Cryopreservation of Roughscale Sole (Clidoderma asperrimum) Sperm: Effects of Cryoprotectant, Diluent, Dilution Ratio, and Thawing Temperature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Handling and Sperm Collection
2.2. Cryoprotective Medium
2.3. Dilution Ratio and Thawing Temperature
2.4. Assessment of Sperm Motility
2.5. Evaluation of Survival Rate
2.6. Single-Cell Gel Electrophoresis
2.7. Fertilization Rate Evaluation
2.8. Statistical Analysis
3. Results
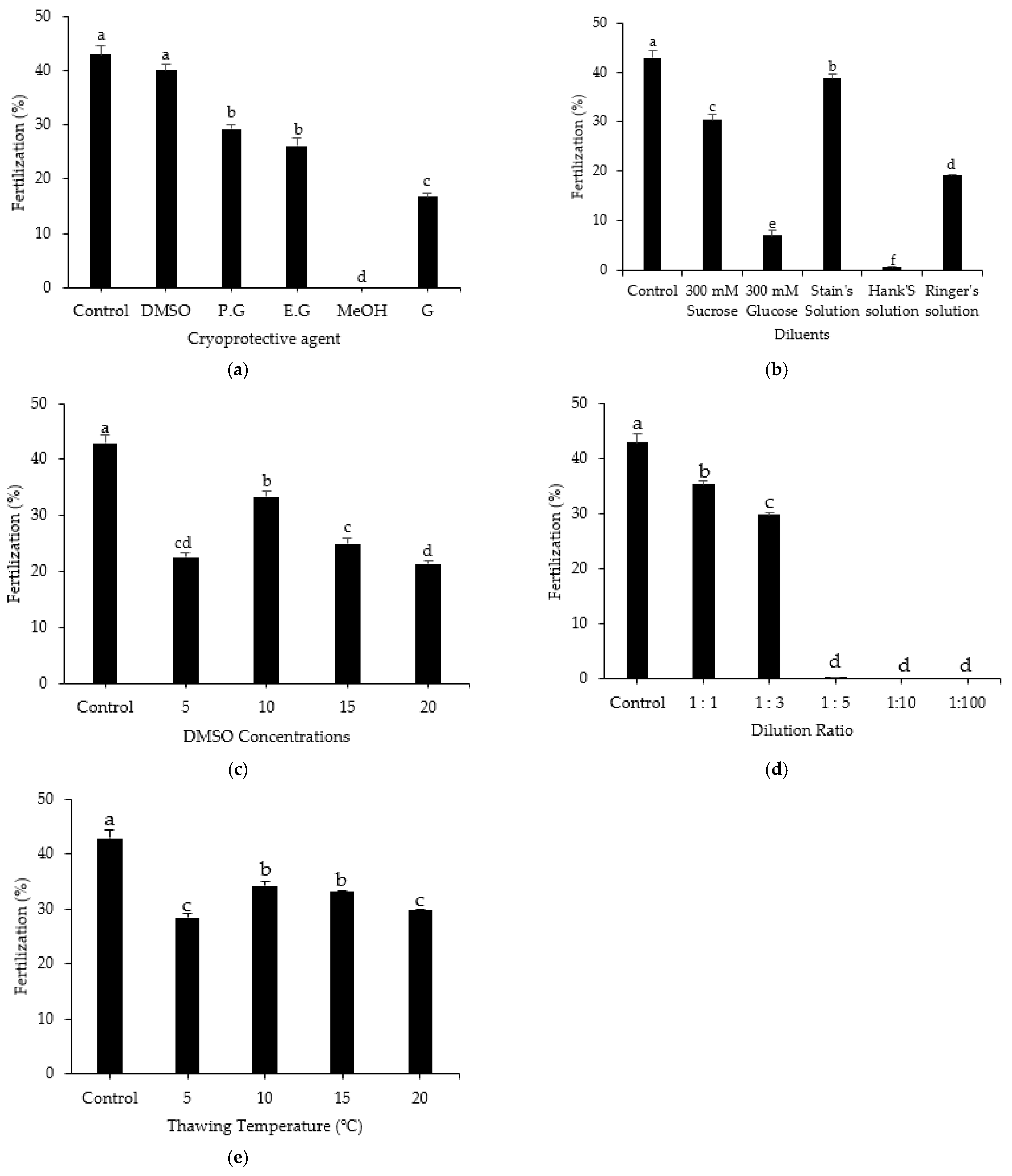
3.1. Effects of Cryoprotective Agent on post-Thaw Motility, Kinematic Parameters, and Cell Survival Rate
3.2. Effects of Diluent on Post-Thaw Motility, Kinematic Parameters, and Cell Survival Rate
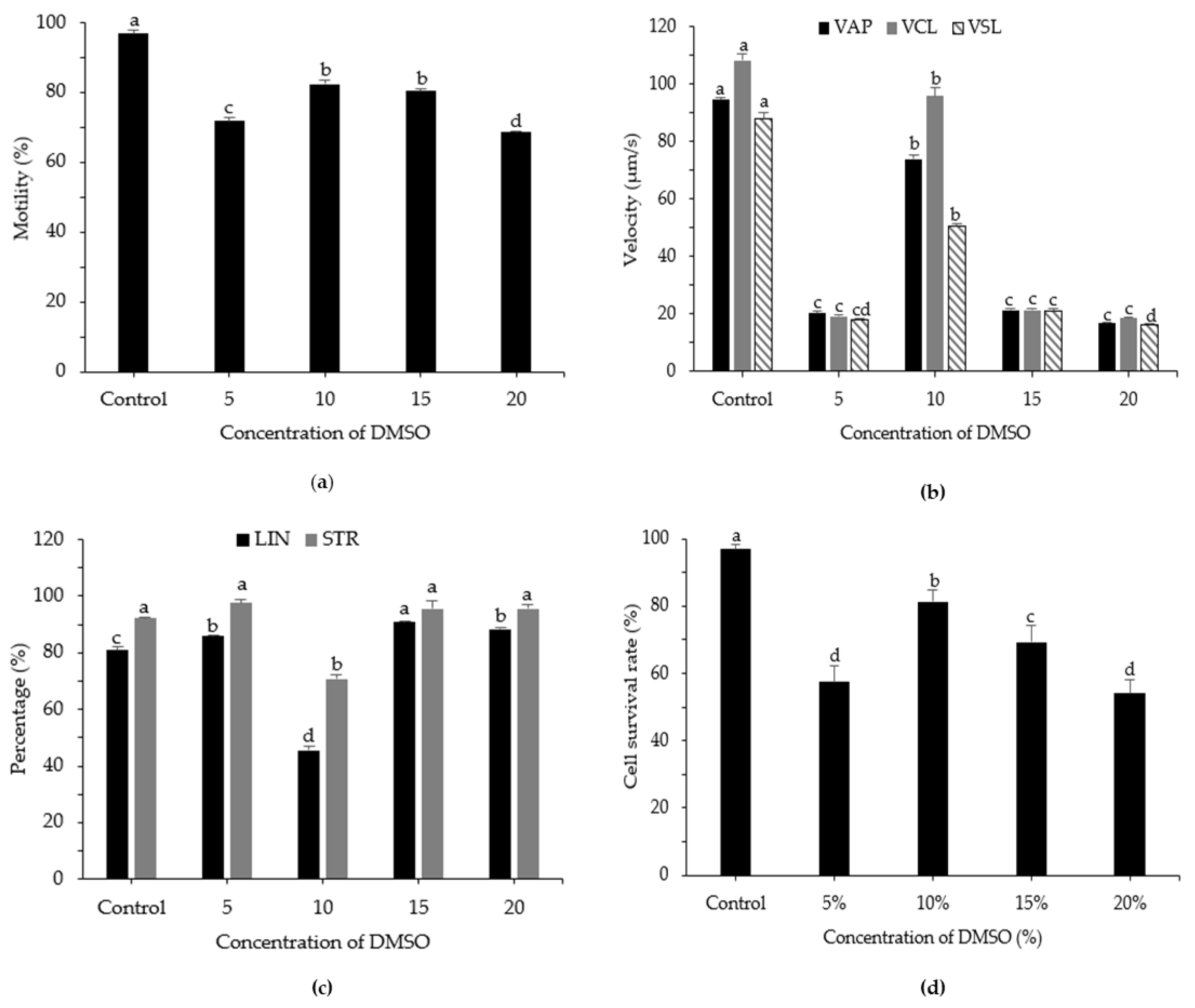
3.3. Effects of DMSO Concentration on Post-Thaw Motility, Kinematic Parameters, and Cell Survival Rate
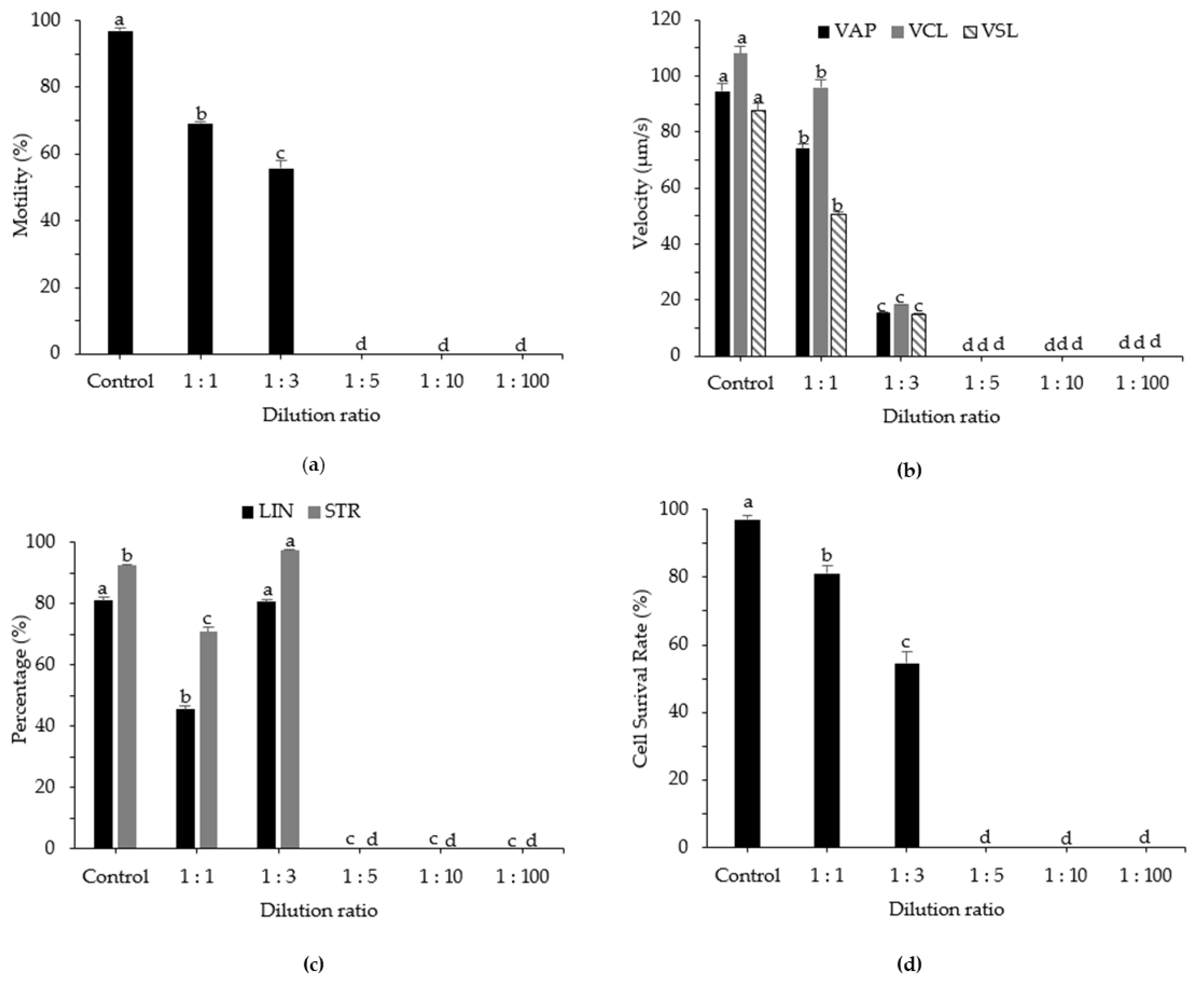
3.4. Effects of Sperm Dilution Ratio on Post-Thaw Motility, Kinematic Parameters, and Cell Survival Rate
3.5. Effects of Thawing Temperature on Post-Thaw Motility, Kinematic Parameters, and Cell Survival Rate
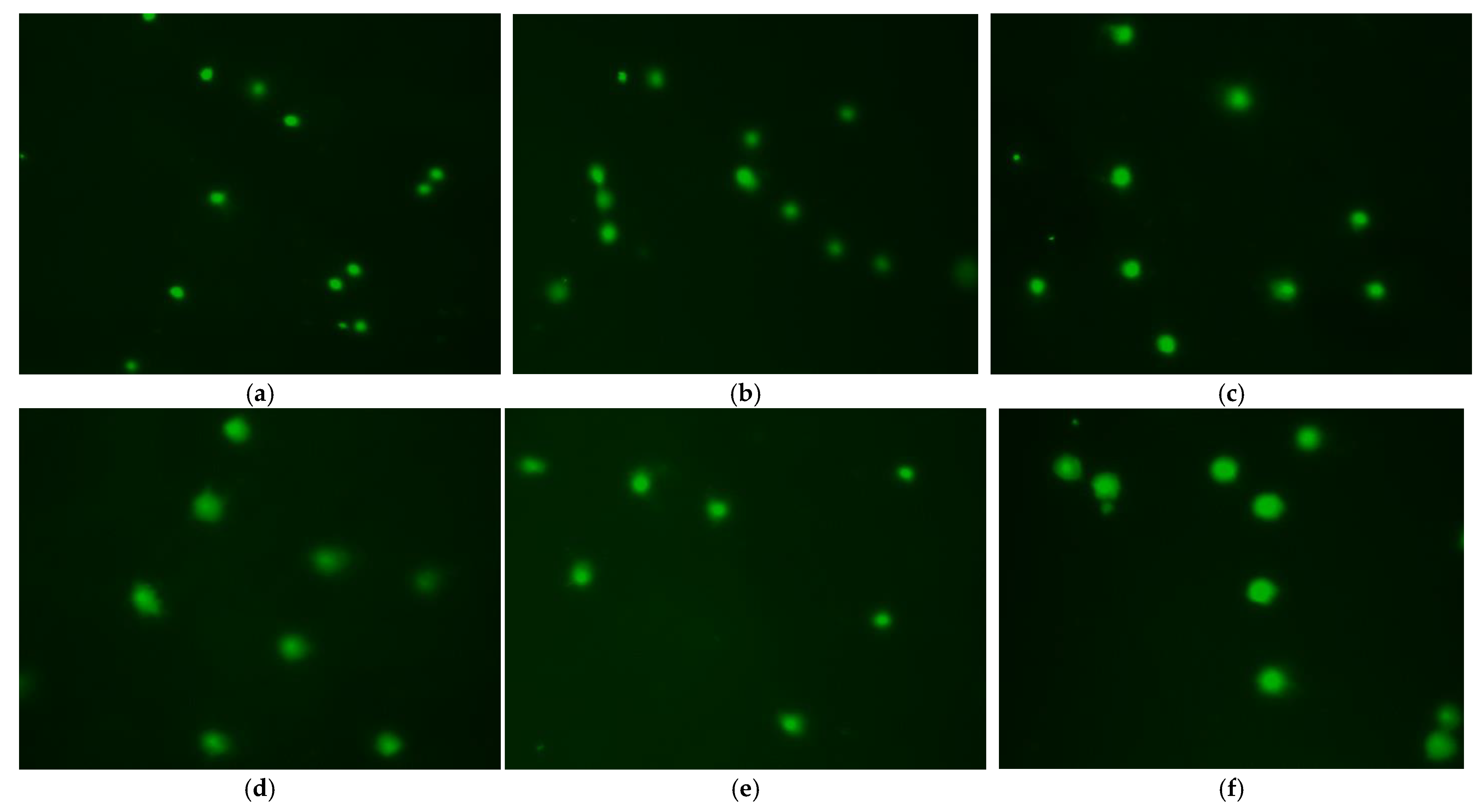
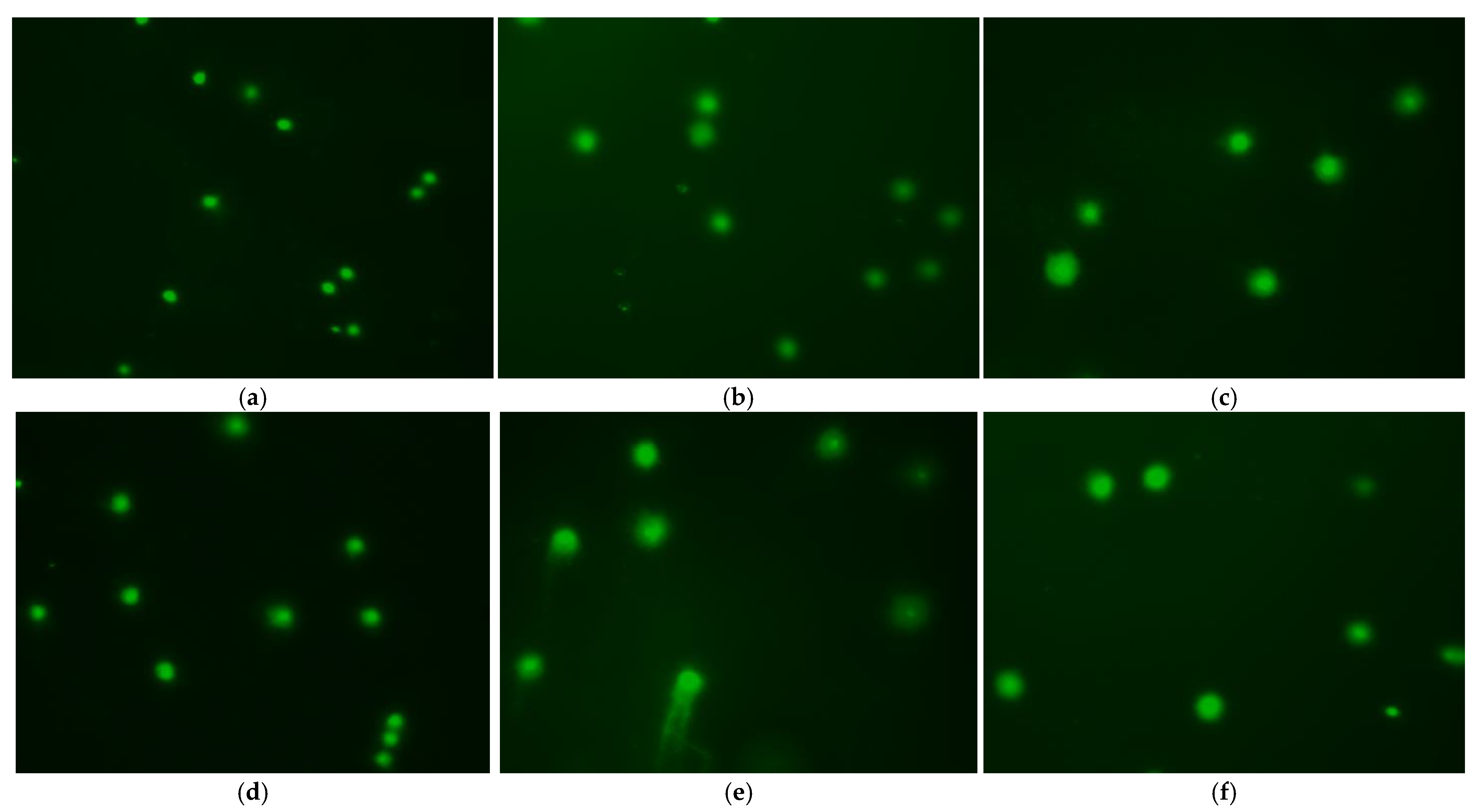
3.6. Effect of Treatment Method on DNA Damage
3.7. Effect of Treatment Method on Fertilization Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Fisheries Research and Development Institute (NFRDI). Aquacultural Technique Development of Roughscale Sole; NFRDI: Busan, Korea, 2010.
- Woo, S.M.; Lee, H.B.; Seo, Y.S.; Lim, H.K. Effects of Exogenous Hormones Treatment on Spermiation and Plasma Levels of Gonadal Steroids in Roughscale Sole, Clidoderma Asperrimum. Fish. Aquat. Sci. 2021, 24, 437–445. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kin, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and Its Clinical Applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Zidni, I.; Lee, Y.H.; Park, J.Y.; Lee, H.B.; Hur, J.W.; Lim, H.K. Effects of Cryoprotective Medium Composition, Dilution Ratio, and Freezing Rates on Spotted Halibut (Verasper Variegatus) Sperm Cryopreservation. Animals 2020, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Gao, D.; Baust, J.M. Cryopreservation: An Emerging Paradigm Change. Organogenesis 2009, 5, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K. Effect of Exogenous Hormones on Ovulation and Gonadal Steroid Plasma Levels in Starry Flounder, Platichthys Stellatus. Aquac. Int. 2016, 24, 1061–1071. [Google Scholar] [CrossRef]
