early-Stage Colorectal Cancer. BioMed Res. Int. 2020, 2020, 2948282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rahimnejad, S.; Wang, L.; Lu, K.; Song, K.; Zhang, C. Substituting fish meal with housefly (Musca domestica) maggot meal in diets for bullfrog Rana (Lithobates) catesbeiana: Effects on growth, digestive enzymes activity, antioxidant capacity and gut health. Aquaculture 2019, 499, 295–305. [Google Scholar] [CrossRef]
- Sudha, C.; Ahilan, B.; Felix, N.; Uma, A.; Prabu, E. Effects of dietary protein substitution of fishmeal with black soldier fly larval meal on growth and physiological responses of juvenile striped catfish, Pangasianodon hypophthalmus. Aquac. Res. 2022, 53, 2204–2217. [Google Scholar] [CrossRef]
- Papackova, Z.; Cahova, M. Fatty Acid Signaling: The New Function of Intracellular Lipases. Int. J. Mol. Sci. 2015, 16, 3831–3855. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, S.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Effects of fishmeal replacement by black soldier fly on growth performance, digestive enzyme activity, intestine morphology, intestinal flora and immune response of pearl gentian grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Fish Shellfish Immunol. 2021, 120, 497–506. [Google Scholar] [CrossRef]
- Yang, S.; Du, J.; Luo, J.; Zhou, Y.; Long, Y.; Xu, G.; Zhao, L.; Du, Z.; Yan, T. Effects of different diets on the intestinal microbiota and immunity of common carp (Cyprinus carpio). J. Appl. Microbiol. 2019, 127, 1327–1338. [Google Scholar] [CrossRef]
- Liu, W.; Guo, X.; Chen, Y.; Tang, Y.; Xiao, H.; Li, S. Carvacrol promotes intestinal health in Pengze crucian carp, enhancing resistance to Aeromonas hydrophila. Aquac. Rep. 2020, 17, 100325. [Google Scholar] [CrossRef]
- Nikoskelainen, S.; Salminen, S.; Bylund, G.; Ouwehand, A.C. Characterization of the properties of human- and dairy-derived probiotics for prevention of infectious diseases in fish. Appl. Environ. Microbiol. 2001, 67, 2430–2435. [Google Scholar] [CrossRef]
- Panigrahi, A.; Kiron, V.; Satoh, S.; Hirono, I.; Kobayashi, T.; Sugita, H.; Puangkaew, J.; Aoki, T. Immune modulation and expression of cytokine genes in rainbow trout Oncorhynchus mykiss upon probiotic feeding. Dev. Comp. Immunol. 2006, 31, 372–382. [Google Scholar] [CrossRef]
- Pirarat, N.; Kobayashi, T.; Katagiri, T.; Maita, M.; Endo, M. Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia (Oreochromis niloticus). Vet. Immunol. Immunopathol. 2006, 113, 339–347. [Google Scholar] [CrossRef]
- Walczak, N.; Puk, K.; Guz, L. Bacterial flora associated with diseased freshwater ornamental fish. J. Vet. Res. 2017, 61, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Sánchez, A.D.J.; Espinosa-Chaurand, L.D.; Díaz-Ramirez, M.; Torres-Ochoa, E. Plesiomonas: A Review on Food Safety, Fish-Borne Diseases, and Tilapia. Sci. World J. 2021, 3119958. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Naya-Català, F.; Piazzon, M.C.; Rimoldi, S.; Calduch-Giner, J.; Giardini, A.; Martínez, I.; Brambilla, F.; Pérez-Sánchez, J.; Terova, G. Evaluating nisin-producing Lactococcus lactis strain as probiotics for gilthead sea bream (Sparus aurata) to determine how growth, gut microbiota, and transcriptional response are affected. Front. Mar. Sci. 2021, 8, 2021. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Simó-Mirabet, P.; Piazzon, M.C.; Calduch-Giner, J.A.; Ortiz, Á.; Puyalto, M.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Sodium salt medium-chain fatty acids and Bacillus-based probiotic strategies to improve growth and intestinal health of gilthead sea bream (Sparus aurata). PeerJ 2017, 5, e4001. [Google Scholar] [CrossRef]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Liu, Z. Fuzhuan Brick Tea Attenuates High-Fat Diet-Induced Obesity and Associated Metabolic Disorders by Shaping Gut Microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef]


| Ingredients (%) | Protein Sources | |