- Zidni, I.; Bin, H.; Hye, J.; Yeol, J.; Dae, Y.; Seok, H.; Su, Y.; Young, I.; Kyu, H. Effect of Antioxidants in Cryopreservation Media on Spotted Halibut ( Verasfer Variegatus ) Sperm Quality during Cryopreservation. Aquaculture 2022, 557, 738351. [Google Scholar] [CrossRef]
- Lim, H.K.; Irfan, Z.; Lee, H.B.; Song, J.H.; Lee, Y.H. Effect of Diluent Variation on Cryopreservation of Large Yellow Croaker Larimichthys Crocea. Fish. Aquat. Sci. 2021, 24, 63–77. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Park, J.Y.; Lim, H.K. Effects of Different Diluents, Cryoprotective Agents, and Freezing Rates on Sperm Cryopreservation in Epinephelus Akaara. Cryobiology 2018, 83, 60–64. [Google Scholar] [CrossRef]
- Herranz-Jusdado, J.G.; Gallego, V.; Rozenfeld, C.; Morini, M.; Pérez, L.; Asturiano, J.F. European Eel Sperm Storage: Optimization of Short-Term Protocols and Cryopreservation of Large Volumes. Aquaculture 2019, 506, 42–50. [Google Scholar] [CrossRef]
- Park, J.Y.; Zidni, I.; Lee, Y.H.; Lee, H.B.; Lim, H.K. Effect of Long-Term Storage on the Quality of Cryopreserved Sperm of the Giant Grouper, Epinephelus Lanceolatus. Aquaculture 2022, 555, 738154. [Google Scholar] [CrossRef]
- Dusinska, M.; Collins, A.R. The Comet Assay in Human Biomonitoring: Gene-Environment Interactions. Mutagenesis 2008, 23, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Nowsheen, S.; Xia, F.; Yang, E.S. Assaying DNA Damage in Hippocampal Neurons Using the Comet Assay. J. Vis. Exp. 2012, e50049. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L. Impact of the Comet Assay in Radiobiology. Mutat. Res.-Rev. Mutat. Res. 2009, 681, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, E.; Robles, V.; Rebordinos, L.; Sarasquete, C.; Herráez, M.P. Evaluation of DNA Damage in Rainbow Trout (Oncorhynchus Mykiss) and Gilthead Sea Bream (Sparus Aurata) Cryopreserved Sperm. Cryobiology 2005, 50, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, J.Y.; Lee, I.Y.; Zidni, I.; Lim, H.K. Effects of Cryoprotective Agents and Treatment Methods on Sperm Cryopreservation of Stone Flounder, Kareius Bicoloratus. Aquaculture 2021, 531, 735969. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Martínez-Páramo, S.; Cabrita, E.; Martínez-Pastor, F.; de Paz, P.; Herráez, M.P. Evaluation of Oxidative DNA Damage Promoted by Storage in Sperm from Sex-Reversed Rainbow Trout. Theriogenology 2009, 71, 605–613. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Chang, Y.-J.; Lim, H.-K. Cryopreservation of Tiger Puffer (Takifugu Rubripes) Sperm. Dev. Reprod. 1997, 1, 29–36. [Google Scholar]
- Koh, I.C.C.; Hamada, D.; Tsuji, Y.A.; Okuda, D.; Nomura, K.; Tanaka, H.; Ohta, H. Sperm Cryopreservation of Japanese Eel, Anguilla Japonica. Aquaculture 2017, 473, 487–492. [Google Scholar] [CrossRef]
- Cabrita, E.; Anel, L.; Herraez, M. Effect of External Cryoprotectant as Membrane Stabilizers on Cryopreserved Rainbow Trout Sperm. Theriogenology 2001, 56, 623–635. [Google Scholar] [CrossRef]
- Lim, H.K.; An, C.M.; Noh, G.A.; Min, B.H. Effects of Diluents and Cryoprotectants on Sperm Cryopreservation in Starry Flounder (Platichthys Stellatus). J. Aquac. 2007, 20, 173–177. [Google Scholar]
- Liu, Q.H.; Ma, D.Y.; Xu, S.H.; Xiao, Z.Z.; Xiao, Y.S.; Song, Z.C.; Li, J. Summer Flounder (Paralichthys Dentatus) Sperm Cryopreservation and Application in Interspecific Hybridization with Olive Flounder (P Olivaceus). Theriogenology 2015, 83, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Lanes, C.F.C.; Okamoto, M.; Cavalcanti, P.V.; Collares, T.; Campos, V.F.; Deschamps, J.C.; Robaldo, R.B.; Marins, L.F.; Sampaio, L.A. Cryopreservation of Brazilian Flounder (Paralichthys Orbignyanus) Sperm. Aquaculture 2008, 275, 361–365. [Google Scholar] [CrossRef]
- Dreanno, C.; Suquet, M.; Quemener, L.; Cosson, J.; Fierville, F.; Normant, Y.; Billard, R. Cryopreservation of Turbot (Scophthalmus Maximus) Spermatozoa. Theriogenology 1997, 48, 589–603. [Google Scholar] [CrossRef]
- Hossen, S.; Kim, S.C.; Cho, Y.; Kho, K.H. Vital Analysis of Cryopreserved Sperm of Marbled Flounder, Pseudopleuronectes Yokohamae. Front. Physiol. 2021, 12, 696737. [Google Scholar] [CrossRef] [PubMed]