|---|---|---|
| FM | TM | |
| Aspartic acid | 6.20 | 4.61 |
| Glutamic acid | 9.45 | 6.93 |
| Serine | 2.02 | 4.91 |
| Histidine | 1.82 | 0.65 |
| Glycine | 4.01 | 4.67 |
| Threonine | 2.33 | 2.30 |
| Arginine | 3.87 | 3.93 |
| Alanine | 4.11 | 3.09 |
| Tyrosine | 1.78 | 1.88 |
| Cystine | 0.52 | 2.14 |
| Valine | 3.54 | 3.99 |
| Methionine | 1.67 | 1.16 |
| Phenylalanine | 3.09 | 3.01 |
| Leucine | 2.85 | 4.74 |
| Isoleucine | 4.86 | 2.65 |
| Lysine | 4.78 | 4.89 |
| Proline | 2.55 | 5.95 |
| Tryptophan | 0.57 | 0.39 |
| Total | 60.02 | 61.89 |
| Proximate analysis | ||
| Crude protein | 68.00 | 63.84 |
| Crude lipid | 9.66 | 1.99 |
| Experimental Diets | ||||
|---|---|---|---|---|
| TM0 | TM15 | TM30 | TM45 | |
| SR (%) | 84.22 ± 3.86 | 89.33 ± 3.06 | 76.33 ± 4.43 | 76.67 ± 1.15 |
| IBW (g) | 11.76 ± 0.06 | 11.78 ± 0.06 | 11.84 ± 0.02 | 11.84 ± 0.04 |
| FBW (g) | 26.59 ± 0.27 b | 26.18 ± 0.31 b | 24.89 ± 0.15 b | 21.31 ± 1.02 a |
| WG (%) | 124.92 ± 2.30 b | 121.47 ± 2.67 b | 110.33 ± 1.23 b | 80.29 ± 8.66 a |
| FE | 0.72 ± 0.02 b | 0.70 ± 0.01 b | 0.57 ± 0.04 a | 0.47 ± 0.03 a |
| FI (%/d) | 2.12 ± 0.08 | 2.00 ± 0.06 | 2.24 ± 0.14 | 2.36 ± 0.04 |
| PER | 1.50 ± 0.04 c | 1.44 ± 0.02 bc | 1.20 ± 0.08 ab | 0.98 ± 0.05 a |
| CF (g cm−3) | 1.91 ± 0.03 | 1.83 ± 0.03 | 1.83 ± 0.03 | 1.81 ± 0.03 |
| HSI (%) | 2.24 ± 0.17 | 2.59 ± 0.01 | 2.61 ± 0.04 | 2.51 ± 0.06 |
| VSI (%) | 7.22 ± 0.16 | 7.63 ± 0.09 | 7.62 ± 0.05 | 7.55 ± 0.09 |
| PDR (%) | 4.19 ± 0.08 b | 3.92 ± 0.03 b | 3.65 ± 0.15 b | 2.61 ± 0.26 a |
| LDR (%) | 17.80 ± 0.85 c | 15.50 ± 0.48 bc | 13.70 ± 0.06 b | 10.75 ± 0.56 a |
| Index (Wet Weight, %) | Experimental Diets | |||
|---|---|---|---|---|
| TM0 | TM15 | TM30 | TM45 | |
| Whole-body | ||||
| Moisture | 74.16 ± 0.18 a | 74.68 ± 0.38 ab | 75.71 ± 0.41 ab | 75.91 ± 0.38 b |
| Crude protein | 13.65 ± 0.22 | 13.44 ± 0.14 | 13.59 ± 0.25 | 13.56 ± 0.06 |
| Crude lipid | 7.71 ± 0.26 | 7.34 ± 0.23 | 7.17 ± 0.02 | 7.19 ± 0.10 |
| Ash | 3.18 ± 0.08 a | 3.16 ± 0.01 a | 3.38 ± 0.02 b | 3.31 ± 0.02 ab |
| Liver | ||||
| Crude lipid | 19.00 ± 0.27 b | 17.27 ± 0.22 a | 19.20 ± 0.19 b | 19.17 ± 0.25 b |
| Index | Experimental Diets | |||
|---|---|---|---|---|
| TM0 | TM15 | TM30 | TM45 | |
| AKP (U/mL) | 25.65 ± 0.47 bc | 25.36 ± 1.15 c | 18.39 ± 0.86 b | 14.13 ± 0.74 a |
| LZM (μg/mL) | 4.96 ± 0.18 a | 5.67 ± 0.11 b | 5.33 ± 0.04 ab | 5.29 ± 0.22 ab |
| C3 (μg/mL) | 208.03 ± 7.42 a | 211.76 ± 9.82 a | 256.64 ± 11.15 b | 224.57 ± 8.03 ab |
| C4 (μg/mL) | 1099.97 ± 25.43 c | 569.28 ± 19.03 b | 508.75 ± 7.34 b | 350.77 ± 12.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Dong, Y.; Song, K.; Wang, L.; Li, X.; Tan, B.; Lu, K.; Zhang, C. Effects of the Replacement of Dietary Fish Meal with Defatted Yellow Mealworm (Tenebrio molitor) on Juvenile Large Yellow Croakers (Larimichthys crocea) Growth and Gut Health. Animals 2022, 12, 2659. https://doi.org/10.3390/ani12192659
Zhang J, Dong Y, Song K, Wang L, Li X, Tan B, Lu K, Zhang C. Effects of the Replacement of Dietary Fish Meal with Defatted Yellow Mealworm (Tenebrio molitor) on Juvenile Large Yellow Croakers (Larimichthys crocea) Growth and Gut Health. Animals. 2022; 12(19):2659. https://doi.org/10.3390/ani12192659
Chicago/Turabian StyleZhang, Jian, Yanzou Dong, Kai Song, Ling Wang, Xueshan Li, Beiping Tan, Kangle Lu, and Chunxiao Zhang. 2022. "Effects of the Replacement of Dietary Fish Meal with Defatted Yellow Mealworm (Tenebrio molitor) on Juvenile Large Yellow Croakers (Larimichthys crocea) Growth and Gut Health" Animals 12, no. 19: 2659. https://doi.org/10.3390/ani12192659
APA StyleZhang, J., Dong, Y., Song, K., Wang, L., Li, X., Tan, B., Lu, K., & Zhang, C. (2022). Effects of the Replacement of Dietary Fish Meal with Defatted Yellow Mealworm (Tenebrio molitor) on Juvenile Large Yellow Croakers (Larimichthys crocea) Growth and Gut Health. Animals, 12(19), 2659. https://doi.org/10.3390/ani12192659