- Yavaş, I.; Bozkurt, Y.; Yildiz, C. Cryopreservation of Scaly Carp (Cyprinus Carpio) Sperm: Effect of Different Cryoprotectant Concentrations on Post-Thaw Motility, Fertilization and Hatching Success of Embryos. Aquac. Int. 2014, 22, 141–148. [Google Scholar] [CrossRef]
- Betsy, J.; Kumar, S. Cryopreservation of Fish Gametes; Springer: Singapore, 2020; ISBN 9789811540240. [Google Scholar]
- Oldenhof, H.; Gojowsky, M.; Wang, S.; Henke, S.; Yu, C.; Rohn, K.; Wolkers, W.F.; Sieme, H. Osmotic Stress and Membrane Phase Changes during Freezing of Stallion Sperm: Mode of Action of Cryoprotective Agents. Biol. Reprod. 2013, 88, 68. [Google Scholar] [CrossRef] [PubMed]
- Akhoondi, M.; Oldenhof, H.; Sieme, H.; Wolkers, W.F. Freezing-Induced Cellular and Membrane Dehydration in the Presence of Cryoprotective Agents. Mol. Membr. Biol. 2012, 29, 197–206. [Google Scholar] [CrossRef]
- Yang, H.; Hu, E.; Tiersch, T.; Carmichael, C.; Matthews, J.; Varga, Z.M. Temporal and Concentration Effects of Methanol on Cryopreservation of Zebrafish (Danio Rerio) Sperm. Zebrafish 2020, 17, 233–242. [Google Scholar] [CrossRef]
- Abinawanto, A.; Vardini, N.; Kristanto, A.H.; Lestari, R.; Bowolaksono, A. Effect of Egg Yolk of Free-Range Chicken and Methanol as a Cryoprotective Agent for the Sperm Preservation of Cyprinid Fish, Neolissochilus Soroides (Valenciennes, 1842). Heliyon 2021, 7, e08158. [Google Scholar] [CrossRef]
- Shirley, C.A.; Colvin, M.E.; Tiersch, T.R.; Allen, P.J. A Generalized Approach for Sperm Cryopreservation in the Genus Pomoxis: Sperm Cryopreservation and Fertilization Efficiency of Black-Stripe Black Crappie, Pomoxis Nigromaculatus. J. World Aquac. Soc. 2021, 52, 405–417. [Google Scholar] [CrossRef]
- Depincé, A.; Gabory, A.; Dziewulska, K.; Le Bail, P.Y.; Jammes, H.; Labbé, C. DNA Methylation Stability in Fish Spermatozoa upon External Constraint: Impact of Fish Hormonal Stimulation and Sperm Cryopreservation. Mol. Reprod. Dev. 2020, 87, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Kawamura, K.; Unuma, T.; Takegoshi, Y. Cryopreservation of the Sperm of the Japanese Bitterling. J. Fish Biol. 2001, 58, 670–681. [Google Scholar] [CrossRef]
- Fujimoto, T.; Kaneyasu, T.; Endoh, M.; Kogame, Y.; Nynca, J.; Ciereszko, A.; Takahashi, E.; Yamaha, E.; Naruse, K.; Arai, K. Cryopreservation of Masu Salmon Sperm Using Glucose-Methanol Extender and Seminal Plasma Biomarkers Related to Post-Thaw Sperm Motility. Aquaculture 2022, 557, 738305. [Google Scholar] [CrossRef]
- Ugwu, S.I.; Kowalska, A.; Morita, M.; Kowalski, R.K. Application of Glucose-Methanol Extender to Cryopreservation of Mozambique Tilapia (Oreochromis Mossambicus) Sperm. Turkish J. Fish. Aquat. Sci. 2019, 19, 41–50. [Google Scholar] [CrossRef]
- Hee Kho, K.; Ho Kang, K. Cryopreservation of Pointhead Flounder (Cleisthenes Pinetorum Herzensteini) Sperm. Korean J. Ichthyol. 2003, 15, 213–218. [Google Scholar]
- Lim, H.K.; Le, M.H. Evaluation of Extenders and Cryoprotectants on Motility and Morphology of Longtooth Grouper (Epinephelus Bruneus) Sperm. Theriogenology 2013, 79, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Bayrle, H. Cryopreservation of the Sperm of Some Freshwater Teleosts. Ann. Biol. Anim. Biochim. Biophys. 1978, 18, 1073–1076. [Google Scholar] [CrossRef]
- Suquet, M.; Normant, Y.; Gaignon, J.L.; Quéméner, L.; Fauvel, C. Effect of Water Temperature on Individual Reproductive Activity of Pollack (Pollachius Pollachius). Aquaculture 2005, 243, 113–120. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Tian, Y.; Li, Z.; Wu, Y.; Li, Z.; Wang, L.; Ma, W.; Zhai, J. Cryopreservation of Kelp Grouper (Epinephelus Moara) Embryos Using Non-Permeating Cryoprotectants. Aquaculture 2020, 519, 734939. [Google Scholar] [CrossRef]
- Rideout, R.M.; Litvak, M.K.; Trippel, E.A. The Development of a Sperm Cryopreservation Protocol for Winter Flounder Pseudopleuronectes Americanus (Walbaum): Evaluation of Cryoprotectants and Diluents. Aquac. Res. 2003, 34, 653–659. [Google Scholar] [CrossRef]
- Brown, R.T.; Colburn, H.R.; Nardi, G.C.; Berlinsky, D.L. Cryopreservation of Summer Flounder, Paralichthys Dentatus l., Sperm. Aquac. Res. 2013, 44, 1560–1567. [Google Scholar] [CrossRef]
- Huang, C.; Dong, Q.; Walter, R.B.; Tiersch, T.R. Sperm Cryopreservation of Green Swordtail Xiphophorus Helleri, a Fish with Internal Fertilization. Cryobiology 2004, 48, 295–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lal, K.K.; Barman, A.S.; Punia, P.; Khare, P.; Mohindra, V.; Lal, B.; Gopalakrishnan, A.; Sah, R.S.; Lakra, W.S. Effect of Extender Composition on Sperm Cryopreservation of Asian Catfish Heteropneustes Fossilis (Bloch) and Clarias Batrachus (Linnaeus). Asian Fish. Sci. 2009, 22, 137–142. [Google Scholar] [CrossRef]
- Cosson, J.; Billard, R.; Cibert, C.; Dreanno, C.; Suquet, M. Ionic Factors Regulating the Motility of Fish Sperm. Int. Symp. Spermatol. 1998, 161–186. [Google Scholar]
- Suquet, M.; Dreanno, C.; Fauvel, C.; Cosson, J.; Billard, R. Cryopreservation of Sperm in Marine Fish. Aquac. Res. 2000, 31, 231–243. [Google Scholar] [CrossRef]
- Correa, J.R.; Zavos, P.M. Frozen-Thawed Bovine Spermatozoa Diluted by Slow or Rapid Dilution Method: Measurements on Occurrence of Osmotic Shock and Sperm Viability. Theriogenology 1995, 44, 963–971. [Google Scholar] [CrossRef]
- Bolla, S.; Holmefjord, I.; Refstie, T. Cryogenic Preservation of Atlantic Halibut Sperm. Aquaculture 1987, 65, 371–374. [Google Scholar] [CrossRef]
- Ding, F.; Lall, S.P.; Li, J.; Lei, J.; Rommens, M.; Milley, J.E. Cryopreservation of Sperm from Atlantic Halibut (Hippoglossus Hippoglossus, L.) for Commercial Application. Cryobiology 2011, 63, 56–60. [Google Scholar] [CrossRef]
- Chereguini, O.; García De La Banda, I.; Herrera, M.; Martinez, C.; De La Hera, M. Cryopreservation of Turbot Scophthalmus Maximus (L.) Sperm: Fertilization and Hatching Rates. Aquac. Res. 2003, 34, 739–747. [Google Scholar] [CrossRef]
- Zilli, L.; Schiavone, R.; Zonno, V.; Storelli, C.; Vilella, S. Evaluation of DNA Damage in Dicentrarchus Labrax Sperm Following Cryopreservation. Cryobiology 2003, 47, 227–235. [Google Scholar] [CrossRef]
- Niu, J.; Wang, X.; Liu, P.; Liu, H.; Li, R.; Li, Z.; He, Y.; Qi, J. Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes Schlegelii). Int. J. Mol. Sci. 2022, 23, 3392. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Zhu, J.; Yang, W.; Wei, P.; Wu, X. Sperm Cryopreservation in Sparus Macrocephalus and DNA Damage Detection with SCGE. Zool. Res. 2009, 30, 151–157. [Google Scholar] [CrossRef]
- Hossen, B.; Hossain, M.S.; Rashid, I.; Hasan, M.N.; Ethin, R.; Thapa, H.; Majumdar, B.C. Effect of Extenders and Storage Periods on Motility and Fertilization Rate of Silver Barb, Barbonymus Gonionotus (Bleeker, 1850). Int. J. Agric. Policy Res. 2017, 5, 178–185. [Google Scholar] [CrossRef]
- Dolfi, L.; Suen, T.K.; Ripa, R.; Antebi, A. Sperm Cryopreservation and in Vitro Fertilization Techniques for the African Turquoise Killifish Nothobranchius Furzeri. Sci. Rep. 2021, 11, 17145. [Google Scholar] [CrossRef]



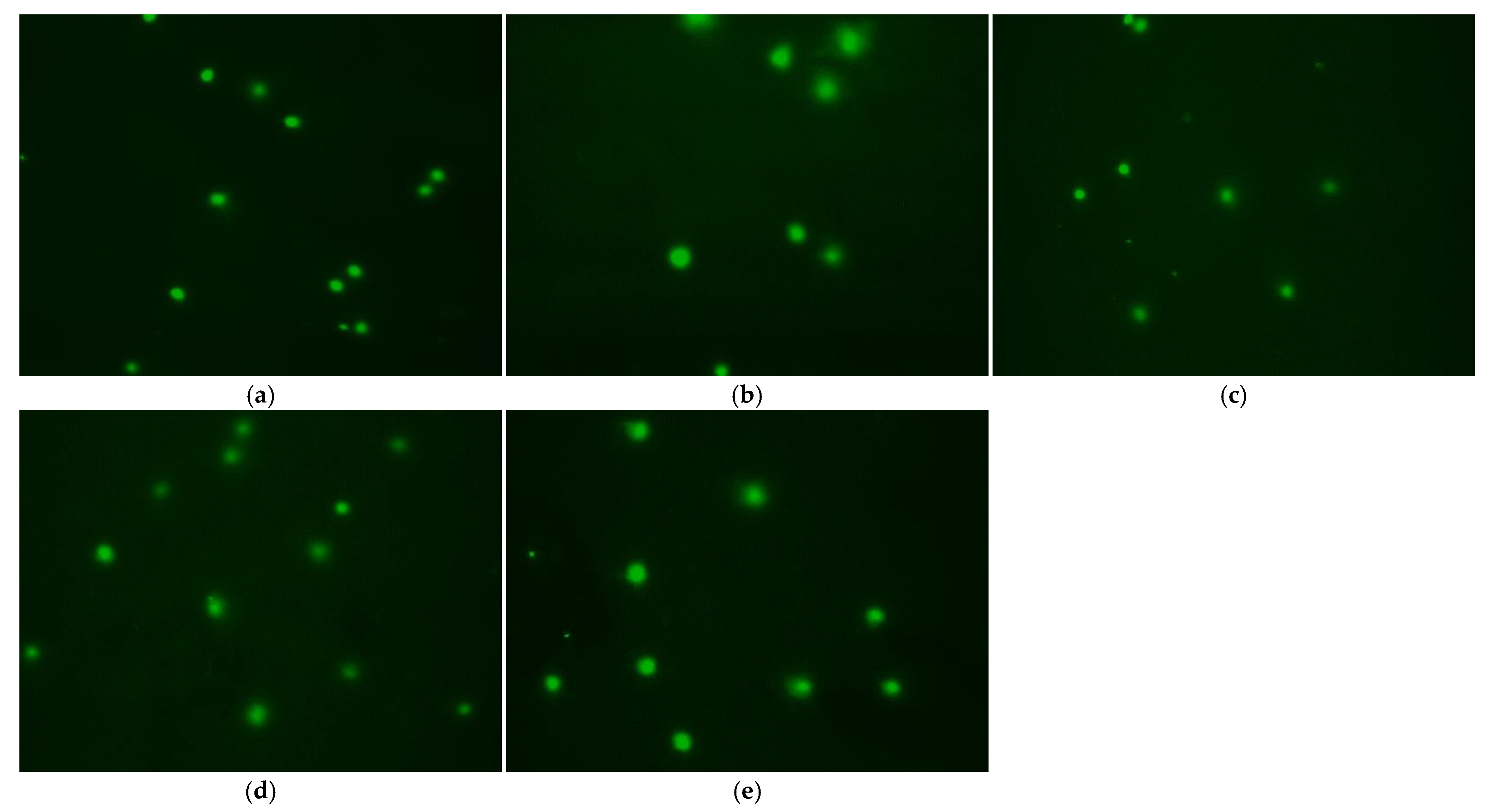
| Control | Different Cryoprotective Agent | |||||
|---|---|---|---|---|---|---|
| DMSO | P.G | E.G | Methanol | Glycerol | ||
| Head Length | 43.87 ± 0.385 c | 49.36 ± 0.501 a | 40.33 ± 0.487 d | 45.27 ± 0.414 b | 45.98 ± 0.489 b | 40.36 ± 0.380 d |
| Tail Length | 24.33 ± 0.219 e | 31.50 ± 0.296 d | 33.62 ± 0.346 c | 32.93 ± 0.253 c | 40.77 ± 0.280 a | 35.85 ± 0.270 b |
| Head Intensity | 94.25 ± 0.263 a | 88.18 ± 0.327 b | 83.23 ± 0.325 c | 82.81 ± 0.265 c | 74.48 ± 0.186 e | 78.71 ± 0.222 d |
| Tail Intensity | 5.70 ± 0.266 a | 11.75 ± 0.322 b | 16.50 ± 0.192 c | 17.19 ± 0.265 d | 25.52 ± 0.186 f | 21.29 ± 0.222 e |
| % Tail DNA | 1.305 ± 0.056 a | 3.319 ± 0.093 b | 5.948 ± 0.132 d | 4.295 ± 0.081 c | 4.488 ± 0.157 c | 6.691 ± 0.142 e |
| Tail Migration | 2.41 ± 0.117 a | 7.06 ± 0.186 b | 12.21 ± 0.145 c | 12.71 ± 0.084 d | 18.14 ± 0.109 f | 15.35 ± 0.137 e |
| Control | Different Diluents | |||||
|---|---|---|---|---|---|---|
| 300 mM Sucrose | 300 mM Glucose | Stain’s Solution | Hank’s Solution | Ringer’s Solution | ||
| Head Length | 43.87 ± 0.385 c | 45.84 ± 0.432 a | 39.24 ± 0.350 c | 46.31 ± 0.444 a | 39.44 ± 0.406 c | 39.91 ± 0.391 c |
| Tail Length | 24.33 ± 0.219 e | 34.80 ± 0.239 d | 37.42 ± 0.221 b | 30.99 ± 0.278 e | 38.73 ± 0.238 a | 36.05 ± 0.233 c |
| Head Intensity | 94.25 ± 0.263 a | 86.18 ± 0.487 c | 70.03 ± 0.221 e | 89.20 ± 0.307 b | 68.82 ± 0.266 f | 72.70 ± 0.245 d |
| Tail Intensity | 5.70 ± 0.266 a | 13.82 ± 0.487 c | 29.97 ± 0.22 e | 10.80 ± 0.307 b | 31.18 ± 0.266 f | 27.30 ± 0.245 d |
| % Tail DNA | 1.305 ± 0.056 a | 3.696 ± 0.127 c | 8.402 ± 0.063 e | 2.835 ± 0.083 b | 8.941 ± 0.065 f | 7.529 ± 0.062 d |
| Tail Migration | 2.41 ± 0.117 a | 11.84 ± 0.12 c | 17.80 ± 0.102 e | 8.01 ± 0.126 b | 19.01 ± 0.099 f | 12.44 ± 0.072 d |
| Control | DMSO Concentrations (%) | ||||
|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | ||
| Head Length | 43.87 ± 0.385 c | 48.56 ± 0.349 b | 49.15 ± 0.370 b | 50.36 ± 0.397 a | 50.56 ± 0.436 a |
| Tail Length | 24.33 ± 0.219 e | 34.91 ± 0.208 b | 30.31 ± 0.211 d | 32.84 ± 0.226 c | 38.67 ± 0.220 a |
| Head Intensity | 94.25 ± 0.263 a | 86.92 ± 0.434 d | 92.11 ± 0.264 b | 90.46 ± 0.355 c | 84.50 ± 0.475 e |
| Tail Intensity | 5.70 ± 0.266 a | 13.08 ± 0.434 d | 7.89 ± 0.264 b | 9.54 ± 0.355 c | 15.50 ± 0.475 e |
| % Tail DNA | 1.305 ± 0.056 a | 3.612 ± 0.124 d | 2.095 ± 0.071 b | 2.626 ± 0.097 c | 4.711 ± 0.165 e |
| Tail Migration | 2.41 ± 0.117 a | 10.65 ± 0.098 b | 6.07 ± 0.067 b | 7.65 ± 0.100 c | 12.44 ± 0.072 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zidni, I.; Lee, H.-B.; Yoon, J.-H.; Park, J.-Y.; Jang, H.-S.; Cho, Y.-S.; Seo, Y.-S.; Lim, H.-K. Cryopreservation of Roughscale Sole (Clidoderma asperrimum) Sperm: Effects of Cryoprotectant, Diluent, Dilution Ratio, and Thawing Temperature. Animals 2022, 12, 2553. https://doi.org/10.3390/ani12192553
Zidni I, Lee H-B, Yoon J-H, Park J-Y, Jang H-S, Cho Y-S, Seo Y-S, Lim H-K. Cryopreservation of Roughscale Sole (Clidoderma asperrimum) Sperm: Effects of Cryoprotectant, Diluent, Dilution Ratio, and Thawing Temperature. Animals. 2022; 12(19):2553. https://doi.org/10.3390/ani12192553
Chicago/Turabian StyleZidni, Irfan, Hyo-Bin Lee, Ji-Hye Yoon, Jung-Yeol Park, Hyun-Seok Jang, Youn-Su Cho, Young-Seok Seo, and Han-Kyu Lim. 2022. "Cryopreservation of Roughscale Sole (Clidoderma asperrimum) Sperm: Effects of Cryoprotectant, Diluent, Dilution Ratio, and Thawing Temperature" Animals 12, no. 19: 2553. https://doi.org/10.3390/ani12192553
APA StyleZidni, I., Lee, H.-B., Yoon, J.-H., Park, J.-Y., Jang, H.-S., Cho, Y.-S., Seo, Y.-S., & Lim, H.-K. (2022). Cryopreservation of Roughscale Sole (Clidoderma asperrimum) Sperm: Effects of Cryoprotectant, Diluent, Dilution Ratio, and Thawing Temperature. Animals, 12(19), 2553. https://doi.org/10.3390/ani12192553






